Mechanical and Contractile Properties of Knee Joint Muscles after Sports-Related Concussions in Women Footballers
Abstract
1. Introduction
2. Methods and Materials
2.1. Subjects
2.2. Instrumentation and Procedures
2.3. Statistical Analysis
3. Results
3.1. TMG Parameters
3.2. Mann–Whitney U Test
- -
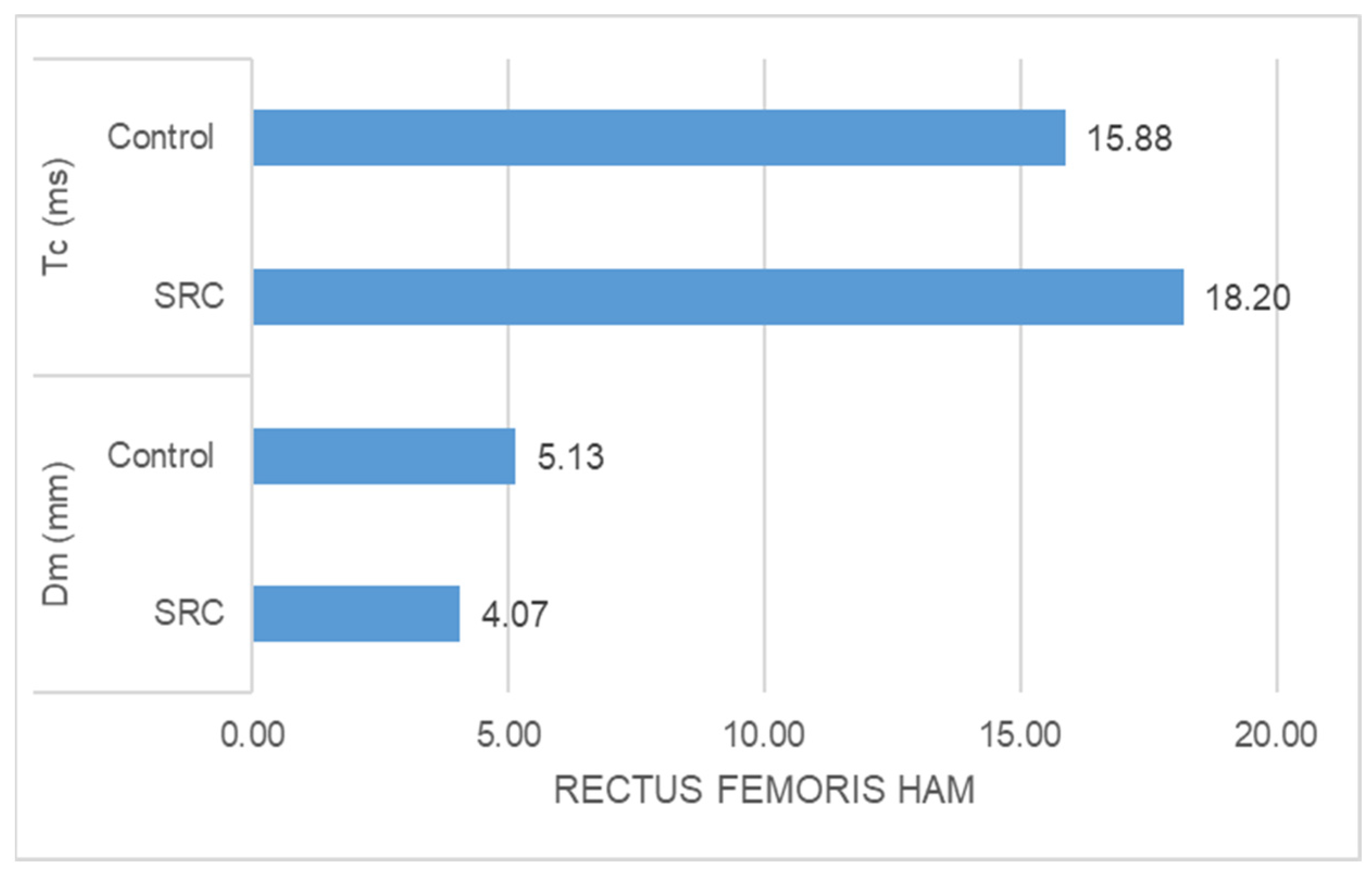
- The SRC group had significantly higher Tc (ms) (z = −5.478, p = 0.000) and significantly lower Dm (mm) (z = −3.835, p = 0.000) than the control group in the case of rectus femoris muscle response (Figure 1).
