Can Perceived Exertion and Velocity Loss Serve as Indirect Indicators of Muscle Fatigue During Explosive Back Squat Exercise?
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Initial Session
2.4. Experimental Session
2.5. Surface Electromyography
2.6. Velocity Loss
2.7. Statistical Analysis
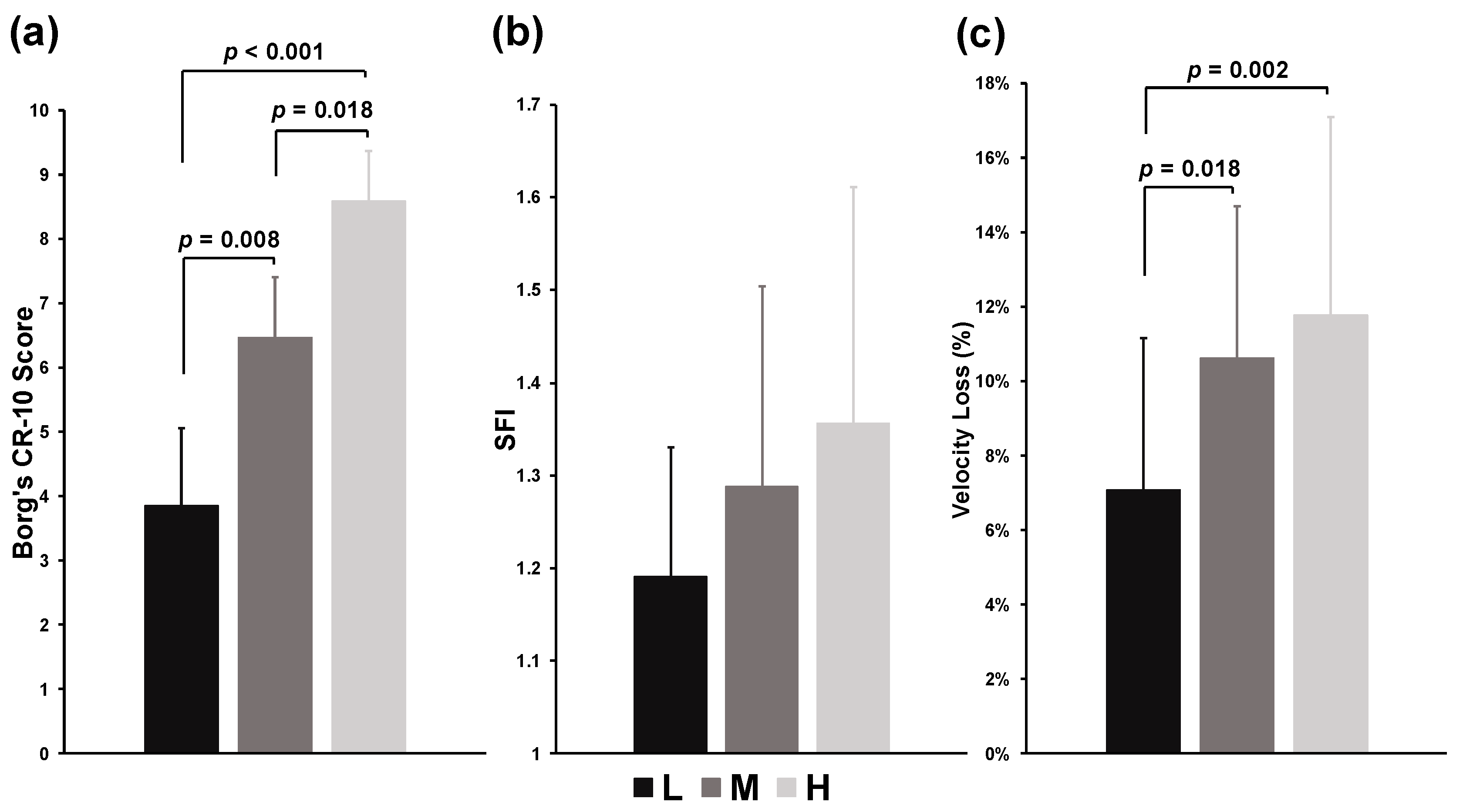
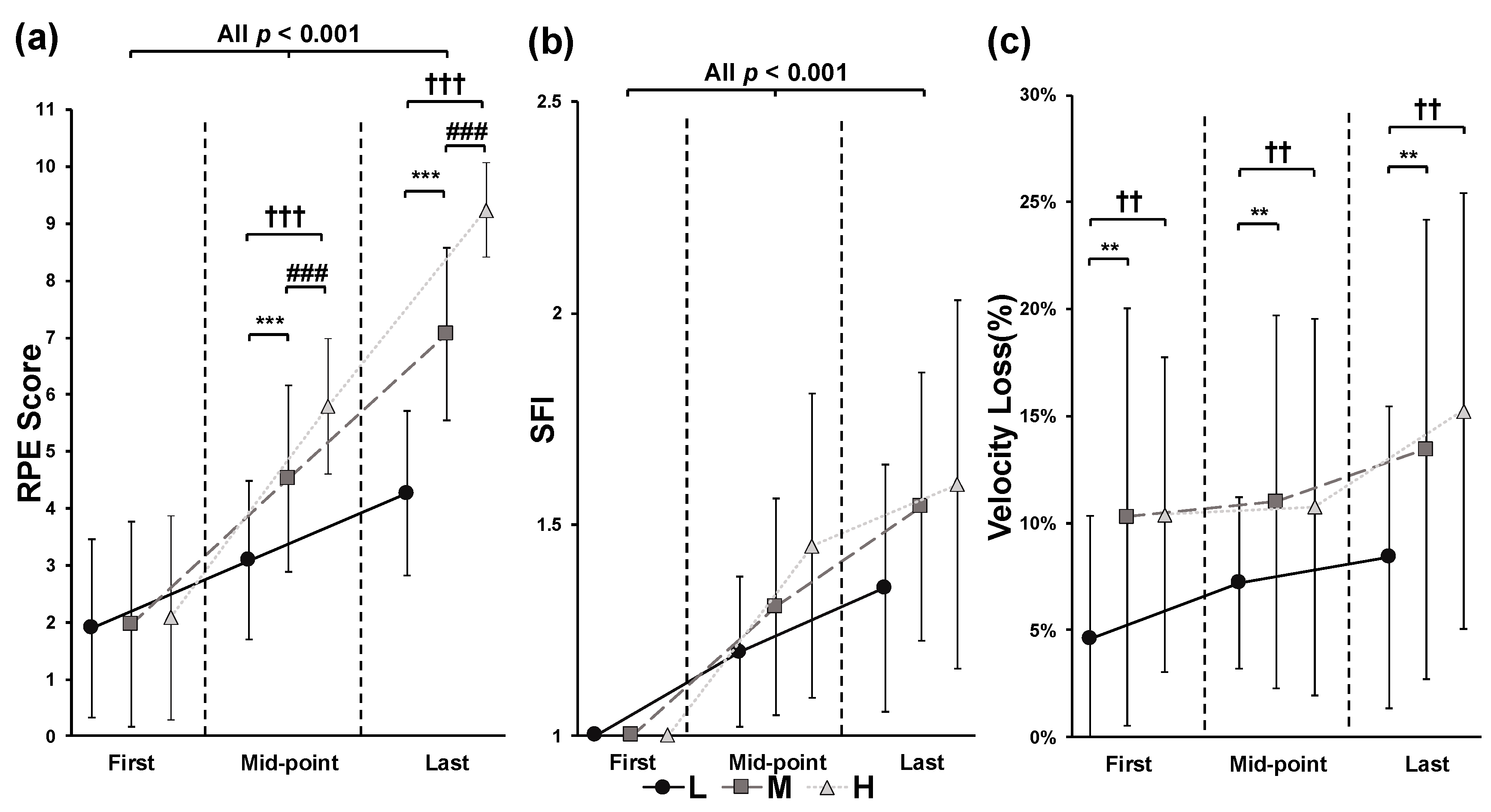
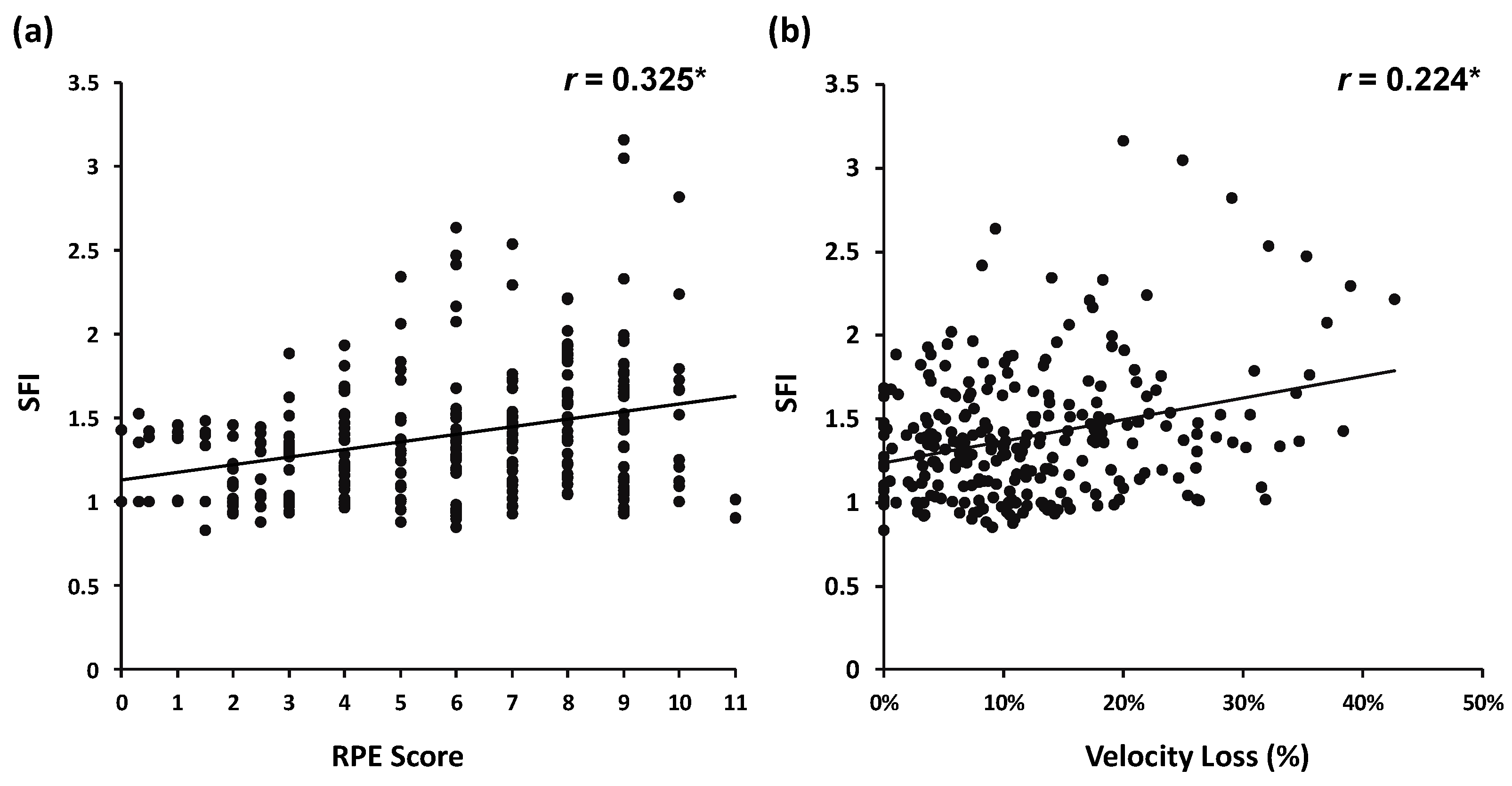
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vieira, J.G.; Sardeli, A.V.; Dias, M.R.; Filho, J.E.; Campos, Y.; Sant’Ana, L.; Leitão, L.; Reis, V.; Wilk, M.; Novaes, J.; et al. Effects of Resistance Training to Muscle Failure on Acute Fatigue: A Systematic Review and Meta-Analysis. Sports Med. 2022, 52, 1103–1125. Available online: https://link.springer.com/article/10.1007/s40279-021-01602-x (accessed on 10 April 2024). [CrossRef] [PubMed]
- Gonzalez-Izal, M.; Falla, D.; Izquierdo, M.; Farina, D. Predicting force loss during dynamic fatiguing exercises from non-linear mapping of features of the surface electromyogram. J. Neurosci. Methods. 2010, 190, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Medina, L.; González-Badillo, J.J. Velocity loss as an indicator of neuromuscular fatigue during resistance training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef] [PubMed]
- Borotikar, B.S.; Newcomer, R.; Koppes, R.; McLean, S.G. Combined effects of fatigue and decision making on female lower limb landing postures: Central and peripheral contributions to ACL injury risk. Clin. Biomech. 2008, 23, 81–92. [Google Scholar] [CrossRef]
