Disc Degeneration and Cervical Spine Intervertebral Motion: A Cross-Sectional Study in Patients with Neck Pain and Matched Healthy Controls
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
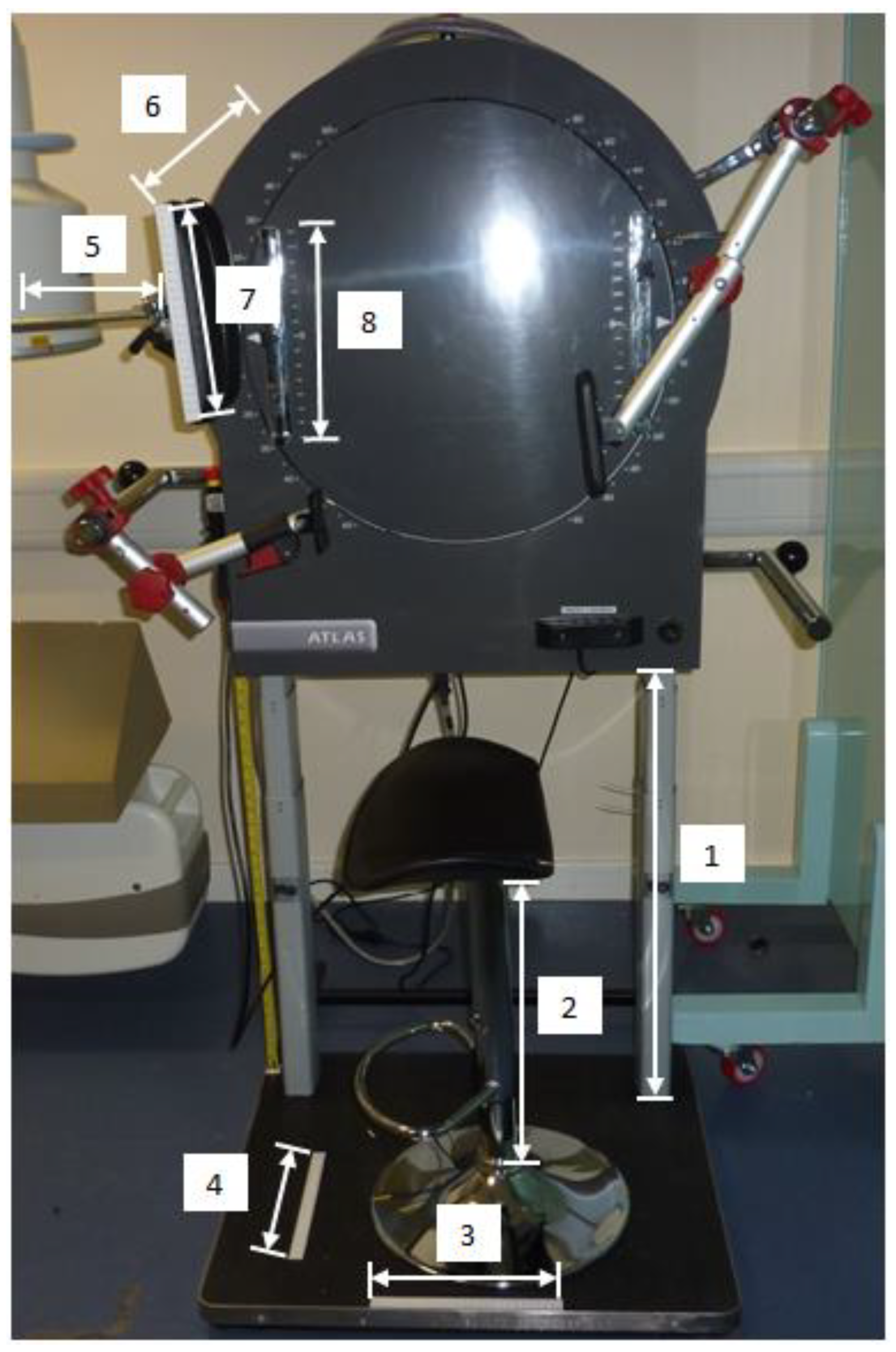
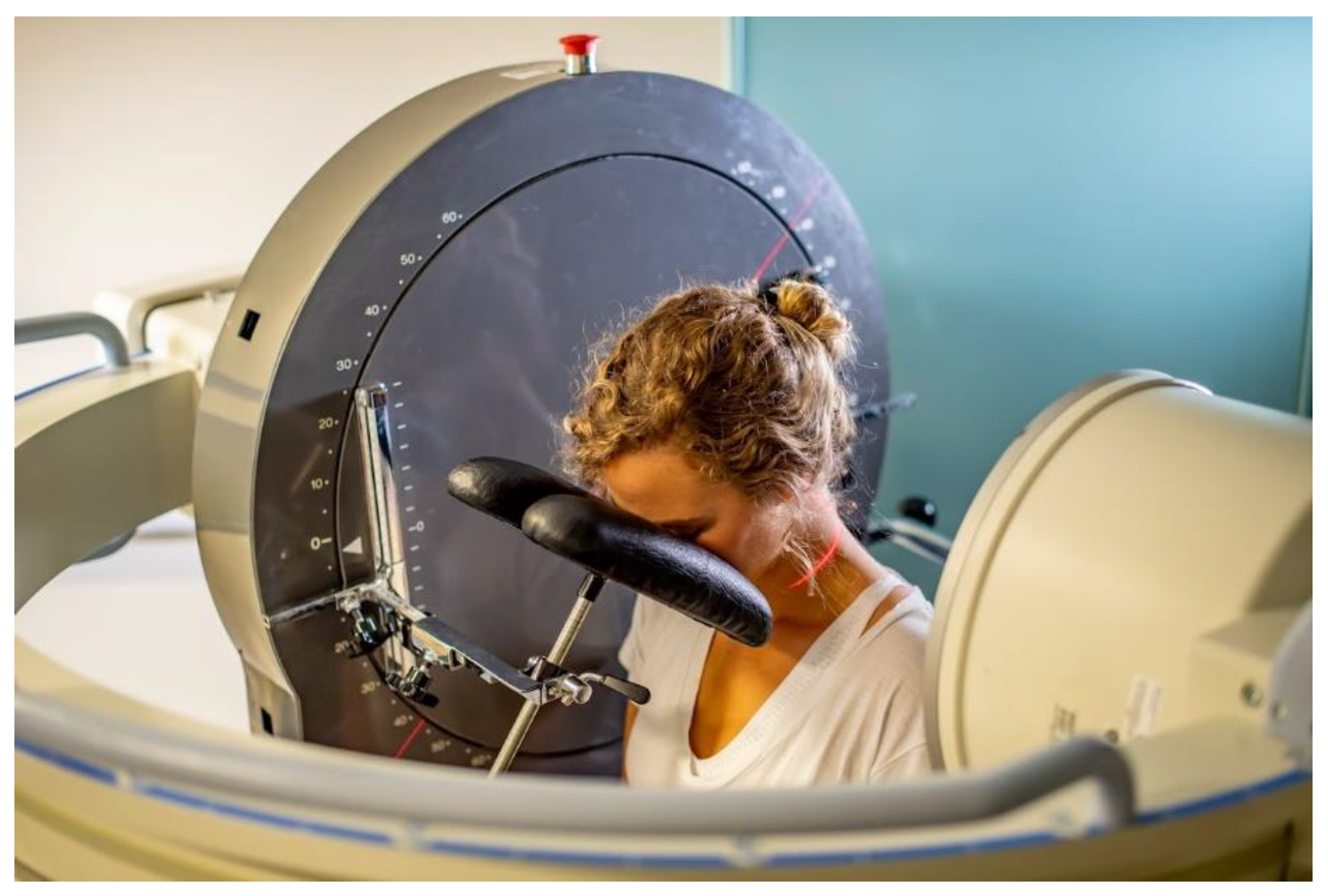
2.2. Image Acquisition
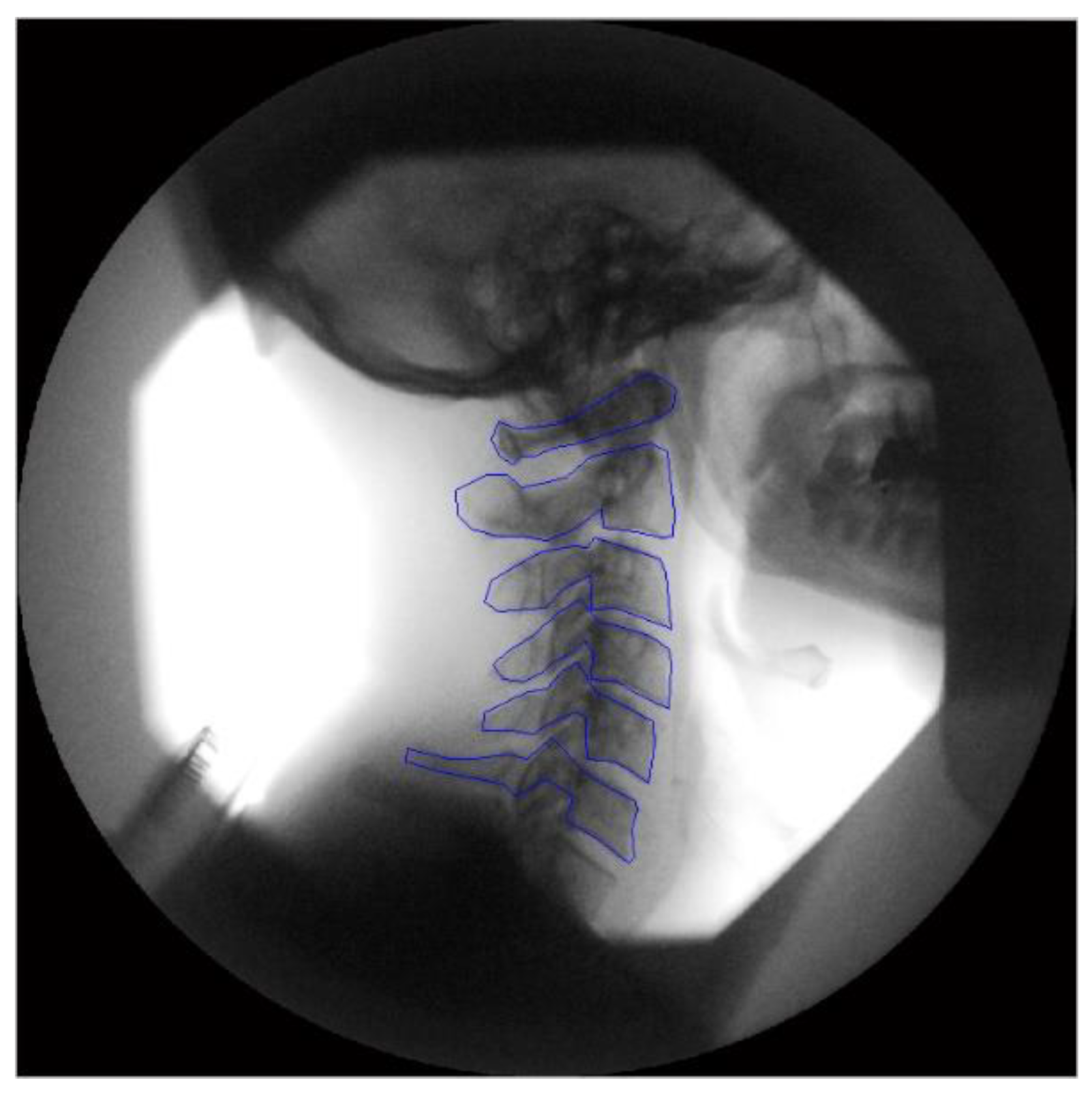
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hoy, D.; March, L.; Woolf, A.; Blyth, F.; Brooks, P.; Smith, E.; Vos, T.; Barendregt, J.; Blore, J.; Murray, C.; et al. The global burden of neck pain: Estimates from the global burden of disease 2010 study. Ann. Rheum. Dis. 2014, 73, 1309–1315. [Google Scholar] [CrossRef]
- Cohen, S.P.; Hooten, W.M. Advances in the diagnosis and management of neck pain. BMJ (Clin. Res. Ed.) 2017, 358, j3221. [Google Scholar] [CrossRef]
- Binder, A.I. Neck pain. BMJ Clin. Evid. 2008, 2008, 1103. [Google Scholar]
- Guzman, J.; Hurwitz, E.L.; Carroll, L.J.; Haldeman, S.; Côté, P.; Carragee, E.J.; Peloso, P.M.; van der Velde, G.; Holm, L.W.; Hogg-Johnson, S.; et al. A new conceptual model of neck pain: Linking onset, course, and care: The Bone and Joint Decade 2000–2010 Task Force on Neck Pain and Its Associated Disorders. Spine 2008, 33, S14–S23. [Google Scholar] [CrossRef]
- Bogduk, N. The anatomy and pathophysiology of neck pain. Phys. Med. Rehabil. Clin. N. Am. 2011, 22, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Kim, S.J.; Lim, S.M. Prevalence of disc degeneration in asymptomatic korean subjects. Part 2: Cervical spine. J. Korean Neurosurg. Soc. 2013, 53, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Onyewu, O.; Manchikanti, L.; Falco, F.; Singh, V.; Geffert, S.; Cohen, S.H.P.; Hirsch, J.A. An update of the appraisal of the accuracy and utility of cervical discography in chronic neck pain. Pain Physician 2012, 15, E777–E806. [Google Scholar] [CrossRef] [PubMed]
- Mellor, F.E.; Thomas, P.W.; Thompson, P.; Breen, A.C. Proportional lumbar spine inter-vertebral motion patterns: A comparison of patients with chronic, non-specific low back pain and healthy controls. Eur. Spine J. 2014, 23, 2059–2067. [Google Scholar] [CrossRef]
