Exercise-Induced Asthma: Managing Respiratory Issues in Athletes
Abstract
1. Introduction
2. Methods
3. Causes and Triggers of EIB in Athletes
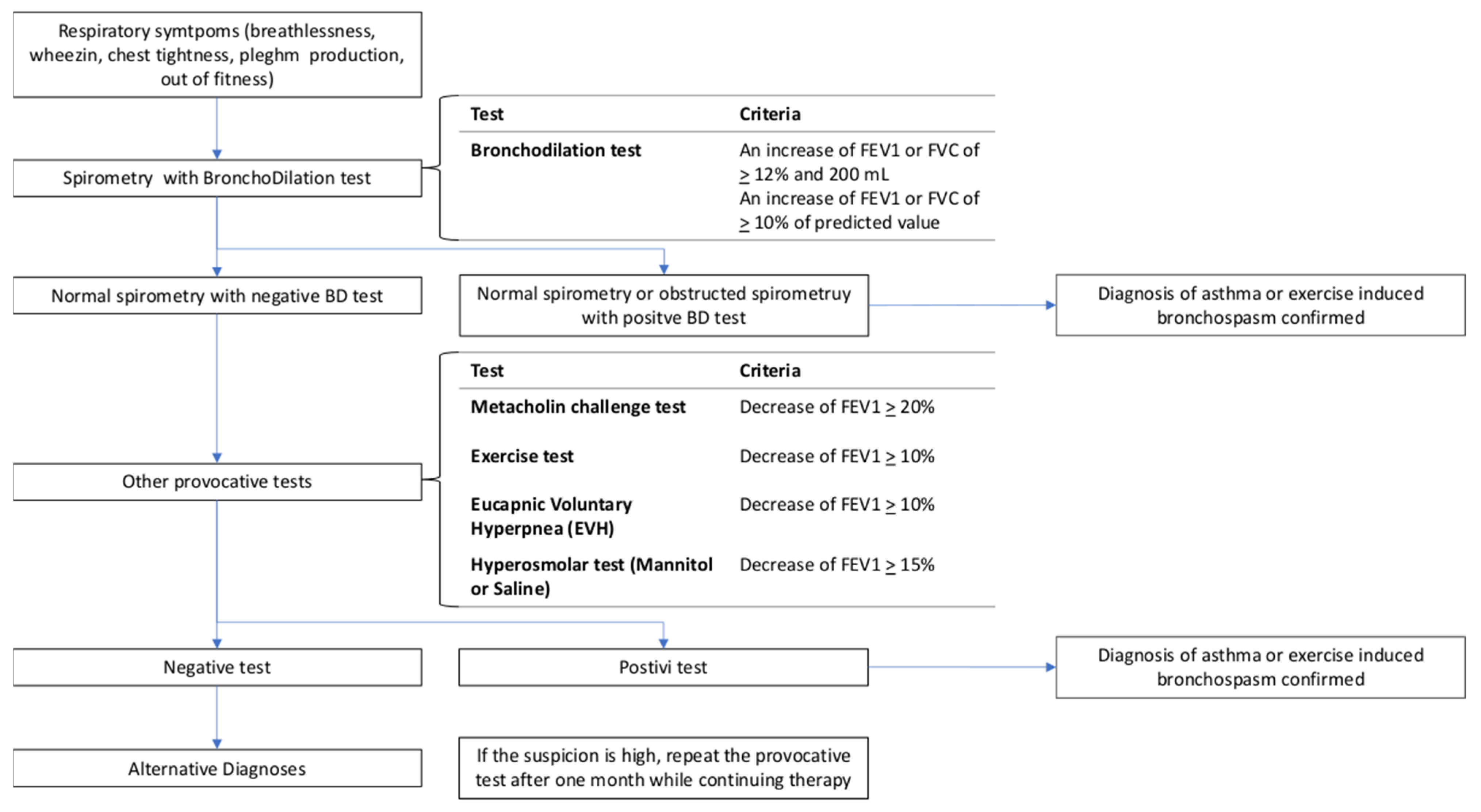
4. Diagnosis of EIA in Athletes
5. Management of EIB in Athletes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
List of Abbreviations
| BHR | Bronchial Hyper-Responsiveness |
| EIA | Exercise-Induced Asthma |
| EIB | Exercise-Induced Bronchoconstriction |
| EIBA | Exercise-Induced Bronchoconstriction with Asthma |
| EIBwA | Exercise-Induced Bronchoconstriction without Asthma |
| EVH | Eucapnic Voluntary Hyperpnea |
| FeNO | Fractional Exhaled Nitric Oxide |
| FEV1 | Forced Expiratory Volume in 1 s |
| FVC | Forced Vital Capacity |
| ICS | Inhaled Corticosteroids |
| LABA | Long-Acting Beta Agonists |
| LTRA | Leukotriene Receptor Antagonists |
| MCSA | Mast Cell Stabilizing Agents |
| SABA | Short-Acting Beta Agonists |
| WADA | World Anti-Doping Agency |
References
- Global Initiative for Asthma GINA 2023. Available online: https://ginasthma.org/2023-gina-main-report/ (accessed on 2 November 2023).