- Roy, S.H.; De Luca, C.J.; Casavant, D.A. Lumbar muscle fatigue and chronic lower back pain. Spine 1989, 14, 992–1001. Available online: https://pubmed.ncbi.nlm.nih.gov/2528828/ (accessed on 12 January 2022). [CrossRef]
- Yoshitake, Y.; Ue, H.; Miyazaki, M.; Moritani, T. Assessment of lower-back muscle fatigue using electromyography, mechanomyography, and near-infrared spectroscopy. Eur. J. Appl. Physiol. 2001, 84, 174–179. [Google Scholar] [CrossRef]
- Desbrosses, K.; Babault, N.; Scaglioni, G.; Meyer, J.P.; Pousson, M. Neural activation after maximal isometric contractions at different muscle lenghts. Med. Sci. Sports Exerc. 2006, 38, 937–944. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Campanini, I.; Disselhorst-Klug, C.; Rymer, W.Z.; Merletti, R. Surface EMG in Clinical Assessment and Neurorehabilitation: Barriers Limiting Its Use. Front. Neurol. 2020, 11, 934. [Google Scholar] [CrossRef]
- Rogers, D.R.; MacIsaac, D.T. A comparison of EMG-based muscle fatigue assessments during dynamic contractions. J. Electromyogr. Kinesiol. 2013, 23, 1004–1011. [Google Scholar] [CrossRef]
- Brody, L.R.; Pollock, M.T.; Roy, S.H.; De Luca, C.J.; Celli, B. pH-induced effects on median frequency and conduction velocity of the myoelectric signal. J. Appl. Physiol. 1991, 71, 1878–1885. [Google Scholar] [CrossRef] [PubMed]
- González-Izal, M.; Malanda, A.; Gorostiaga, E.; Izquierdo, M. Electromyographic models to assess muscle fatigue. J. Electromyogr. Kinesiol. 2012, 22, 501–512. [Google Scholar] [CrossRef]
