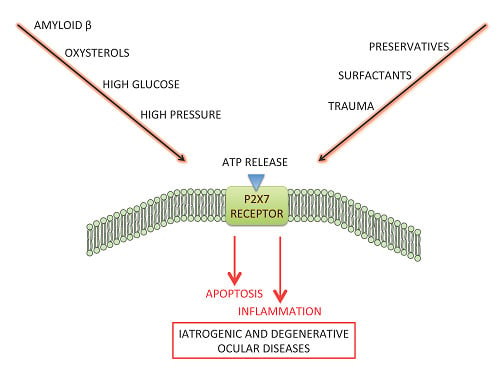
The Role of the P2X7 Receptor in Ocular Stresses: A Potential Therapeutic Target
Abstract
:1. Introduction
2. P2X7 Receptor Activation in the Case of Ocular Stresses
2.1. Exogenous Stresses: Chemical and Mechanical Injuries
2.1.1. Preservatives
2.1.2. Surfactants
2.1.3. Trauma
2.2. Endogenous Stresses: Biological Stresses
2.2.1. AMD
2.2.2. Diabetic Retinopathy
2.2.3. Glaucoma
3. Anti-P2X7 Strategies in Ophthalmology
3.1. Topical Administration
3.2. Oral Administration
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- North, R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002, 82, 1013–1067. [Google Scholar] [CrossRef] [PubMed]
- Ralevic, V.; Burnstock, G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998, 50, 413–492. [Google Scholar] [PubMed]
- Lundy, P.M.; Hamilton, M.G.; Mi, L.; Gong, W.; Vair, C.; Sawyer, T.W.; Frew, R. Stimulation of Ca(2+) influx through ATP receptors on rat brain synaptosomes: Identification of functional P2X(7) receptor subtypes. Br. J. Pharmacol. 2002, 135, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Miras-Portugal, M.T.; Diaz-Hernandez, M.; Giraldez, L.; Hervas, C.; Gomez-Villafuertes, R.; Sen, R.P.; Gualix, J.; Pintor, J. P2X7 receptors in rat brain: Presence in synaptic terminals and granule cells. Neurochem. Res. 2003, 28, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Deuchars, S.A.; Atkinson, L.; Brooke, R.E.; Musa, H.; Milligan, C.J.; Batten, T.F.; Buckley, N.J.; Parson, S.H.; Deuchars, J. Neuronal P2X7 receptors are targeted to presynaptic terminals in the central and peripheral nervous systems. J. Neurosci. 2001, 21, 7143–7152. [Google Scholar] [PubMed]
- Li, J.; Liu, D.; Ke, H.Z.; Duncan, R.L.; Turner, C.H. The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J. Biol. Chem. 2005, 280, 42952–42959. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, N.R.; Henriksen, Z.; Sorensen, O.H.; Eriksen, E.F.; Civitelli, R.; Steinberg, T.H. Intercellular calcium signaling occurs between human osteoblasts and osteoclasts and requires activation of osteoclast P2X7 receptors. J. Biol. Chem. 2002, 277, 7574–7580. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Luo, X.; Zeng, W.; Muallem, S. Cell-specific behavior of P2X7 receptors in mouse parotid acinar and duct cells. J. Biol. Chem. 2003, 278, 47554–47561. [Google Scholar] [CrossRef] [PubMed]
- Bardini, M.; Lee, H.Y.; Burnstock, G. Distribution of P2X receptor subtypes in the rat female reproductive tract at late pro-oestrus/early oestrus. Cell Tissue Res. 2000, 299, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wang, L.; Feng, Y.H.; Li, X.; Zeng, R.; Gorodeski, G.I. P2X7 receptor-mediated apoptosis of human cervical epithelial cells. Am. J. Physiol. Cell Physiol. 2004, 287, C1349–C1358. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Liang, H.; Pauloin, T.; Brignole-Baudouin, F.; Baudouin, C.; Warnet, J.M.; Rat, P. Effects of toxic cellular stresses and divalent cations on the human P2X7 cell death receptor. Mol. Vis. 2008, 14, 889–897. [Google Scholar] [PubMed]
- Mayo, C.; Ren, R.; Rich, C.; Stepp, M.A.; Trinkaus-Randall, V. Regulation by P2X7: Epithelial migration and stromal organization in the cornea. Investig. Ophthalmol. Vis. Sci. 2008, 49, 4384–4391. [Google Scholar] [CrossRef] [PubMed]
- Minns, M.S.; Teicher, G.; Rich, C.B.; Trinkaus-Randall, V. Purinoreceptor P2X7 Regulation of Ca(2+) Mobilization and Cytoskeletal Rearrangement Is Required for Corneal Reepithelialization after Injury. Am. J. Pathol. 2016, 186, 285–296. [Google Scholar] [CrossRef] [PubMed]
- McGilligan, V.E.; Gregory-Ksander, M.S.; Li, D.; Moore, J.E.; Hodges, R.R.; Gilmore, M.S.; Moore, T.C.; Dartt, D.A. Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells. PLoS ONE 2013, 8, e74010. [Google Scholar] [CrossRef] [PubMed]