- GBD 2019 Diseases and Injuries Collaborators. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef] [PubMed]
- Price, O.J.; Sewry, N.; Schwellnus, M.; Backer, V.; Reier-Nilsen, T.; Bougault, V.; Pedersen, L.; Chenuel, B.; Larsson, K.; Hull, J.H. Prevalence of lower airway dysfunction in athletes: A systematic review and meta-analysis by a subgroup of the IOC consensus group on ‘acute respiratory illness in the athlete’. Br. J. Sports Med. 2021, 56, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Melsom, H.S.; Randa, A.; Hisdal, J.; Stang, J.S.; Stensrud, T. Prevalence of Asthma among Norwegian Elite Athletes. Calbet JAL, editor. Transl. Sports Med. 2022, 2022, 1–10. Available online: https://www.hindawi.com/journals/tsmed/2022/3887471/ (accessed on 5 October 2023). [CrossRef]
- Lund, T.; Pedersen, L.; Larsson, B.; Backer, V. Prevalence of asthma-like symptoms, asthma and its treatment in elite athletes. Scand. J. Med. Sci. Sports 2008, 19, 174–178. [Google Scholar] [CrossRef] [PubMed]
- Dharmage, S.C.; Perret, J.L.; Custovic, A. Epidemiology of Asthma in Children and Adults. Front. Pediatr. 2019, 7, 246. Available online: https://www.frontiersin.org/article/10.3389/fped.2019.00246/full (accessed on 1 October 2023). [CrossRef]
- Abbasi, A.; Vieira, R.D.P.; Northoff, H. Letter to the editor: The evidence of exercise-induced bronchoconstriction in endurance runners; genetic basis and gender differences. Exerc. Immunol. Rev. 2015, 21, 186–188. [Google Scholar]
- Jayasinghe, H.; Kopsaftis, Z.; Carson, K. Asthma Bronchiale and Exercise-Induced Bronchoconstriction. Respiration 2015, 89, 505–512. [Google Scholar] [CrossRef]
- Weiler, J.M.; Anderson, S.D.; Randolph, C.; Bonini, S.; Craig, T.J.; Pearlman, D.S.; Rundell, K.W.; Silvers, W.S.; Storms, W.W.; Bernstein, D.I.; et al. Pathogenesis, prevalence, diagnosis, and management of exercise-induced bronchoconstriction: A practice parameter. Ann. Allergy Asthma Immunol. 2010, 105, S1–S47. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1081120610008914 (accessed on 15 October 2023). [CrossRef]
- Price, O.J.; Hull, J.H.; Backer, V.; Hostrup, M.; Ansley, L. The impact of exercise-induced bronchoconstriction on athletic performance: A systematic review. Sports Med. 2014, 44, 1749–1761. [Google Scholar] [CrossRef]
- Weiler, J.M.; Bonini, S.; Coifman, R.; Craig, T.; Delgado, L.; Capão-Filipe, M.; Passali, D.; Randolph, C.; Storms, W. American Academy of Allergy, Asthma & Immunology Work Group Report: Exercise-induced asthma. J. Allergy Clin. Immunol. 2007, 119, 1349–1358. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0091674907005702 (accessed on 1 October 2023).