- -
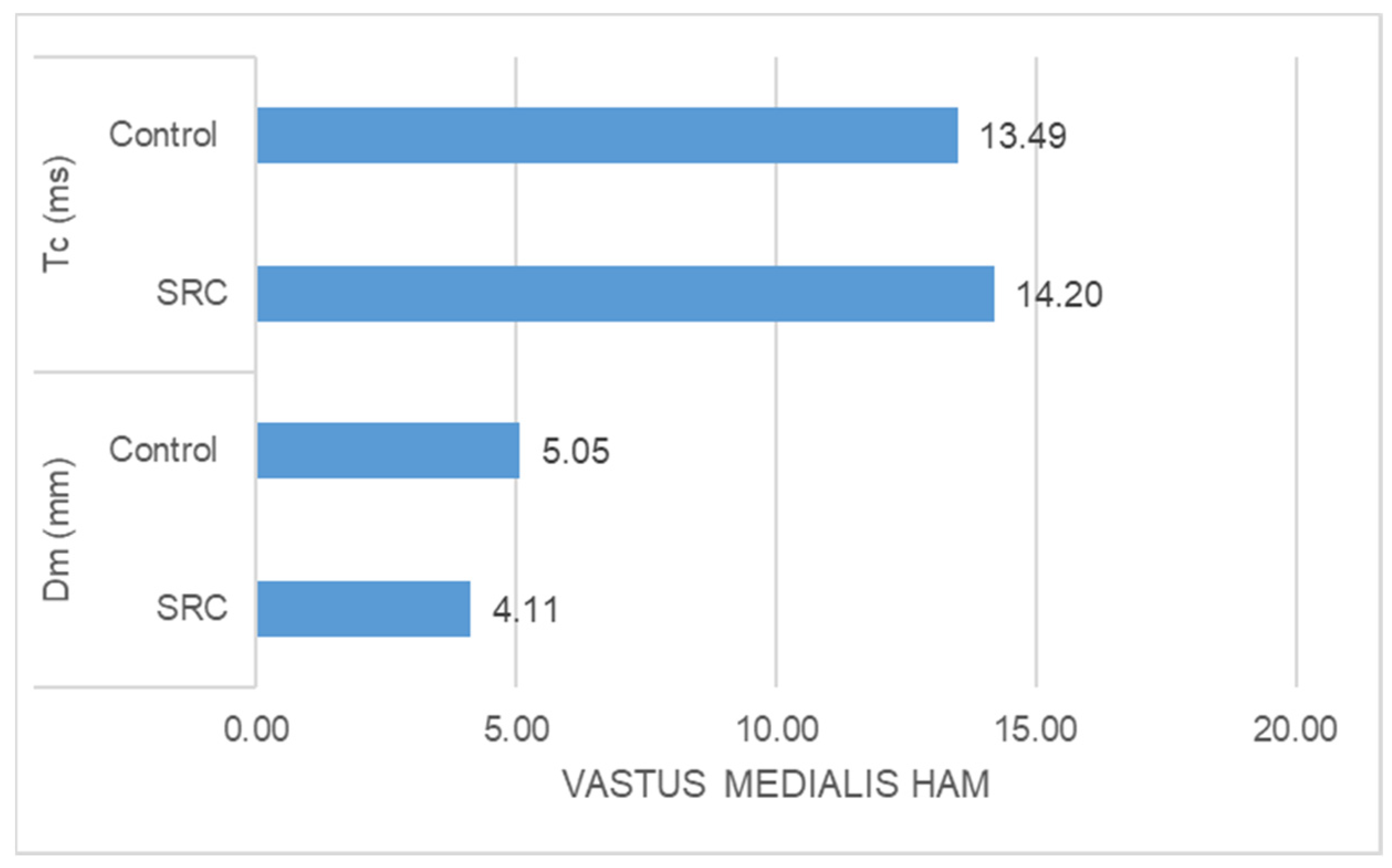
- The SRC group had significantly higher Tc (ms) (z = −2.348, p = 0.016) and significantly lower Dm (mm) (z = −4.776, p = 0.000) than the control group in the case of vastus medialis muscle response (Figure 2)