- Branney, J.; Breen, A.C. Does inter-vertebral range of motion increase after spinal manipulation? A prospective cohort study. Chiropr. Man. Ther. 2014, 22, 24. [Google Scholar] [CrossRef]
- Anderst, W.J.; Donaldson, W.F., 3rd; Lee, J.Y.; Kang, J.D. Continuous cervical spine kinematics during in vivo dynamic flexion-extension. Spine J. 2014, 14, 1221–1227. [Google Scholar] [CrossRef]
- Lindstrøm, R.; Breen, A.; Qu, N.; Rose, A.D.; Andersen, V.B.; Breen, A. Novel assessment of the variation in cervical inter-vertebral motor control in a healthy pain-free population. Sci. Rep. 2021, 11, 10769. [Google Scholar] [CrossRef] [PubMed]
- Boselie, T.F.M.; van Santbrink, H.; de Bie, R.A.; van Mameren, H. Pilot Study of Sequence of Segmental Contributions in the Lower Cervical Spine During Active Extension and Flexion: Healthy Controls Versus Cervical Degenerative Disc Disease Patients. Spine 2017, 42, E642–E647. [Google Scholar] [CrossRef] [PubMed]
- Breen, A.; Breen, A. Uneven intervertebral motion sharing is related to disc degeneration and is greater in patients with chronic, non-specific low back pain: An in vivo, cross-sectional cohort comparison of intervertebral dynamics using quantitative fluoroscopy. Eur. Spine J. 2018, 27, 145–153. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet 2007, 370, 1453–1457. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, R.H.; Turk, D.C.; Farrar, J.T.; Haythornthwaite, J.; Jensen, M.P.; Katz, N.P.; Kerns, R.D.; Stucki, G.; Allen, R.R.; Bellamy, N.; et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain 2005, 113, 9–19. [Google Scholar] [CrossRef]
- MacDermid, J.C.; Walton, D.M.; Avery, S.; Blanchard, A.; Etruw, E.; Mcalpine, C.; Goldsmith, C.H. Measurement properties of the neck disability index: A systematic review. J. Orthop. Sports Phys. Ther. 2009, 39, 400–417. [Google Scholar] [CrossRef]