- Bowes Rickman, C.; Farsiu, S.; Toth, C.A.; Klingeborn, M. Dry age-related macular degeneration: Mechanisms, therapeutic targets, and imaging. Investig. Ophthalmol. Vis. Sci. 2013, 54, ORSF68–ORSF80. [Google Scholar] [CrossRef] [PubMed]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Paterno, J.J.; Blasiak, J.; Salminen, A.; Kaarniranta, K. Inflammation and its role in age-related macular degeneration. Cell. Mol. Life Sci. 2016, 73, 1765–1786. [Google Scholar] [CrossRef] [PubMed]
- Pannicke, T.; Fischer, W.; Biedermann, B.; Schadlich, H.; Grosche, J.; Faude, F.; Wiedemann, P.; Allgaier, C.; Illes, P.; Burnstock, G.; et al. P2X7 receptors in Muller glial cells from the human retina. J. Neurosci. 2000, 20, 5965–5972. [Google Scholar] [PubMed]
- Vessey, K.A.; Fletcher, E.L. Rod and cone pathway signalling is altered in the P2X7 receptor knock out mouse. PLoS ONE 2012, 7, e29990. [Google Scholar] [CrossRef] [PubMed]
- Hodges, R.R.; Vrouvlianis, J.; Shatos, M.A.; Dartt, D.A. Characterization of P2X7 purinergic receptors and their function in rat lacrimal gland. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5681–5689. [Google Scholar] [CrossRef] [PubMed]
- Suzuki-Kerr, H.; Vlajkovic, S.; Donaldson, P.J.; Lim, J. Molecular identification and localization of P2X receptors in the rat lens. Exp. Eye Res. 2008, 86, 844–855. [Google Scholar] [CrossRef] [PubMed]
- Sluyter, R.; Stokes, L. Significance of P2X7 receptor variants to human health and disease. Recent Pat. DNA Gene Seq. 2011, 5, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Dubyak, G.R.; el-Moatassim, C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am. J. Physiol. 1993, 265, C577–C606. [Google Scholar] [PubMed]
- Di Virgilio, F. The P2Z purinoceptor: An intriguing role in immunity, inflammation and cell death. Immunol. Today 1995, 16, 524–528. [Google Scholar] [CrossRef]
- Rat, P.; Olivier, E.; Tanter, C.; Wakx, A.; Dutot, M. A fast and reproducible cell- and 96-well plate-based method for the evaluation of P2X7 receptor activation using YO-PRO-1 fluorescent dye. J. Biol. Methods 2017, 4, e64. [Google Scholar] [CrossRef]
- Ferrari, D.; Los, M.; Bauer, M.K.; Vandenabeele, P.; Wesselborg, S.; Schulze-Osthoff, K. P2Z purinoreceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999, 447, 71–75. [Google Scholar] [CrossRef]
- Kong, Q.; Wang, M.; Liao, Z.; Camden, J.M.; Yu, S.; Simonyi, A.; Sun, G.Y.; Gonzalez, F.A.; Erb, L.; Seye, C.I.; et al. P2X(7) nucleotide receptors mediate caspase-8/9/3-dependent apoptosis in rat primary cortical neurons. Purinergic Signal. 2005, 1, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, Z.A.; Aga, M.; Prabhu, U.; Watters, J.J.; Hall, D.J.; Bertics, P.J. The nucleotide receptor P2X7 mediates actin reorganization and membrane blebbing in RAW 264.7 macrophages via p38 MAP kinase and Rho. J. Leukoc. Biol. 2004, 75, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Wesselborg, S.; Bauer, M.K.; Schulze-Osthoff, K. Extracellular ATP activates transcription factor NF-kappaB through the P2Z purinoreceptor by selectively targeting NF-kappaB p65. J. Cell Biol. 1997, 139, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Lemoli, R.M.; Curti, A.; Idzko, M.; Panther, E.; Di Virgilio, F. The P2X7 receptor: A key player in IL-1 processing and release. J. Immunol. 2006, 176, 3877–3883. [Google Scholar] [CrossRef] [PubMed]
- Labasi, J.M.; Petrushova, N.; Donovan, C.; McCurdy, S.; Lira, P.; Payette, M.M.; Brissette, W.; Wicks, J.R.; Audoly, L.; Gabel, C.A. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J. Immunol. 2002, 168, 6436–6445. [Google Scholar] [CrossRef] [PubMed]