- -
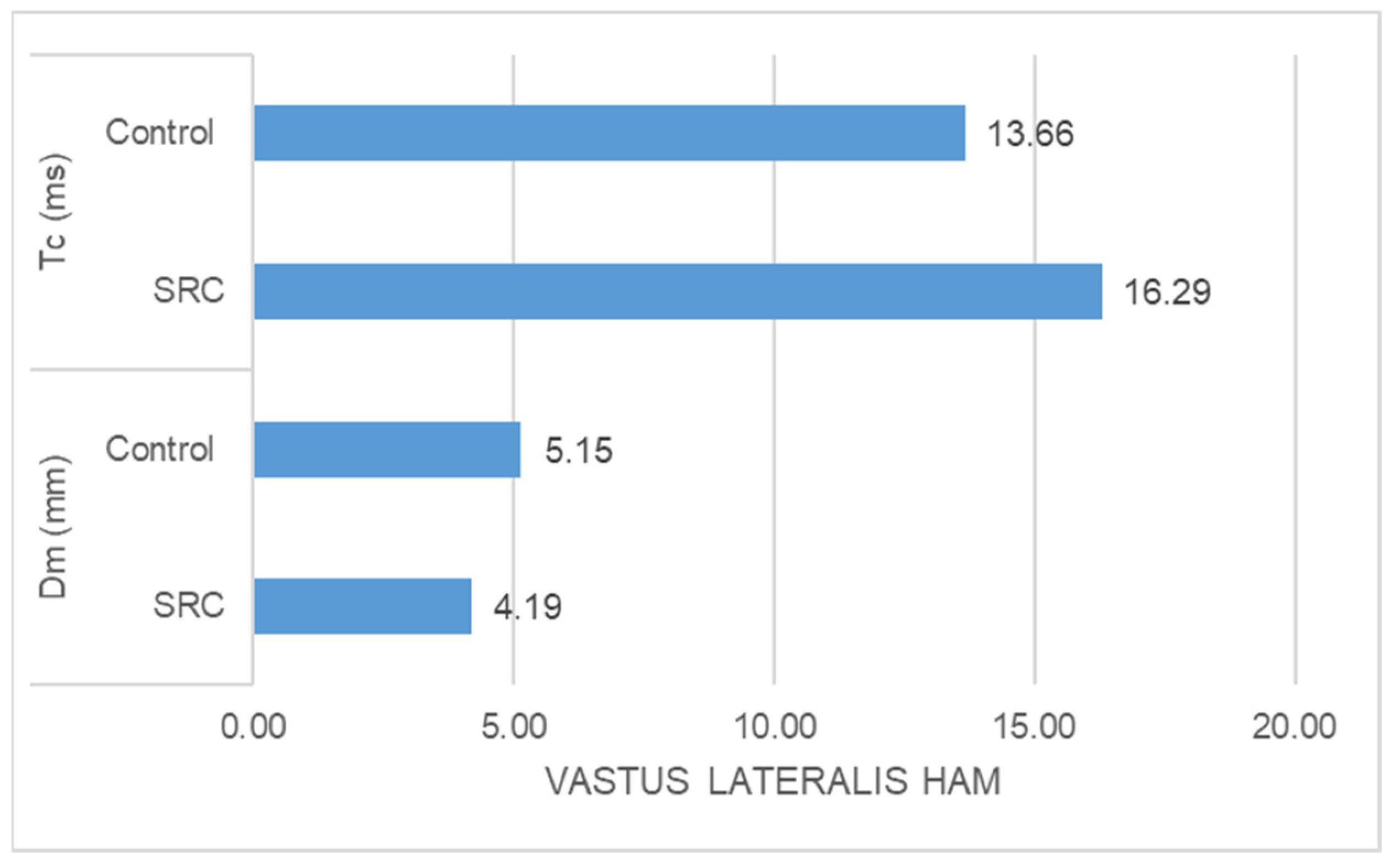
- The SRC group had significantly higher Tc (ms) (z = −5.400, p = 0.000) and significantly lower Dm (mm) (z = −4.971, p = 0.000) than the control group in the case of vastus lateralis muscle response (Figure 3).