- Luo, N.; Li, M.; Chevalier, J.; Lloyd, A.; Herdman, M. A comparison of the scaling properties of the English, Spanish, French, and Chinese EQ-5D descriptive systems. Qual. Life Res. 2013, 22, 2237–2243. [Google Scholar] [CrossRef]
- Breen, A.C.; Teyhen, D.S.; Mellor, F.E.; Breen, A.C.; Wong, K.W.; Deitz, A. Measurement of intervertebral motion using quantitative fluoroscopy: Report of an international forum and proposal for use in the assessment of degenerative disc disease in the lumbar spine. Adv. Orthop. 2012, 2012, 802350. [Google Scholar] [CrossRef]
- de Vet, H.C.; Terwee, C.B.; Knol, D.L.; Bouter, L.M. When to use agreement versus reliability measures. J. Clin. Epidemiol. 2006, 59, 1033–1039. [Google Scholar] [CrossRef]
- To, D.; Breen, A.; Breen, A.; Mior, S.; Howarth, S.J. Investigator analytic repeatability of two new intervertebral motion biomarkers for chronic, nonspecific low back pain in a cohort of healthy controls. Chiropr. Man. Ther. 2020, 28, 62. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological assessment of osteo-arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Kettler, A.; Wilke, H.J. Review of existing grading systems for cervical or lumbar disc and facet joint degeneration. Eur. Spine J. 2006, 15, 705–718. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Sim, J.; Wright, C.C. The kappa statistic in reliability studies: Use, interpretation, and sample size requirements. Phys. Ther. 2005, 85, 257–268. [Google Scholar] [CrossRef]
- D’Hooge, R.; Cagnie, B.; Crombez, G.; Vanderstraeten, G.; Achten, E.; Danneels, L. Lumbar muscle dysfunction during remission of unilateral recurrent nonspecific low-back pain: Evaluation with muscle functional MRI. Clin. J. Pain 2013, 29, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. A hypothesis of chronic back pain: Ligament subfailure injuries lead to muscle control dysfunction. Eur. Spine J. 2006, 15, 668–676. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Pollintine, P.; Hole, B.D.; Dolan, P.; Adams, M.A. Discogenic origins of spinal instability. Spine 2005, 30, 2621–2630. [Google Scholar] [CrossRef] [PubMed]