- Pelegrin, P.; Barroso-Gutierrez, C.; Surprenant, A. P2X7 receptor differentially couples to distinct release pathways for IL-1beta in mouse macrophage. J. Immunol. 2008, 180, 7147–7157. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Chiozzi, P.; Morelli, A.; Fellin, R.; Di Virgilio, F. Human primary fibroblasts in vitro express a purinergic P2X7 receptor coupled to ion fluxes, microvesicle formation and IL-6 release. J. Cell Sci. 1999, 112, 297–305. [Google Scholar] [PubMed]
- Solle, M.; Labasi, J.; Perregaux, D.G.; Stam, E.; Petrushova, N.; Koller, B.H.; Griffiths, R.J.; Gabel, C.A. Altered cytokine production in mice lacking P2X(7) receptors. J. Biol. Chem. 2001, 276, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Franceschini, A.; Capece, M.; Chiozzi, P.; Falzoni, S.; Sanz, J.M.; Sarti, A.C.; Bonora, M.; Pinton, P.; Di Virgilio, F. The P2X7 receptor directly interacts with the NLRP3 inflammasome scaffold protein. FASEB J. 2015, 29, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Kahlenberg, J.M.; Dubyak, G.R. Mechanisms of caspase-1 activation by P2X7 receptor-mediated K+ release. Am. J. Physiol. Cell Physiol. 2004, 286, C1100–C1108. [Google Scholar] [CrossRef] [PubMed]
- Baricordi, O.R.; Ferrari, D.; Melchiorri, L.; Chiozzi, P.; Hanau, S.; Chiari, E.; Rubini, M.; Di Virgilio, F. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood 1996, 87, 682–690. [Google Scholar] [PubMed]
- Baricordi, O.R.; Melchiorri, L.; Adinolfi, E.; Falzoni, S.; Chiozzi, P.; Buell, G.; Di Virgilio, F. Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J. Biol. Chem. 1999, 274, 33206–33208. [Google Scholar] [CrossRef] [PubMed]
- Ghazi, K.; Deng-Pichon, U.; Warnet, J.M.; Rat, P. Hyaluronan fragments improve wound healing on in vitro cutaneous model through P2X7 purinoreceptor basal activation: Role of molecular weight. PLoS ONE 2012, 7, e48351. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Raffaghello, L.; Giuliani, A.L.; Cavazzini, L.; Capece, M.; Chiozzi, P.; Bianchi, G.; Kroemer, G.; Pistoia, V.; Di Virgilio, F. Expression of P2X7 receptor increases in vivo tumor growth. Cancer Res. 2012, 72, 2957–2969. [Google Scholar] [CrossRef] [PubMed]
- Giannuzzo, A.; Pedersen, S.F.; Novak, I. The P2X7 receptor regulates cell survival, migration and invasion of pancreatic ductal adenocarcinoma cells. Mol. Cancer 2015, 14, 203. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Pizzirani, C.; Idzko, M.; Panther, E.; Norgauer, J.; Di Virgilio, F.; Ferrari, D. P2X(7) receptor: Death or life? Purinergic Signal. 2005, 1, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, E.; Cirillo, M.; Woltersdorf, R.; Falzoni, S.; Chiozzi, P.; Pellegatti, P.; Callegari, M.G.; Sandona, D.; Markwardt, F.; Schmalzing, G.; et al. Trophic activity of a naturally occurring truncated isoform of the P2X7 receptor. FASEB J. 2010, 24, 3393–3404. [Google Scholar] [CrossRef] [PubMed]
- Pisella, P.J.; Pouliquen, P.; Baudouin, C. Prevalence of ocular symptoms and signs with preserved and preservative free glaucoma medication. Br. J. Ophthalmol. 2002, 86, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Jaenen, N.; Baudouin, C.; Pouliquen, P.; Manni, G.; Figueiredo, A.; Zeyen, T. Ocular symptoms and signs with preserved and preservative-free glaucoma medications. Eur. J. Ophthalmol. 2007, 17, 341–349. [Google Scholar] [PubMed]