- -
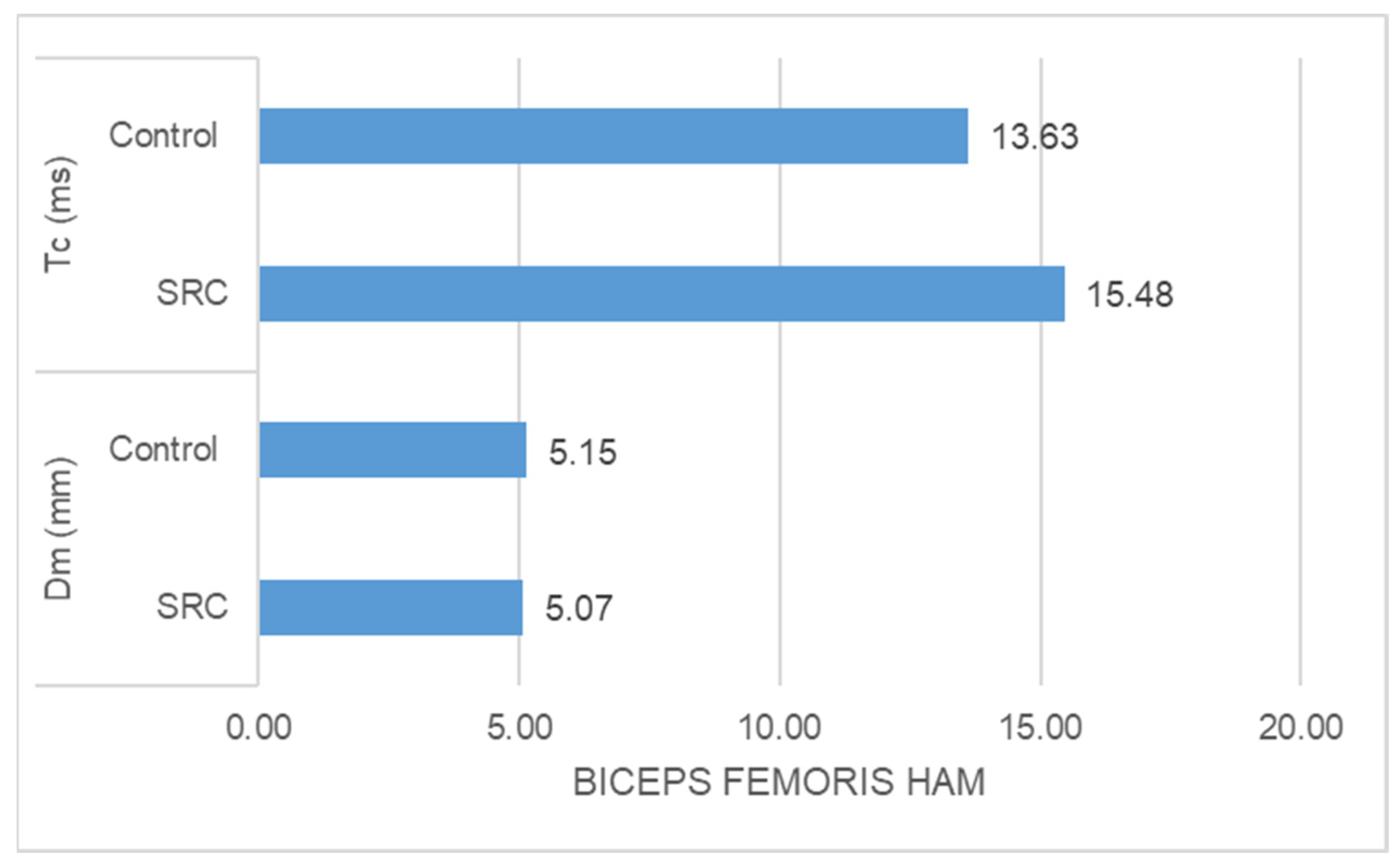
- The SRC group had significantly higher Tc (ms) (z = −5.349, p = 0.000) than the control group in the case of biceps femoris muscle response, whereas no significant difference was found in Dm (mm) (z = −0.198, p = 0.853) between the groups (Figure 4).
4. Discussion
4.1. Limitations of the Study
4.2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fait, P.; McFadyen, B.J.; Swaine, B.; Cantin, J.F. Alterations to locomotor navigation in a complex environment at 7 and 30 days following a concussion in an elite athlete. Brain Inj. 2009, 23, 362–369. [Google Scholar] [CrossRef]
- Fait, P.; Swaine, B.; Cantin, J.F.; Leblond, J.; McFadyen, B. Altered integrated locomotor and cognitive function in elite athletes 30 days postconcussion: A preliminary study. J. Head Trauma Rehabil. 2013, 28, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Dahmane, R.; Djordjevič, S.; Šimunič, B.; Valenčič, V. Spatial fiber type distribution in normal human muscle: Histochemical and tensiomyographical evaluation. J. Biomech. 2005, 38, 2451–2459. [Google Scholar] [CrossRef]
- García-Manso, J.M.; Rodríguez-Ruiz, D.; Rodríguez-Matoso, D.; de Saa, Y.; Sarmiento, S.; Quiroga, M. Assessment of muscle fatigue after an ultra-endurance triathlon using tensiomyography (TMG). J. Sports Sci. 2011, 29, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Catena, R.D.; van Donkelaar, P.; Chou, L.-S. Different gait tasks distinguish immediate vs. long-term effects of concussion on balance control. J. Neuroeng. Rehabil. 2009, 6, 25. [Google Scholar] [CrossRef]
- Martini, D.N.; Sabin, M.J.; DePesa, S.A.; Leal, E.W.; Negrete, T.N.; Sosnoff, J.J.; Broglio, S.P. The Chronic Effects of Concussion on Gait. Arch. Phys. Med. Rehabil. 2011, 92, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Hewett, T.E.; Myer, G.D.; Ford, K.R.; Heidt, R.S., Jr.; Colosimo, A.J.; McLean, S.G.; Van Den Bogert, A.J.; Paterno, M.V.; Succop, P. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: A prospective study. Am. J. Sports Med. 2005, 33, 492–501. [Google Scholar] [CrossRef]
- Zazulak, B.T.; Hewett, T.E.; Reeves, N.P.; Goldberg, B.; Cholewicki, J. Deficits in neuromuscular control of the trunk predict knee injury risk: A prospective biomechanical-epidemiologic study. Am. J. Sports Med. 2007, 35, 1123–1130. [Google Scholar] [CrossRef]