- Gonzalez-Izal, M.; Malanda, A.; Navarro-Amezqueta, I.; Gorostiaga, E.M.; Mallor, F.; Ibanez, J.; Izquierdo, M. EMG spectral indices and muscle power fatigue during dynamic contractions. J. Electromyogr. Kinesiol. 2010, 20, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, G.V.; Arabadzhiev, T.I.; Mileva, K.N.; Bowtell, J.L.; Crichton, N.; Dimitrova, N.A. Muscle fatigue during dynamic contractions assessed by new spectral indices. Med. Sci. Sports Exerc. 2006, 38, 1971–1979. [Google Scholar] [CrossRef]
- Gonzalez-Izal, M.; Malanda, A.; Rodríguez-Carreño, I.; Navarro-Amézqueta, I.; Gorostiaga, E.M.; Farina, D.; Falla, D.; Izquierdo, M. Linear vs. non-linear mapping of peak power using surface EMG features during dynamic fatiguing contractions. J. Biomech. 2010, 43, 2589–2594. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Montecinos, C.; Bustamante, A.; Candia-González, M.; González-Bravo, C.; Gallardo-Molina, P.; Andersen, L.L.; Calatayud, J. Perceived physical exertion is a good indicator of neuromuscular fatigue for the core muscles. J. Electromyogr. Kinesiol. 2019, 49, 102360. [Google Scholar] [CrossRef]
- Hiscock, D.J.; Dawson, B.; Clarke, M.; Peeling, P. Can changes in resistance exercise workload influence internal load, countermovement jump performance and the endocrine response? J. Sports Sci. 2018, 36, 191–197. [Google Scholar] [CrossRef]
- Hiscock, D.J.; Dawson, B.; Donnelly, C.J.; Peeling, P. Muscle activation, blood lactate, and perceived exertion responses to changing resistance training programming variables. Eur. J. Sport Sci. 2016, 16, 536–544. [Google Scholar] [CrossRef]