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J. Grad. Med. Educ. 2022, 14, 565–567. Available online: https://meridian.allenpress.com/jgme/article/14/5/565/487459/Steps-for-Conducting-a-Scoping-Review (accessed on 18 October 2023). [CrossRef] [PubMed]
- Sadeh, J.; Israel, E. Airway narrowing in athletes: A different kettle of fish? Am. J. Respir. Crit. Care Med. 2003, 15, 1146–1147. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Daviskas, E. The mechanism of exercise-induced asthma is. J. Allergy Clin. Immunol. 2000, 106, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Holzer, K. Exercise-induced asthma: Is it the right diagnosis in elite athletes? J. Allergy Clin. Immunol. 2000, 106, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Helenius, I.J.; Rytilä, P.; Metso, T.; Haahtela, T.; Venge, P.; Tikkanen, H.O. Respiratory symptoms, bronchial responsiveness, and cellular characteristics of induced sputum in elite swimmers. Allergy 1998, 53, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, E.M.; Laitinen, A.; Sue-Chu, M.; Altraja, A.; Bjermer, L.; Laitinen, L.A. Evidence of airway inflammation and remodeling in ski athletes with and without bronchial hyperresponsiveness to methacholine. Am. J. Respir. Crit. Care Med. 2000, 161, 2086–2091. [Google Scholar] [CrossRef] [PubMed]
- Sue-Chu, M.; Karjalainen, E.M.; Laitinen, A.; Larsson, L.; Laitinen, L.A.; Bjermer, L. Placebo-controlled study of inhaled budesonide on indices of airway inflammation in bronchoalveolar lavage fluid and bronchial biopsies in cross-country skiers. Respiration 2000, 67, 417–425. [Google Scholar] [CrossRef]
- Helenius, I.; Haahtela, T. Allergy and asthma in elite summer sport athletes. J. Allergy Clin. Immunol. 2000, 106, 444–452. [Google Scholar] [CrossRef]
- Sue-Chu, M.; Karjalainen, E.-M.; Altraja, A.; Laitinen, A.; Laitinen, L.A.; Ss, A.B.N.; Larsson, L.; Bjermer, L. Lymphoid aggregates in endobronchial biopsies from young elite cross-country skiers. Am. J. Respir. Crit. Care Med. 1998, 158, 597–601. [Google Scholar] [CrossRef]
- Sue-Chu, M.; Larsson, L.; Moen, T.; Rennard, S.I.; Bjermer, L. Bronchoscopy and bronchoalveolar lavage findings in cross-country skiers with and without ‘ski asthma’. Eur. Respir. J. 1999, 13, 626–632. [Google Scholar] [CrossRef]
- Hallstrand, T.S.; Moody, M.W.; Wurfel, M.M.; Schwartz, L.B.; Henderson, W.R.; Aitken, M.L. Inflammatory basis of exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2005, 172, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Vergès, S.; Devouassoux, G.; Flore, P.; Rossini, E.; Fior-Gozlan, M.; Levy, P.; Wuyam, B. Bronchial hyperresponsiveness, airway inflammation, and airflow limitation in endurance athletes. Chest 2005, 127, 1935–1941. [Google Scholar] [CrossRef] [PubMed]
- Helenius, I.; Rytilä, P.; Sarna, S.; Lumme, A.; Helenius, M.; Remes, V.; Haahtela, T. Effect of continuing or finishing high-level sports on airway inflammation, bronchial hyperresponsiveness, and asthma: A 5-year prospective follow-up study of 42 highly trained swimmers. J. Allergy Clin. Immunol. 2002, 109, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Kippelen, P. Exercise-induced bronchoconstriction: Pathogenesis. Curr. Allergy Asthma Rep. 2005, 5, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, K.H.; Engh, G.; Mørk, M. Exercise-induced bronchoconstriction depends on exercise load. Respir. Med. 2000, 94, 750–755. [Google Scholar] [CrossRef]