- Nordstrom, A.; Nordstrom, P.; Ekstrand, J. Sports-related concussion increases the risk of subsequent injury by about 50% in elite male football players. Br. J. Sports Med. 2014, 48, 1447–1450. [Google Scholar] [CrossRef]
- Cross, M.; Kemp, S.; Smith, A.; Trewartha, G.; Stokes, K. Professional Rugby Union players have a 60% greater risk of time loss injury after concussion: A 2-season prospective study of clinical outcomes. Br. J. Sports Med. 2015, 50, 926–931. [Google Scholar] [CrossRef]
- Asken, B.M.; McCrea, M.A.; Clugston, J.R.; Snyder, A.R.; Houck, Z.M.; Bauer, R.M. “Playing Through It”: Delayed Reporting and Removal From Athletic Activity After Concussion Predicts Prolonged Recovery. J. Athl. Train. 2016, 51, 329–335. [Google Scholar] [CrossRef]
- Dorminy, M.; Hoogeveen, A.; Tierney, R.T.; Higgins, M.; McDevitt, J.K.; Kretzschmar, J. Effect of soccer heading ball speed on S100B, sideline concussion assessments and head impact kinematics. Brain Inj. 2015, 29, 1158–1164. [Google Scholar] [CrossRef]
- Kaminski, T.W.; Cousino, E.S.; Glutting, J.J. Examining the relationship between purposeful heading in soccer and computerised neuropsychological test performance. Res. Q. Exerc. Sport 2008, 79, 235–244. [Google Scholar] [CrossRef]
- Baker, J.G.; Leddy, J.J.; Darling, S.R.; Shucard, J.; Makdissi, M.; Willer, B.S. Gender Differences in Recovery From Sports-Related Concussion in Adolescents. Clin. Pediatr. 2016, 55, 771–775. [Google Scholar] [CrossRef]
- Catenaccio, E.; Caccese, J.; Wakschlag, N.; Fleysher, R.; Kim, N.; Kim, M.; Buckley, T.A.; Stewart, W.F.; Lipton, R.B.; Kaminski, T.; et al. Validation and calibration of HeadCount, a self-report measure for quantifying heading exposure in soccer players. Res. Sports Med. 2016, 24, 416–425. [Google Scholar] [CrossRef]
- Putukian, M.; Echemendia, R.J.; Mackin, S. The acute neuropsychological effects of heading in soccer: A pilot study. Clin. J. Sport Med. 2000, 10, 104–109. [Google Scholar] [CrossRef]
- Bakhos, L.L.; Lockhart, G.R.; Myers, R.; Linakis, J.G. Emergency Department Visits for Concussion in Young Child Athletes. Pediatrics 2010, 126, e550–e556. [Google Scholar] [CrossRef]