- Vøllestad, N.K. Measurement of human muscle fatigue. J. Neurosci. Methods 1997, 74, 219–227. Available online: https://pubmed.ncbi.nlm.nih.gov/9219890/ (accessed on 23 January 2022). [CrossRef]
- Lagally, K.M.; Gallagher, K.I.; Robertson, R.J.; Gearhart, R.; Goss, F.L. Ratings of perceived exertion during low- and high-intensity resistance exercise by young adults. Percept. Mot. Skills. 2002, 94, 723–731. [Google Scholar] [CrossRef]
- Zhao, H.; Yamaguchi, S.; Okada, J. Effects of rest interval array on training volume, perceived exertion, neuromuscular fatigue, and metabolic responses during agonist-antagonist muscle alternative training. J. Sports Med. Phys. Fitness. 2020, 60, 536–543. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, M.; Adamus, P.; Zieliński, J.; Kantanista, A. Effects of Velocity-Based Training on Strength and Power in Elite Athletes-A Systematic Review. Int. J. Environ. Res. Public Health 2021, 18, 5257. Available online: https://pubmed.ncbi.nlm.nih.gov/34069249/ (accessed on 1 February 2022). [CrossRef] [PubMed]
- Evandro, C.S.; Adré, R.M.; Adré, S.F.; Amador, G.R.; Dnica, J.; Daniel, B. Validity of the iLOAD® app for resistance training monitoring. PeerJ 2019, 7, e7372. Available online: https://pubmed.ncbi.nlm.nih.gov/31410306/ (accessed on 6 August 2021).
- Pérez-Castilla, A.; Piepoli, A.; Delgado-García, G.; Garrido-Blanca, G.; García-Ramos, A. Reliability and concurrent validity of seven commercially available devices for the assessment of movement velocity at different intensities during the bench press. J. Strength. Cond. Res. 2019, 33, 1258–1265. Available online: https://journals.lww.com/nsca-jscr/fulltext/2019/05000/reliability_and_concurrent_validity_of_seven.13.aspx (accessed on 19 October 2023). [CrossRef]