- Panjabi, M.M. The stabilizing system of the spine. Part I. Function, dysfunction, adaptation, and enhancement. J. Spinal Disord. 1992, 5, 383–389; discussion 397. [Google Scholar] [CrossRef]
- Anderst, W.J.; Donaldson, W.F., 3rd; Lee, J.Y.; Kang, J.D. Cervical motion segment percent contributions to flexion-extension during continuous functional movement in control subjects and arthrodesis patients. Spine 2013, 38, E533–E539. [Google Scholar] [CrossRef]
- Lee, M.J.; Dettori, J.R.; Standaert, C.J.; Brodt, E.D.; Chapman, J.R. The natural history of degeneration of the lumbar and cervical spines: A systematic review. Spine 2012, 37, S18–S30. [Google Scholar] [CrossRef]
- Kolstad, F.; Nygaard, Ø.P.; Leivseth, G. Segmental motion adjacent to anterior cervical arthrodesis: A prospective study. Spine 2007, 32, 512–517. [Google Scholar] [CrossRef]
- Franov, E.; Straub, M.; Bauer, C.M.; Ernst, M.J. Head kinematics in patients with neck pain compared to asymptomatic controls: A systematic review. BMC Musculoskelet. Disord. 2022, 23, 156. [Google Scholar] [CrossRef]
- du Rose, A.; Breen, A. Relationships between Paraspinal Muscle Activity and Lumbar Inter-Vertebral Range of Motion. Healthcare 2016, 4, 4. [Google Scholar] [CrossRef]
- Suzuki, A.; Daubs, M.D.; Hayashi, T.; Ruangchainikom, M.; Xiong, C.; Phan, K.; Scott, T.P.; Wang, J.C. Patterns of Cervical Disc Degeneration: Analysis of Magnetic Resonance Imaging of Over 1000 Symptomatic Subjects. Glob. Spine J. 2018, 8, 254–259. [Google Scholar] [CrossRef]
- Stenneberg, M.S.; Rood, M.; de Bie, R.; Schmitt, M.A.; Cattrysse, E.; Scholten-Peeters, G.G. To What Degree Does Active Cervical Range of Motion Differ between Patients with Neck Pain, Patients with Whiplash, and Those without Neck Pain? A Systematic Review and Meta-Analysis. Arch. Phys. Med. Rehabil. 2017, 98, 1407–1434. [Google Scholar] [CrossRef]