- Stephens, R.; Rutherford, A.; Potter, D.; Fernie, G. Neuropsychological impairment as a consequence of football (soccer) play and football heading: A preliminary analysis and report on school students (13–16 years). Child Neuropsychol. 2005, 11, 513–526. [Google Scholar] [CrossRef]
- Maher, M.E.; Hutchison, M.; Cusimano, M.; Comper, P.; Schweizer, T.A. Concussions and heading in soccer: A review of the evidence of incidence, mechanisms, biomarkers and neurocognitive outcomes. Brain Inj. 2014, 28, 271–285. [Google Scholar] [CrossRef] [PubMed]
- Kakavas, G.; Giannakopoulos, I.; Tsiokanos, A.; Potoupnis, M.; Tsaklis, P.V. The Effect of Ball Heading and Subclinical Concussion On the Neuromuscular Control Of The Lower Limb: A Systematic Review. Int. J. Sports Phys. Ther. 2023, 18, 1054–1064. [Google Scholar] [CrossRef] [PubMed]
- Barnes, B.C.; Cooper, L.; Kirkendall, D.T.; McDermott, T.P.; Jordan, B.D.; Garrett, W.E. Concussion History in Elite Male and Female Soccer Players. Am. J. Sports Med. 1998, 26, 433–438. [Google Scholar] [CrossRef]
- Kakavas, G.; Malliaropoulos, N.; Blach, W.; Bikos, G.; Migliorini, F.; Maffulli, N. Ball heading and subclinical concussion in soccer as a risk factor for anterior cruciate ligament injury. J. Orthop. Surg. Res. 2021, 16, 566. [Google Scholar] [CrossRef]
- Barr, C.C.; Schultheis, L.W.; Robinson, D.A. Voluntary, non-visual control of the human vestibulo-ocular reflex. Acta Oto-Laryngol. 1976, 81, 365–375. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Reneker, J.C.; Babl, R.; Flowers, M.M. History of concussion and risk of subsequent injury in athletes and service members: A systematic review and meta-analysis. Musculoskelet. Sci. Pract. 2019, 42, 173–185. [Google Scholar] [CrossRef]
- Renström, P.; Arms, S.W.; Stanwyck, T.S.; Johnson, R.J.; Pope, M.H. Strain within the anterior cruciate ligament during hamstring and quadriceps activity. Am. J. Sports Med. 1986, 14, 83–87. [Google Scholar] [CrossRef]
- Barr, W.B. Neuropsychological testing of high school athletes. Preliminary norms and test-retest indices. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 2003, 18, 91–101. [Google Scholar]