- Baudouin, C.; Labbe, A.; Liang, H.; Pauly, A.; Brignole-Baudouin, F. Preservatives in eyedrops: The good, the bad and the ugly. Prog. Retinal Eye Res. 2010, 29, 312–334. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Dong, N.; Huang, C.; Zhang, Z.; Hu, J.; Xie, H.; Pan, J.; Liu, Z. Corneal alterations induced by topical application of commercial latanoprost, travoprost and bimatoprost in rabbit. PLoS ONE 2014, 9, e89205. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.J.; Kim, Y.H.; Kang, S.H.; Lee, K.W.; Park, Y.J. In vitro effects of preservative-free and preserved prostaglandin analogs on primary cultured human conjunctival fibroblast cells. Korean J. Ophthalmol. 2013, 27, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Pouzaud, F.; Larosche, I.; Brignole-Baudouin, F.; Warnet, J.M.; Rat, P. Fluoroquinolone eye drop-induced cytotoxicity: Role of preservative in P2X7 cell death receptor activation and apoptosis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Brasnu, E.; Brignole-Baudouin, F.; Riancho, L.; Guenoun, J.M.; Warnet, J.M.; Baudouin, C. In vitro effects of preservative-free tafluprost and preserved latanoprost, travoprost, and bimatoprost in a conjunctival epithelial cell line. Curr. Eye Res. 2008, 33, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Mallari, P.L.T.; McCarty, D.J.; Daniell, M.; Taylor, H. Increased incidence of corneal perforation after topical fluoroquinolone treatment for microbial keratitis. Am. J. Ophthalmol. 2001, 131, 131–133. [Google Scholar] [CrossRef]
- Dutot, M.; Warnet, J.M.; Baudouin, C.; Rat, P. Cytotoxicity of contact lens multipurpose solutions: Role of oxidative stress, mitochondrial activity and P2X7 cell death receptor activation. Eur. J. Pharm. Sci. 2008, 33, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Paillet, H.; Chaumeil, C.; Warnet, J.M.; Rat, P. Severe ocular infections with contact lens: Role of multipurpose solutions. Eye (Lond.) 2009, 23, 470–476. [Google Scholar] [CrossRef] [PubMed]
- Dutot, M.; Vincent, J.; Martin-Brisac, N.; Fabre, I.; Grasmick, C.; Rat, P. Ocular cytotoxicity evaluation of medical devices such as contact lens solutions and benefits of a rinse step in cleaning procedure. Altex 2013, 30, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.K.; Cho, P.; Boost, M.V. Cytotoxicity of rigid gas-permeable lens care solutions. Clin. Exp. Optom. 2013, 96, 467–471. [Google Scholar] [CrossRef] [PubMed]
- Choy, C.K.; Cho, P.; Boost, M.V. Cytotoxicity and effects on metabolism of contact lens care solutions on human corneal epithelium cells. Clin. Exp. Optom. 2012, 95, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Cater, K.C.; Harbell, J.W. Prediction of eye irritation potential of surfactant-based rinse-off personal care formulations by the bovine corneal opacity and permeability (BCOP) assay. Cutan. Ocul. Toxicol. 2006, 25, 217–233. [Google Scholar] [CrossRef] [PubMed]
- Tachon, P.; Cotovio, J.; Dossou, K.G.; Prunieras, M. Assessment of surfactant cytotoxicity: Comparison with the Draize eye test. Int. J. Cosmet. Sci. 1989, 11, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Pauloin, T.; Dutot, M.; Liang, H.; Chavinier, E.; Warnet, J.M.; Rat, P. Corneal protection with high-molecular-weight hyaluronan against in vitro and in vivo sodium lauryl sulfate-induced toxic effects. Cornea 2009, 28, 1032–1041. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.I.; Yang, J.S.; Lee, G.H.; Choi, B.S.; Ryu, J.H. Nanoemulsion-type ophthalmic composition. No. EP2659903 A2, 6 November 2013. [Google Scholar]
- Burgalassi, S.; Chetoni, P.; Monti, D.; Saettone, M.F. Cytotoxicity of potential ocular permeation enhancers evaluated on rabbit and human corneal epithelial cell lines. Toxicol. Lett. 2001, 122, 1–8. [Google Scholar] [CrossRef]
- Ujhelyi, Z.; Fenyvesi, F.; Varadi, J.; Feher, P.; Kiss, T.; Veszelka, S.; Deli, M.; Vecsernyes, M.; Bacskay, I. Evaluation of cytotoxicity of surfactants used in self-micro emulsifying drug delivery systems and their effects on paracellular transport in Caco-2 cell monolayer. Eur. J. Pharm. Sci. 2012, 47, 564–573. [Google Scholar] [CrossRef] [PubMed]