- Külkamp, W.; Bishop, C.; Kons, R.; Antunes, L.; Carmo, E.; Hizume-Kunzler, D.; Dal Pupo, J. Concurrent Validity and Technological Error-Based Reliability of a Novel Device for Velocity-Based Training. Meas. Phys. Educ. Exerc. Sci. 2023, 28, 15–26. Available online: https://www.tandfonline.com/doi/abs/10.1080/1091367X.2023.2207570 (accessed on 19 October 2023). [CrossRef]
- Mayo, X.; Iglesias-Soler, E.; Kingsley, J.D. Perceived exertion is affected by the submaximal set configuration used in resistance exercise. J. Strength. Cond. Res. 2019, 33, 426–432. Available online: https://journals.lww.com/nsca-jscr/Fulltext/2019/02000/Perceived_Exertion_Is_Affected_by_the_Submaximal.15.aspx (accessed on 18 July 2021). [CrossRef]
- Izquierdo, M.; González-Badillo, J.J.; Häkkinen, K.; Ibáñez, J.; Kraemer, W.J.; Altadill, A.; Eslava, J.; Gorostiaga, E.M. Effect of loading on unintentional lifting velocity declines during single sets of repetitions to failure during upper and lower extremity muscle actions. Int. J. Sports Med. 2006, 27, 718–724. Available online: https://pubmed.ncbi.nlm.nih.gov/16944400/ (accessed on 31 January 2022). [CrossRef]
- Thomas, R.B.; Roger, W.E. Essentials of Strength Training and Conditioning, 3rd ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Ratamess, N.A.; Alvar, B.A.; Tammy, K.E.; Housh, T.J.; Ben, K.W.; Kraemer, W.J.; Triplett, N.T. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. Available online: https://pubmed.ncbi.nlm.nih.gov/19204579/ (accessed on 27 May 2022).