- Shimokochi, Y.; Yong Lee, S.; Shultz, S.J.; Schmitz, R.J. The relationships among sagittal-plane lower extremity moments: Implications for landing strategy in anterior cruciate ligament injury prevention. J. Athl. Train. 2009, 44, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Barr, W.B.; Prichep, L.S.; Chabot, R.; Powell, M.R.; McCrea, M. Measuring brain electrical activity to track recovery from sport-related concussion. Brain Inj. 2012, 26, 58–66. [Google Scholar] [CrossRef]
- Swanik, C.B. Brains and sprains: The brain’s role in noncontact anterior cruciate ligament injuries. J. Athl. Train. 2015, 50, 1100–1102. [Google Scholar] [CrossRef]
- Basford, J.R.; Chou, L.S.; Kaufman, K.R.; Brey, R.H.; Walker, A.; Malec, J.F.; Moessner, A.M.; Brown, A.W. An assessment of gait and balance deficits after traumatic brain injury. Arch. Phys. Med. Rehabil. 2003, 84, 343–349. [Google Scholar] [CrossRef]
- Swanik, C.; Covassin, T.; Stearne, D.J.; Schatz, P. The relationship between neurocognitive function and noncontact anterior cruciate ligament injuries. Am. J. Sports Med. 2007, 35, 943–948. [Google Scholar] [CrossRef]
- Baugh, C.M.; Kroshus, E.; Stamm, J.M.; Daneshvar, D.H.; Pepin, M.J.; Meehan, W.P. Clinical Practices in Collegiate Concussion Management. Am. J. Sports Med. 2016, 44, 1391–1399. [Google Scholar] [CrossRef]
- Wilkerson, G.B. Neurocognitive reaction time predicts lower extremity sprains and strains. Int. J. Athl. Ther. Train. 2012, 17, 4–9. [Google Scholar] [CrossRef]
- Beidler, E.; Bretzin, A.C.; Hanock, C.; Covassin, T. Sport-related concussion: Knowledge and reporting behaviors among collegiate club-sport athletes. J. Athl. Train. 2018, 53, 866–872. [Google Scholar] [CrossRef]
- Bell, D.R.; Guskiewicz, K.M.; Clark, M.A.; Padua, D.A. Systematic review of the balance error scoring system. Sports Health 2011, 3, 287–295. [Google Scholar] [CrossRef]
| Sample | SRC Group | Control Group | ||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |
| n | (n = 41) | (n = 20) | (n = 21) | |||
| Age | 19.73 | 1.23 | 19.35 | 1.18 | 20.10 | 1.18 |
| Weight (Kg) | 61.95 | 7.62 | 59.15 | 8.22 | 64.62 | 6.05 |
| Height (cm) | 166.76 | 7.54 | 163.45 | 7.44 | 169.90 | 6.32 |
| BMI | 22.21 | 1.67 | 22.07 | 2.03 | 22.35 | 1.27 |
| Values | |||||||
|---|---|---|---|---|---|---|---|
| Muscle | Variable | N | Group | Mean | SD | Median | Mean Rank |
| Rectus Femoris | Tc (ms) | 20 | SRC | 18.20 | 0.09 | 18.21 | 31.50 |
| 21 | Control | 15.88 | 0.61 | 15.88 | 11.00 | ||
| Dm (mm) | 20 | SRC | 4.07 | 0.78 | 4.10 | 13.65 | |