- Goodman, M.; Hays, S. Asthma and swimming: A meta-analysis. J. Asthma 2008, 45, 639–647. [Google Scholar] [CrossRef]
- Andersson, M.; Hedman, L.; Nordberg, G.; Forsberg, B.; Eriksson, K.; Rönmark, E. Swimming pool attendance is related to asthma among atopic school children: A population-based study. Environ. Health 2015, 15, 37. [Google Scholar] [CrossRef]
- Bernard, A.; Nickmilder, M.; Voisin, C.; Sardella, A. Impact of chlorinated swimming pool attendance on the respiratory health of adolescents. Pediatrics 2009, 124, 1110–1118. [Google Scholar] [CrossRef]
- Sue-Chu, M. Winter sports athletes: Long-term effects of cold air exposure. Br. J. Sports Med. 2012, 46, 397–401. Available online: https://bjsm.bmj.com/lookup/doi/10.1136/bjsports-2011-090822 (accessed on 1 October 2023). [CrossRef]
- Rundell, K.W.; Im, J.; Mayers, L.B.; Wilber, R.L.; Szmedra, L.; Schmitz, H.R. Self-reported symptoms and exercise-induced asthma in the elite athlete. Med. Sci. Sports Exerc. 2001, 33, 208–213. [Google Scholar] [CrossRef]
- Atchley, T.J.; Smith, D.M. Exercise-induced bronchoconstriction in elite or endurance athletes: Pathogenesis and diagnostic considerations. Ann. Allergy Asthma Immunol. 2020, 125, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Weiler, J.M.; Hallstrand, T.S.; Parsons, J.P.; Randolph, C.; Silvers, W.S.; Storms, W.W.; Bronstone, A. Improving screening and diagnosis of exercise-induced bronchoconstriction: A call to action. J. Allergy Clin. Immunol. Pract. 2014, 2, 275–280.e7. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.P.; Hallstrand, T.S.; Mastronarde, J.G.; Kaminsky, D.A.; Rundell, K.W.; Hull, J.H.; Storms, W.W.; Weiler, J.M.; Cheek, F.M.; Wilson, K.C.; et al. An official American Thoracic Society clinical practice guideline: Exercise-induced bronchoconstriction. Am. J. Respir. Crit. Care Med. 2013, 187, 1016–1027. [Google Scholar] [CrossRef]
- Aggarwal, B.; Mulgirigama, A.; Berend, N. Exercise-induced bronchoconstriction: Prevalence, pathophysiology, patient impact, diagnosis and management. NPJ Prim. Care Respir. Med. 2018, 28, 31. [Google Scholar] [CrossRef]
- Stanojevic, S.; Kaminsky, D.A.; Miller, M.R.; Thompson, B.; Aliverti, A.; Barjaktarevic, I.; Cooper, B.G.; Culver, B.; Derom, E.; Hall, G.L.; et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur. Respir. J. 2021, 60, 2101499. Available online: http://erj.ersjournals.com/lookup/doi/10.1183/13993003.01499-2021 (accessed on 14 January 2022). [CrossRef] [PubMed]
- Pellegrino, R. Interpretative strategies for lung function tests. Eur. Respir. J. 2005, 26, 948–968. Available online: http://erj.ersjournals.com/cgi/doi/10.1183/09031936.05.00035205 (accessed on 18 September 2023). [CrossRef] [PubMed]
- Rundell, K.W.; Slee, J.B. Exercise and other indirect challenges to demonstrate asthma or exercise-induced bronchoconstriction in athletes. J. Allergy Clin. Immunol. 2008, 122, 238–246. [Google Scholar] [CrossRef] [PubMed]
- Stickland, M.K.; Rowe, B.H.; Spooner, C.H.; Vandermeer, B.; Dryden, D.M. Accuracy of eucapnic hyperpnea or mannitol to diagnose exercise-induced bronchoconstriction: A systematic review. Ann. Allergy Asthma Immunol. 2011, 107, 229–234.e8. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1081120611004613 (accessed on 15 October 2023). [CrossRef]
- Crapo, R.O.; Casaburi, R.; Coates, A.L.; Enright, P.L.; Hankinson, J.L.; Irvin, C.G.; MacIntyre, N.R.; McKay, R.T.; Wanger, J.S.; Anderson, S.D.; et al. Guidelines for methacholine and exercise challenge testing-1999. This official statement of the American Thoracic Society was adopted by the ATS Board of Directors, July 1999. Am. J. Respir. Crit. Care Med. 2000, 161, 309–329. [Google Scholar]