- Said, T.; Dutot, M.; Christon, R.; Beaudeux, J.L.; Martin, C.; Warnet, J.M.; Rat, P. Benefits and side effects of different vegetable oil vectors on apoptosis, oxidative stress, and P2X7 cell death receptor activation. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5000–5006. [Google Scholar] [CrossRef] [PubMed]
- Sigward, E.; Mignet, N.; Rat, P.; Dutot, M.; Muhamed, S.; Guigner, J.M.; Scherman, D.; Brossard, D.; Crauste-Manciet, S. Formulation and cytotoxicity evaluation of new self-emulsifying multiple W/O/W nanoemulsions. Int. J. Nanomed. 2013, 8, 611–625. [Google Scholar]
- Jayamanne, D.G.; Fitt, A.W.; Dayan, M.; Andrews, R.M.; Mitchell, K.W.; Griffiths, P.G. The effectiveness of topical diclofenac in relieving discomfort following traumatic corneal abrasions. Eye 1997, 11, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Mankus, C.; Rich, C.; Minns, M.; Trinkaus-Randall, V. Corneal epithelium expresses a variant of P2X(7) receptor in health and disease. PLoS ONE 2011, 6, e28541. [Google Scholar] [CrossRef] [PubMed]
- Minns, M.S.; Trinkaus-Randall, V. Purinergic Signaling in Corneal Wound Healing: A Tale of 2 Receptors. J. Ocul. Pharmacol. Ther. 2016, 32, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Bruban, J.; Glotin, A.L.; Dinet, V.; Chalour, N.; Sennlaub, F.; Jonet, L.; An, N.; Faussat, A.M.; Mascarelli, F. Amyloid-beta(1–42) alters structure and function of retinal pigmented epithelial cells. Aging Cell 2009, 8, 162–177. [Google Scholar] [CrossRef] [PubMed]
- Ratnayaka, J.A.; Serpell, L.C.; Lotery, A.J. Dementia of the eye: The role of amyloid beta in retinal degeneration. Eye (Lond.) 2015, 29, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Wakx, A.; Dutot, M.; Massicot, F.; Mascarelli, F.; Limb, G.A.; Rat, P. Amyloid beta Peptide Induces Apoptosis Through P2X7 Cell Death Receptor in Retinal Cells: Modulation by Marine Omega-3 Fatty Acid DHA and EPA. Appl. Biochem. Biotechnol. 2016, 178, 368–381. [Google Scholar] [CrossRef] [PubMed]
- Dunaief, J.L.; Dentchev, T.; Ying, G.S.; Milam, A.H. The role of apoptosis in age-related macular degeneration. Arch. Ophthalmol. 2002, 120, 1435–1442. [Google Scholar] [CrossRef] [PubMed]
- Parvathenani, L.K.; Tertyshnikova, S.; Greco, C.R.; Roberts, S.B.; Robertson, B.; Posmantur, R. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer’s disease. J. Biol. Chem. 2003, 278, 13309–13317. [Google Scholar] [CrossRef] [PubMed]
- McLarnon, J.G.; Ryu, J.K.; Walker, D.G.; Choi, H.B. Upregulated expression of purinergic P2X(7) receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J. Neuropathol. Exp. Neurol. 2006, 65, 1090–1097. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, P.; Zhang, J.; Chen, W.; Gu, L. Silencing of the P2X(7) receptor enhances amyloid-beta phagocytosis by microglia. Biochem. Biophys. Res. Commun. 2013, 434, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Lordan, S.; Mackrill, J.J.; O’Brien, N.M. Oxysterols and mechanisms of apoptotic signaling: Implications in the pathology of degenerative diseases. J. Nutr. Biochem. 2009, 20, 321–336. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, A.; Vejux, A.; Mackrill, J.; O’Callaghan, Y.; Hammami, M.; O’Brien, N.; Lizard, G. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014, 18, 148–162. [Google Scholar] [CrossRef] [PubMed]
- Javitt, N.B.; Javitt, J.C. The retinal oxysterol pathway: A unifying hypothesis for the cause of age-related macular degeneration. Curr. Opin. Ophthalmol. 2009, 20, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.R.; Clark, M.E.; Lee, J.W.; Curcio, C.A. 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp. Eye Res. 2014, 128, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, I.R.; Larrayoz, I.M. Cholesterol oxidation in the retina: Implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J. Lipid Res. 2010, 51, 2847–2862. [Google Scholar] [CrossRef] [PubMed]