| 21 | Control | 5.13 | 0.77 | 5.10 | 28.00 | ||
| Vastus Medialis | Tc (ms) | 20 | SRC | 14.20 | 0.06 | 14.20 | 25.50 |
| 21 | Control | 13.49 | 0.76 | 13.34 | 16.71 | ||
| Dm (mm) | 20 | SRC | 4.11 | 0.45 | 4.10 | 11.85 | |
| 21 | Control | 5.05 | 0.38 | 4.99 | 29.71 | ||
| Vastus Lateralis | Tc (ms) | 20 | SRC | 16.29 | 0.58 | 16.31 | 31.35 |
| 21 | Control | 13.66 | 0.87 | 13.70 | 11.14 | ||
| Dm (mm) | 20 | SRC | 4.19 | 0.39 | 4.12 | 11.48 | |
| 21 | Control | 5.15 | 0.42 | 5.19 | 30.07 | ||
| Biceps Femoris | Tc (ms) | 20 | SRC | 15.48 | 0.32 | 15.55 | 31.25 |
| 21 | Control | 13.63 | 0.75 | 13.76 | 11.24 | ||
| Dm (mm) | 20 | SRC | 5.07 | 0.15 | 5.17 | 21.38 | |
| 21 | Control | 5.15 | 0.51 | 5.15 | 20.64 | ||
| Muscle | Variable | z | p Value |
|---|---|---|---|
| Rectus Femoris Ham | Tc (ms) | −5.478 | 0.000 |
| Dm (mm) | −3.835 | 0.000 | |
| Vastus Medialis Ham | Tc (ms) | −2.348 | 0.016 |
| Dm (mm) | −4.776 | 0.000 | |
| Vastus Lateralis Ham | Tc (ms) | −5.400 | 0.000 |
| Dm (mm) | −4.971 | 0.000 b | |
| Biceps Femoris Ham | Tc (ms) | −5.349 | 0.00 |
| Dm (mm) | −0.198 | 0.853 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kakavas, G.; Tsiokanos, A.; Potoupnis, M.; Tsaklis, P.V. Mechanical and Contractile Properties of Knee Joint Muscles after Sports-Related Concussions in Women Footballers. J. Funct. Morphol. Kinesiol. 2024, 9, 65. https://doi.org/10.3390/jfmk9020065
Kakavas G, Tsiokanos A, Potoupnis M, Tsaklis PV. Mechanical and Contractile Properties of Knee Joint Muscles after Sports-Related Concussions in Women Footballers. Journal of Functional Morphology and Kinesiology. 2024; 9(2):65. https://doi.org/10.3390/jfmk9020065
Chicago/Turabian StyleKakavas, Georgios, Athanasios Tsiokanos, Michael Potoupnis, and Panagiotis V. Tsaklis. 2024. "Mechanical and Contractile Properties of Knee Joint Muscles after Sports-Related Concussions in Women Footballers" Journal of Functional Morphology and Kinesiology 9, no. 2: 65. https://doi.org/10.3390/jfmk9020065
APA StyleKakavas, G., Tsiokanos, A., Potoupnis, M., & Tsaklis, P. V. (2024). Mechanical and Contractile Properties of Knee Joint Muscles after Sports-Related Concussions in Women Footballers. Journal of Functional Morphology and Kinesiology, 9(2), 65. https://doi.org/10.3390/jfmk9020065