- Cheung, K.M.; Samartzis, D.; Karppinen, J.; Luk, K.D. Are “patterns” of lumbar disc degeneration associated with low back pain? New insights based on skipped level disc pathology. Spine 2012, 37, E430–E438. [Google Scholar] [CrossRef]
- Von Forell, G.A.; Stephens, T.K.; Samartzis, D.; Bowden, A.E. Low Back Pain: A Biomechanical Rationale Based on “Patterns” of Disc Degeneration. Spine 2015, 40, 1165–1172. [Google Scholar] [CrossRef]

| Patients (n = 29) | Healthy Controls (n = 30) | p | |
|---|---|---|---|
| Age, mean (SD) | 40 years (13.1) | 41 years (13.1) | 0.72 † |
| Female, n | 21 | 21 | >0.99 ‡ |
| Mean (SD) regional cervical spine ROM (°) | Flexion 49 (6.7) | 53 (7.2) | 0.04 † |
| Extension 51 (7.2) | 56 (6.6) | 0.03 † | |
| CDD/16, median (25, 75) | 2 (1, 4) | 2 (1, 3) | 0.94 †† |
| Mean (SD) NRS score | 5 (1.5) | - | - |
| Mean (SD) NDI score | 13 (6.7) | - | - |
| Mean (SD) EQ-5D 5L VAS | 75 (15.5) | - | - |
| Mean (SD) EQ-5D 5L Index | 0.74 (0.09) | - | - |
| Intervertebral Ranges of Motion | Number of Degenerated Intervertebral Discs (K-L 1 or Above) | Number of Degenerated Intervertebral Discs (K-L ≥ 2) | ||||||
|---|---|---|---|---|---|---|---|---|
| Flexion, Degrees Mean (SD) | Extension, Degrees Mean (SD) | |||||||
| Level | Patients | Healthy Controls | Patients | Healthy Controls | Patients | Healthy Controls | Patients | Healthy Controls |
| C2/3 | 6 (3.1) | 6 (3.5) | 4 (3.2) | 5 (3.7) | 16 | 13 | 2 | 0 |
| C3/4 | 7 (3.8) | 7 (2.8) | 7 (3.7) | 8 (5.5) | 5 | 6 | 1 | 1 |
| C4/5 | 6 (2.8) | 6 (3.4) | 8 (4.7) | 11 (5.8) | 7 | 5 | 1 | 3 |
| C5/6 | 5 (2.9) | 6 (3.9) | 9 (4.9) | 8 (4.9) | 14 | 11 | 5 | 4 |
| C6/7 | - | - | - | - | 11 | 12 | 4 | 7 |
| C7/T1 | - | - | - | - | 9 | 8 | 0 | 0 |
| Total | 24 (8.9) | 25 (11.3) | 28 (12.5) | 32 (16.0) | 62 | 55 | 13 | 15 |
| Patients (n = 29) | Healthy Controls (n = 30) | p †† | ||
|---|---|---|---|---|
| Flexion, median (25, 75) | MSI | 0.34 (0.28, 0.41) | 0.32 (0.24, 0.41) | 0.38 |
| MSV | 0.14 (0.09, 0.19) | 0.13 (0.08, 0.21) | 0.75 | |