- Olivier, E.; Dutot, M.; Regazzetti, A.; Leguillier, T.; Dargere, D.; Auzeil, N.; Laprevote, O.; Rat, P. P2X7-pannexin-1 and amyloid beta-induced oxysterol input in human retinal cell: Role in age-related macular degeneration? Biochimie 2016, 127, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Solini, A.; Chiozzi, P.; Falzoni, S.; Morelli, A.; Fellin, R.; Di Virgilio, F. High glucose modulates P2X7 receptor-mediated function in human primary fibroblasts. Diabetologia 2000, 43, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Pereira Tde, O.; da Costa, G.N.; Santiago, A.R.; Ambrosio, A.F.; dos Santos, P.F. High glucose enhances intracellular Ca2+ responses triggered by purinergic stimulation in retinal neurons and microglia. Brain Res. 2010, 1316, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, T.; Kobayashi, M.; Kawamura, H.; Li, Q.; Puro, D.G. Enhancement of P2X(7)-induced pore formation and apoptosis: An early effect of diabetes on the retinal microvasculature. Investig. Ophthalmol. Vis. Sci. 2004, 45, 1026–1032. [Google Scholar] [CrossRef]
- Sugiyama, T.; Lee, S.Y.; Horie, T.; Oku, H.; Takai, S.; Tanioka, H.; Kuriki, Y.; Kojima, S.; Ikeda, T. P2X(7) receptor activation may be involved in neuronal loss in the retinal ganglion cell layer after acute elevation of intraocular pressure in rats. Mol. Vis. 2013, 19, 2080–2091. [Google Scholar] [PubMed]
- Xia, J.; Lim, J.C.; Lu, W.; Beckel, J.M.; Macarak, E.J.; Laties, A.M.; Mitchell, C.H. Neurons respond directly to mechanical deformation with pannexin-mediated ATP release and autostimulation of P2X7 receptors. J. Physiol. 2012, 590, 2285–2304. [Google Scholar] [CrossRef] [PubMed]
- Niyadurupola, N.; Sidaway, P.; Ma, N.; Rhodes, J.D.; Broadway, D.C.; Sanderson, J. P2X7 receptor activation mediates retinal ganglion cell death in a human retina model of ischemic neurodegeneration. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2163–2170. [Google Scholar] [CrossRef] [PubMed]
- Perez de Lara, M.J.; Guzman-Aranguez, A.; de la Villa, P.; Diaz-Hernandez, J.I.; Miras-Portugal, M.T.; Pintor, J. Increased levels of extracellular ATP in glaucomatous retinas: Possible role of the vesicular nucleotide transporter during the development of the pathology. Mol. Vis. 2015, 21, 1060–1070. [Google Scholar] [PubMed]
- Stuart, J.C.; Linn, J.G. Dilute sodium hyaluronate (Healon) in the treatment of ocular surface disorders. Ann. Ophthalmol. 1985, 17, 190–192. [Google Scholar] [PubMed]
- Johnson, M.E.; Murphy, P.J.; Boulton, M. Effectiveness of sodium hyaluronate eyedrops in the treatment of dry eye. Graefes Arch. Clin. Exp. Ophthalmol. 2006, 244, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Hamano, T.; Horimoto, K.; Lee, M.; Komemushi, S. Sodium hyaluronate eyedrops enhance tear film stability. Jpn. J. Ophthalmol. 1996, 40, 62–65. [Google Scholar] [PubMed]
- Prabhasawat, P.; Tesavibul, N.; Kasetsuwan, N. Performance profile of sodium hyaluronate in patients with lipid tear deficiency: Randomised, double-blind, controlled, exploratory study. Br. J. Ophthalmol. 2007, 91, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Pauloin, T.; Dutot, M.; Warnet, J.M.; Rat, P. In vitro modulation of preservative toxicity: High molecular weight hyaluronan decreases apoptosis and oxidative stress induced by benzalkonium chloride. Eur. J. Pharm. Sci. 2008, 34, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Rat, P.; Dutot, M.; Watzinger, M.; Baudouin, C.; Warnet, J.M. Cytoprotective Effects of Four Different Hyaluronic Acids: Role of Molecular Weight. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4944. [Google Scholar]