- Borg, G.A.V. Borg’s Perceived Exertion and Pain Scales; Human kinetics: Champaign, IL, USA, 1998. [Google Scholar]
- Robertson, R.J.; Goss, F.L.; Rutkowski, J.; Lenz, B.; Dixon, C.; Timmer, J.; Frazee, K.; Dube, J.; Andreacci, J. Concurrent validation of the OMNI perceived exertion scale for resistance exercise. Med. Sci. Sports Exerc. 2003, 35, 333–341. [Google Scholar] [CrossRef]
- Lagally, K.M.; Robertson, R.J. Construct validity of the OMNI Resistance Exercise Scale. J. Strength Cond. Res. 2006, 20, 252–256. [Google Scholar]
- Robertson, R.J.; Goss, F.L.; Dube, J.; Rutkowski, J.; Dupain, M.; Brennan, C.; Andreacci, J. Validation of the Adult OMNI Scale of Perceived Exertion for Cycle Ergometer Exercise. Med. Sci. Sports Exerc. 2004, 36, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Fontes, E.B.; Smirmaul, B.P.C.; Nakamura, F.Y.; Pereira, G.; Okano, A.H.; Altimari, L.R.; Dantas, J.L.; de Moraes, A.C. The relationship between rating of perceived exertion and muscle activity during exhaustive constant-load cycling. Int. J. Sports Med. 2010, 31, 683–688. [Google Scholar] [CrossRef] [PubMed]
- Lagally, K.M.; Robertson, R.J.; Gallagher, K.I.; Goss, F.L.; Jakicic, J.M.; Lephart, S.M.; McCaw, S.T.; Goodpaster, B. Perceived exertion, electromyography, and blood lactate during acute bouts of resistance exercise. Med. Sci. Sports Exerc. 2002, 34, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Weakley, J.J.S.; Till, K.; Read, D.B.; Roe, G.A.B.; Darrall-Jones, J.; Phibbs, P.J.; Jones, B. The effects of traditional, superset, and tri-set resistance training structures on perceived intensity and physiological responses. Eur. J. Appl. Physiol. 2017, 117, 1877–1889. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Seo, D.; Okada, J. Validity of using perceived exertion to assess muscle fatigue during bench press exercise. Isokinet. Exerc. Sci. 2024, 32, 73–83. [Google Scholar] [CrossRef]
- Zhao, H.; Seo, D.; Okada, J. Validity of using perceived exertion to assess muscle fatigue during back squat exercise. BMC Sports Sci. Med. Rehabil. 2023, 15, 14. Available online: https://bmcsportsscimedrehabil.biomedcentral.com/articles/10.1186/s13102-023-00620-8 (accessed on 7 February 2023). [CrossRef]
- Zhao, H.; Nishioka, T.; Okada, J. Validity of using perceived exertion to assess muscle fatigue during resistance exercises. PeerJ 2022, 10, e13019. Available online: https://peerj.com/articles/13019 (accessed on 11 March 2022). [CrossRef]
- George, J.D.; Tolley, J.R.; Vehrs, P.R.; Reece, J.D.; Fatih Akay, M.; Cambridge, E.D.J. New approach in assessing core muscle endurance using ratings of perceived exertion. J. Strength Cond. Res. 2018, 32, 1081–1088. [Google Scholar] [CrossRef]
- Turki, O.; Chaouachi, A.; Drinkwater, E.J.; Chtara, M.; Chamari, K.; Amri, M.; Behm, D.G. Ten minutes of dynamic stretching is sufficient to potentiate vertical jump performance characteristics. J. Strength Cond. Res. 2011, 25, 2453–2463. Available online: https://pubmed.ncbi.nlm.nih.gov/21792071/ (accessed on 25 July 2024). [CrossRef]
- Eston, R.; James, H.; Evans, L. The validity of submaximal ratings of perceived exertion to predict one repetition maximum. J. Sport. Sci. Med. 2009, 8, 567–573. Available online: https://pubmed.ncbi.nlm.nih.gov/24149599/ (accessed on 29 October 2024).