| Extension, median (25, 75) | MSI | 0.41 (0.32, 0.52) | 0.36 (0.29, 0.45) | 0.28 |
| MSV | 0.10 (0.06, 0.21) | 0.09 (0.06, 0.16) | 0.34 |
| Patients | Healthy Controls | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CDD | MSI | MSV | CDD | MSI | MSV | ||||||||
| rs | p | rs | p | rs | p | rs | p | rs | p | rs | p | ||
| Flex | Age | 0.29 | 0.13 | −0.05 | 0.79 | −0.16 | 0.40 | 0.52 | <0.001 | −0.10 | 0.62 | −0.21 | 0.27 |
| CDD | 0.23 | 0.23 | −0.06 | 0.77 | −0.25 | 0.18 | −0.16 | 0.41 | |||||
| MSI | 0.53 | <0.001 | 0.44 | 0.02 | |||||||||
| Ext | Age | 0.24 | 0.21 | 0.63 | <0.001 | 0.28 | 0.14 | 0.24 | 0.20 | ||||
| CDD | −0.08 | 0.70 | 0.09 | 0.63 | −0.19 | 0.32 | 0.10 | 0.59 | |||||
| MSI | 0.39 | 0.04 | 0.29 | 0.12 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Branney, J.; Breen, A.; du Rose, A.; Mowlem, P.; Breen, A. Disc Degeneration and Cervical Spine Intervertebral Motion: A Cross-Sectional Study in Patients with Neck Pain and Matched Healthy Controls. J. Funct. Morphol. Kinesiol. 2024, 9, 55. https://doi.org/10.3390/jfmk9010055
Branney J, Breen A, du Rose A, Mowlem P, Breen A. Disc Degeneration and Cervical Spine Intervertebral Motion: A Cross-Sectional Study in Patients with Neck Pain and Matched Healthy Controls. Journal of Functional Morphology and Kinesiology. 2024; 9(1):55. https://doi.org/10.3390/jfmk9010055
Chicago/Turabian StyleBranney, Jonathan, Alexander Breen, Alister du Rose, Philip Mowlem, and Alan Breen. 2024. "Disc Degeneration and Cervical Spine Intervertebral Motion: A Cross-Sectional Study in Patients with Neck Pain and Matched Healthy Controls" Journal of Functional Morphology and Kinesiology 9, no. 1: 55. https://doi.org/10.3390/jfmk9010055
APA StyleBranney, J., Breen, A., du Rose, A., Mowlem, P., & Breen, A. (2024). Disc Degeneration and Cervical Spine Intervertebral Motion: A Cross-Sectional Study in Patients with Neck Pain and Matched Healthy Controls. Journal of Functional Morphology and Kinesiology, 9(1), 55. https://doi.org/10.3390/jfmk9010055