- Jiang, L.H. Inhibition of P2X(7) receptors by divalent cations: Old action and new insight. Eur. Biophys. J. 2009, 38, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Rassendren, F.; Buell, G.N.; Virginio, C.; Collo, G.; North, R.A.; Surprenant, A. The permeabilizing ATP receptor, P2X7. Cloning and expression of a human cDNA. J. Biol. Chem. 1997, 272, 5482–5486. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, T.H.; Newman, A.S.; Swanson, J.A.; Silverstein, S.C. ATP4-permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J. Biol. Chem. 1987, 262, 8884–8888. [Google Scholar] [PubMed]
- Michel, A.D.; Chessell, I.P.; Humphrey, P.P. Ionic effects on human recombinant P2X7 receptor function. Naunyn Schmiedebergs Arch. Pharmacol. 1999, 359, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Virginio, C.; Church, D.; North, R.A.; Surprenant, A. Effects of divalent cations, protons and calmidazolium at the rat P2X7 receptor. Neuropharmacology 1997, 36, 1285–1294. [Google Scholar] [CrossRef]
- Falsini, B.; Piccardi, M.; Minnella, A.; Savastano, C.; Capoluongo, E.; Fadda, A.; Balestrazzi, E.; Maccarone, R.; Bisti, S. Influence of saffron supplementation on retinal flicker sensitivity in early age-related macular degeneration. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6118–6124. [Google Scholar] [CrossRef] [PubMed]
- Piccardi, M.; Marangoni, D.; Minnella, A.M.; Savastano, M.C.; Valentini, P.; Ambrosio, L.; Capoluongo, E.; Maccarone, R.; Bisti, S.; Falsini, B. A longitudinal follow-up study of saffron supplementation in early age-related macular degeneration: Sustained benefits to central retinal function. Evid. Based Complement. Alternat. Med. 2012, 2012, 429124. [Google Scholar] [CrossRef] [PubMed]
- Corso, L.; Cavallero, A.; Baroni, D.; Garbati, P.; Prestipino, G.; Bisti, S.; Nobile, M.; Picco, C. Saffron reduces ATP-induced retinal cytotoxicity by targeting P2X7 receptors. Purinergic Signal. 2016, 12, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.; SanGiovanni, J.P.; Danis, R.; Domalpally, A.; McBee, W.; Sperduto, R.; Ferris, F.L. The Age-Related Eye Disease Study 2 (AREDS2): Study design and baseline characteristics (AREDS2 report number 1). Ophthalmology 2012, 119, 2282–2289. [Google Scholar] [CrossRef] [PubMed]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Ferris, F.L.; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- SanGiovanni, J.P.; Agron, E.; Clemons, T.E.; Chew, E.Y. Omega-3 long-chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration. Arch. Ophthalmol. 2009, 127, 110–112. [Google Scholar] [CrossRef] [PubMed]
- Seddon, J.M.; George, S.; Rosner, B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: The US Twin Study of Age-Related Macular Degeneration. Arch. Ophthalmol. 2006, 124, 995–1001. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.J.; Calippe, B.; Lavalette, S.; Roubeix, C.; Montassar, F.; Housset, M.; Levy, O.; Delarasse, C.; Paques, M.; Sahel, J.A.; et al. Upregulation of P2RX7 in Cx3cr1-Deficient Mononuclear Phagocytes Leads to Increased Interleukin-1beta Secretion and Photoreceptor Neurodegeneration. J. Neurosci. 2015, 35, 6987–6996. [Google Scholar] [CrossRef] [PubMed]
- Notomi, S.; Hisatomi, T.; Murakami, Y.; Terasaki, H.; Sonoda, S.; Asato, R.; Takeda, A.; Ikeda, Y.; Enaida, H.; Sakamoto, T.; et al. Dynamic increase in extracellular ATP accelerates photoreceptor cell apoptosis via ligation of P2RX7 in subretinal hemorrhage. PLoS ONE 2013, 8, e53338. [Google Scholar] [CrossRef] [PubMed]
- Notomi, S.; Hisatomi, T.; Kanemaru, T.; Takeda, A.; Ikeda, Y.; Enaida, H.; Kroemer, G.; Ishibashi, T. Critical involvement of extracellular ATP acting on P2RX7 purinergic receptors in photoreceptor cell death. Am. J. Pathol. 2011, 179, 2798–2809. [Google Scholar] [CrossRef] [PubMed]