- Hollander, D.B.; Worley, J.R.; Asoodeh, M.; Wakesa, D.; Magnuson, M.; Dantzler, D.K.; Didier, J.J.; Kraemer, R.R. Comparison of resistance exercise perceived exertion and muscle activation at varied submaximal durations, loads, and muscle actions. J. Strength Cond. Res. 2017, 31, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- St Clair, G.; Timothy, D.N. Evidence for complex system integration and dynamic neural regulation of skeletal muscle recruitment during exercise in humans. Br. J. Sports Med. 2004, 38, 797–806. Available online: https://bjsm.bmj.com/content/38/6/797.long (accessed on 29 October 2024). [CrossRef] [PubMed]
- Noakes, T.D.; St, A.; Gibson, C. From catastrophe to complexity: A novel model of integrative central neural regulation of effort and fatigue during exercise in humans: Summary and conclusions. Br. J. Sports Med. 2005, 39, 120–124. Available online: https://bjsm.bmj.com/content/39/2/120.long (accessed on 11 June 2021). [CrossRef]
- Walker, S.; Davis, L.; Avela, J.; Häkkinen, K. Neuromuscular fatigue during dynamic maximal strength and hypertrophic resistance loadings. J. Electromyogr. Kinesiol. 2012, 22, 356–362. [Google Scholar] [CrossRef]
- Blain, G.M.; Mangum, T.S.; Sidhu, S.K.; Weavil, J.C.; Hureau, T.J.; Jessop, J.E.; Bledsoe, A.D.; Richardson, R.S.; Amann, M. Group III/IV muscle afferents limit the intramuscular metabolic perturbation during whole body exercise in humans. J. Physiol. 2016, 594, 5303–5315. [Google Scholar] [CrossRef]
- Barbero, M.; Merletti, R.; Rainoldi, A. Atlas of Muscle Innervation Zones; Springer Science & Business Media: Lavis, Italy, 2012. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European recommendations for surface electromyography. Roessingh Res. Dev. 1999, 8. Available online: http://www.seniam.org/ (accessed on 30 October 2024).
- Ang, C.L.; Kong, P.W. Field-Based Biomechanical Assessment of the Snatch in Olympic Weightlifting Using Wearable In-Shoe Sensors and Videos—A Preliminary Report. Sensors 2023, 23, 1171. Available online: https://www.mdpi.com/1424-8220/23/3/1171/htm (accessed on 16 November 2023). [CrossRef] [PubMed]
- Migliaccio, G.M.; Dello Iacono, A.; Ardigò, L.P.; Samozino, P.; Iuliano, E.; Grgantov, Z.; Padulo, J. Leg press vs. smith machine: Quadriceps activation and overall perceived effort profiles. Front. Physiol. 2018, 9, 1481. [Google Scholar]
- de Morree, H.M.; Klein, C.; Marcora, S.M. Perception of effort reflects central motor command during movement execution. Psychophysiology. 2012, 49, 1242–1253. [Google Scholar] [CrossRef]
- Hiscock, D.J.; Dawson, B.; Peeling, P. Perceived exertion responses to changing resistance training programming variables. J. Strength Cond. Res. 2015, 29, 1564–1569. Available online: https://journals.lww.com/nsca-jscr/Fulltext/2015/06000/Perceived_Exertion_Responses_to_Changing.14.aspx (accessed on 20 October 2021). [CrossRef]
- Esteban, M.G.; Navarro-Amézqueta, I.; Calbet, J.A.L.; Hellsten, Y.; Cusso, R.; Guerrero, M.; Granados, C.; González-Izal, M.; Ibañez, J.; Izquierdo, M. Energy metabolism during repeated sets of leg press exercise leading to failure or not. PLoS ONE 2012, 7, e40621. Available online: https://pubmed.ncbi.nlm.nih.gov/22808209/ (accessed on 20 October 2021).