- Rundell, K.W.; Wilber, R.L.; Szmedra, L.; Jenkinson, D.M.; Mayers, L.B.; Im, J. Exercise-induced asthma screening of elite athletes: Field versus laboratory exercise challenge. Med. Sci. Sports Exerc. 2000, 32, 309–316. [Google Scholar] [CrossRef]
- Anderson, S.D.; Pearlman, D.S.; Rundell, K.W.; Perry, C.P.; Boushey, H.; A Sorkness, C.; Nichols, S.; Weiler, J.M. Reproducibility of the airway response to an exercise protocol standardized for intensity, duration, and inspired air conditions, in subjects with symptoms suggestive of asthma. Respir. Res. 2010, 11, 120. [Google Scholar] [CrossRef] [PubMed]
- Anderson, S.D.; Schoeffel, R.E.; Follet, R.; Perry, C.P.; Daviskas, E.; Kendall, M. Sensitivity to heat and water loss at rest and during exercise in asthmatic patients. Eur. J. Respir. Dis. 1982, 63, 459–471. [Google Scholar] [PubMed]
- Fitch, K.D.; Sue-Chu, M.; Anderson, S.D.; Boulet, L.P.; Hancox, R.J.; McKenzie, D.C.; Ljungqvist, A. Asthma and the elite athlete: Summary of the International Olympic Committee’s consensus conference, Lausanne, Switzerland, January 22–24, 2008. J. Allergy Clin. Immunol. 2008, 122, 254–260.e1–7. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.; Gowers, W.; Sturridge, S.; Williams, N.; Kippelen, P.; Simpson, A.; Jackson, A.; Hull, J.H.; Price, O.J. Fractional exhaled nitric oxide in the assessment of exercise-induced bronchoconstriction: A multicenter retrospective analysis of UK-based athletes. Scand. J. Med. Sci. Sports 2023, 33, 1221–1230. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.G.; Weiler, J.M.; Baker, R.; Collins, J.; D’Alonzo, G. National Athletic Trainers’ Association position statement: Management of asthma in athletes. J. Athl. Train. 2005, 40, 224–245. [Google Scholar] [PubMed]
- Greiwe, J.; Cooke, A.; Nanda, A.; Epstein, S.Z.; Wasan, A.N.; Shepard, K.V.; Capão-Filipe, M.; Nish, A.; Rubin, M.; Gregory, K.L.; et al. Work Group Report: Perspectives in Diagnosis and Management of Exercise-Induced Bronchoconstriction in Athletes. J. Allergy Clin. Immunol. Pract. 2020, 8, 2542–2555. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.P.; Mastronarde, J.G. Exercise-induced bronchoconstriction in athletes. Chest 2005, 128, 3966–3974. [Google Scholar] [CrossRef]
- McCreanor, J.; Cullinan, P.; Nieuwenhuijsen, M.J.; Stewart-Evans, J.; Malliarou, E.; Jarup, L.; Harrington, R.; Svartengren, M.; Han, I.-K.; Ohman-Strickland, P.; et al. Respiratory effects of exposure to diesel traffic in persons with asthma. N. Engl. J. Med. 2007, 357, 2348–2358. [Google Scholar] [CrossRef]
- Millqvist, E.; Bake, B.; Bengtsson, U.; Löwhagen, O. A breathing filter exchanging heat and moisture prevents asthma induced by cold air. Allergy 1995, 50, 225–228. [Google Scholar] [CrossRef]
- Beuther, D.A.; Martin, R.J. Efficacy of a heat exchanger mask in cold exercise-induced asthma. Chest 2006, 129, 1188–1193. [Google Scholar] [CrossRef]
- Elkins, M.R.; Brannan, J.D. Warm-up exercise can reduce exercise-induced bronchoconstriction. Br. J. Sports Med. 2012, 47, 657–658. [Google Scholar] [CrossRef] [PubMed]