- Guzman-Aranguez, A.; Perez de Lara, M.J.; Pintor, J. Hyperosmotic stress induces ATP release and changes in P2X7 receptor levels in human corneal and conjunctival epithelial cells. Purinergic Signal. 2017. [Google Scholar] [CrossRef] [PubMed]
- Brandle, U.; Kohler, K.; Wheeler-Schilling, T.H. Expression of the P2X7-receptor subunit in neurons of the rat retina. Brain Res. Mol. Brain Res. 1998, 62, 106–109. [Google Scholar] [CrossRef]
- Wheeler-Schilling, T.H.; Marquordt, K.; Kohler, K.; Guenther, E.; Jabs, R. Identification of purinergic receptors in retinal ganglion cells. Brain Res. Mol. Brain Res. 2001, 92, 177–180. [Google Scholar] [CrossRef]
- Yang, D.; Elner, S.G.; Clark, A.J.; Hughes, B.A.; Petty, H.R.; Elner, V.M. Activation of P2X receptors induces apoptosis in human retinal pigment epithelium. Investig. Ophthalmol. Vis. Sci. 2011, 52, 1522–1530. [Google Scholar] [CrossRef] [PubMed]
- Guha, S.; Baltazar, G.C.; Coffey, E.E.; Tu, L.A.; Lim, J.C.; Beckel, J.M.; Patel, S.; Eysteinsson, T.; Lu, W.; O’Brien-Jenkins, A.; et al. Lysosomal alkalinization, lipid oxidation, and reduced phagosome clearance triggered by activation of the P2X7 receptor. FASEB J. 2013, 27, 4500–4509. [Google Scholar] [CrossRef] [PubMed]
- Puthussery, T.; Fletcher, E.L. Synaptic localization of P2X7 receptors in the rat retina. J. Comp. Neurol. 2004, 472, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Leung, C.T.; Peterson-Yantorno, K.; Mitchell, C.H.; Civan, M.M. Pathways for ATP release by bovine ciliary epithelial cells, the initial step in purinergic regulation of aqueous humor inflow. Am. J. Physiol. Cell Physiol. 2010, 299, C1308–C1317. [Google Scholar] [CrossRef] [PubMed]
| Tissue | Cells | mRNA | Protein | Species | References |
|---|---|---|---|---|---|
| Cornea | Corneal epithelial cells (Human Corneal Epithelial (HCE) cell line) | + | Human | [11] | |
| Cornea section | + | Mouse | [12] | ||
| Corneolimbal epithelial cells (telomerase immortalized cells) | + | + | Human | [66,109] | |
| Conjunctiva | Conjunctival epithelial cells | + | Human | [109] | |
| Conjunctival epithelial cells (Wong-Kilbourne derivative of Chang conjunctiva (WKD) cell line) | + | Human | [11] | ||
| Goblet cells | + | Rat | [14] | ||
| Lens | Lens fiber cells | + | + | Rat | [21] |
| Lens epithelial cells | + | Human | [11] | ||
| Retina | Müller glial cells | + | + | Human | [18,70] |
| Retina section | + | + | Rat | [110] | |
| Retinal ganglion cells | + | Rat | [111] | ||
| Retinal pigmented epithelial cells (Acute Retinal Pigment Epithelial-19 (ARPE-19) cell line) | + | Human | [11] | ||
| Retinal pigmented epithelial cells (primary culture) | + | + | Human | [112] | |
| Retinal pigmented epithelial cells | + | + | Mouse | [113] | |
| Photoreceptors | + | + | Rat | [114] | |
| Ciliary body | Ciliary epithelial cells | + | Bovine | [115] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dutot, M.; Olivier, E.; Wakx, A.; Rat, P. The Role of the P2X7 Receptor in Ocular Stresses: A Potential Therapeutic Target. Vision 2017, 1, 14. https://doi.org/10.3390/vision1020014
Dutot M, Olivier E, Wakx A, Rat P. The Role of the P2X7 Receptor in Ocular Stresses: A Potential Therapeutic Target. Vision. 2017; 1(2):14. https://doi.org/10.3390/vision1020014
Chicago/Turabian StyleDutot, Mélody, Elodie Olivier, Anaïs Wakx, and Patrice Rat. 2017. "The Role of the P2X7 Receptor in Ocular Stresses: A Potential Therapeutic Target" Vision 1, no. 2: 14. https://doi.org/10.3390/vision1020014
APA StyleDutot, M., Olivier, E., Wakx, A., & Rat, P. (2017). The Role of the P2X7 Receptor in Ocular Stresses: A Potential Therapeutic Target. Vision, 1(2), 14. https://doi.org/10.3390/vision1020014