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J. Physiol. 2011, 589, 5299–5309. [Google Scholar] [CrossRef] [PubMed]
- Broxterman, R.M.; Hureau, T.J.; Layec, G.; Morgan, D.E.; Bledsoe, A.D.; Jessop, J.E.; Amann, M.; Richardson, R.S. Influence of group III/IV muscle afferents on small muscle mass exercise performance: A bioenergetics perspective. J. Physiol. 2018, 596, 2301–2314. [Google Scholar] [CrossRef] [PubMed]
- Gorostiaga, E.M.; Navarro-Amézqueta, I.; González-Izal, M.; Malanda, A.; Granados, C.; Ibáñez, J.; Setuain, I.; Izquierdo, M. Blood lactate and sEMG at different knee angles during fatiguing leg press exercise. Eur. J. Appl. Physiol. 2012, 112, 1349–1358. [Google Scholar] [CrossRef]
- Camic, C.L.; Housh, T.J.; Johnson, G.O.; Hendrix, C.R.; Zuniga, J.M.; Mielke, M.; Schmidt, R.J. An EMG frequency-based test for estimating the neuromuscular fatigue threshold during cycle ergometry. Eur. J. Appl. Physiol. 2010, 108, 337–345. [Google Scholar] [CrossRef]
- Sanchis-Moysi, J.; Idoate, F.; Olmedillas, H.; Guadalupe-Grau, A.; Alayón, S.; Carreras, A.; Dorado, C.; Calbet, J.A.L. The upper extremity of the professional tennis player: Muscle volumes, fiber-type distribution and muscle strength. Scand. J. Med. Sci. Sports 2010, 20, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G.; Jensen-Urstad, M.; Rosdahl, H.; Holmberg, H.C.; Saltin, B.; Calbet, J.A.L. Leg and arm lactate and substrate kinetics during exercise. Am. J. Physiol.-Endocrinol. Metab. 2003, 284, E193–E205. [Google Scholar] [CrossRef]
- Samozino, P.; Peyrot, N.; Edouard, P.; Nagahara, R.; Jimenez-Reyes, P.; Vanwanseele, B.; Morin, J.B. Optimal mechanical force-velocity profile for sprint acceleration performance. Scand. J. Med. Sci. Sports 2022, 32, 559–575. Available online: https://pubmed.ncbi.nlm.nih.gov/34775654/ (accessed on 6 October 2024). [CrossRef]
- Fernandes, J.F.T.; Lamb, K.L.; Clark, C.C.T.; Moran, J.; Drury, B.; Garcia-Ramos, A.; Twist, C. Comparison of the FitroDyne and GymAware Rotary Encoders for Quantifying Peak and Mean Velocity During Traditional Multijointed Exercises. J. Strength Cond. Res. 2021, 35, 1760–1765. Available online: https://pubmed.ncbi.nlm.nih.gov/30399117/ (accessed on 6 August 2021). [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, H.; Kurokawa, T.; Tajima, M.; Liu, Z.; Okada, J. Can Perceived Exertion and Velocity Loss Serve as Indirect Indicators of Muscle Fatigue During Explosive Back Squat Exercise? J. Funct. Morphol. Kinesiol. 2024, 9, 238. https://doi.org/10.3390/jfmk9040238
Zhao H, Kurokawa T, Tajima M, Liu Z, Okada J. Can Perceived Exertion and Velocity Loss Serve as Indirect Indicators of Muscle Fatigue During Explosive Back Squat Exercise? Journal of Functional Morphology and Kinesiology. 2024; 9(4):238. https://doi.org/10.3390/jfmk9040238
Chicago/Turabian StyleZhao, Hanye, Takanori Kurokawa, Masayoshi Tajima, Zijian Liu, and Junichi Okada. 2024. "Can Perceived Exertion and Velocity Loss Serve as Indirect Indicators of Muscle Fatigue During Explosive Back Squat Exercise?" Journal of Functional Morphology and Kinesiology 9, no. 4: 238. https://doi.org/10.3390/jfmk9040238
APA StyleZhao, H., Kurokawa, T., Tajima, M., Liu, Z., & Okada, J. (2024). Can Perceived Exertion and Velocity Loss Serve as Indirect Indicators of Muscle Fatigue During Explosive Back Squat Exercise? Journal of Functional Morphology and Kinesiology, 9(4), 238. https://doi.org/10.3390/jfmk9040238