- Boulet, L.P.; O’Byrne, P.M. Asthma and exercise-induced bronchoconstriction in athletes. N. Engl. J. Med. 2015, 372, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, J.; Amirav, I.; Hostrup, M. Nonpharmacologic Strategies to Manage Exercise-Induced Bronchoconstriction. Immunol. Allergy Clin. N. Am. 2018, 38, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Stickland, M.K.; Rowe, B.H.; Spooner, C.H.; Vandermeer, B.; Dryden, D.M. Effect of warm-up exercise on exercise-induced bronchoconstriction. Med. Sci. Sports Exerc. 2012, 44, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Page, C.P.; Calzetta, L.; Matera, M.G. Pharmacology and therapeutics of bronchodilators. Pharmacol. Rev. 2012, 64, 450–504. [Google Scholar] [CrossRef] [PubMed]
- Papi, A.; Ferreira, D.S.; Agache, I.; Baraldi, E.; Beasley, R.; Brusselle, G.; Coleman, C.; Gaga, M.; Maria, C.; Rivera, G.; et al. European Respiratory Society short guidelines for the use of as-needed ICS/formoterol in mild asthma. Eur. Respir. J. 2023, 62, 2300047. [Google Scholar] [CrossRef] [PubMed]
- Bordeleau, M.; Turmel, J.; Boulet, L.P. Effects of ipratropium on exercise-induced cough in winter athletes: A hypothesis-generating study. Physician Sportsmed. 2014, 42, 7–13. [Google Scholar] [CrossRef]
- Boaventura, L.C.; Araujo, A.C.; Martinez, J.B.; Vianna, E.O. Effects of ipratropium on exercise-induced bronchospasm. Int. J. Sports Med. 2010, 31, 516–520. [Google Scholar] [CrossRef]
- Cazzola, M.; Ora, J.; Rogliani, P.; Matera, M.G. Role of muscarinic antagonists in asthma therapy. Expert Rev. Respir. Med. 2017, 11, 239–253. [Google Scholar] [CrossRef]
- Wada Prohibited List 2024. Available online: https://www.wada-ama.org/en/news/wada-publishes-2024-prohibited-list (accessed on 2 November 2023).
| Topic | Problem | Clinical Relevance |
|---|---|---|
| Pathogenesis | EIBA and EIBwA may exhibit distinct inflammatory patterns | Variations in disease response and management strategies |
| Hyper-responsiveness | Athletes demonstrate heightened airway sensitivity | Greater susceptibility to EIB during extended exercise or specific environmental conditions |
| Diagnosis | Aspecific symptoms or symptoms misattributed to other causes | Risk of delayed EIB diagnosis leading to sport changes for undiagnosed asthma |
| Normal pulmonary function tests or situational variability | Potential for EIB to remain undetected in some athletes, requiring multiple tests for conclusive diagnosis | |
| Complex diagnosis of asthma or concurrent conditions | The risk of under- or over-treatment | |
| Therapy | Overreliance on short-acting β2 agonists | Risk of tachyphylaxis and treatment unresponsiveness, potentially culminating in fatal asthma |
| Treatment ambiguity for EIBwA | Lack of clarity on whether short-acting β2 agonists or a combination therapy with inhaled corticosteroids is more effective |
| Class | Name | Pharmacological Effect | Indication |
|---|---|---|---|
| Short-Acting Beta 2 Agonist | Salbutamol, Terbutaline | Quick relief from bronchoconstriction | Suitable for rapid relief but not intended for chronic usage unless the individual is concurrently on ICS or ICS/LABA maintenance therapy |
| Long-Acting Beta 2 Agonist | Formoterol, Vilanterol, Olodaterol | Maintenance treatment for bronchoconstriction | Not intended for chronic usage, except when used in combination with ICS |
| Inhaled Corticosteroids (ICS) | Beclometasone, Budesonide, Fluticasone Furoate, Fluticasone Propionate | Reduces airway inflammation | Used as monotherapy or in combination and not intended for rapid relief |
| Short-Acting Muscarinic Agent | Ipratropium, Oxitropium | Provides bronchodilation | The use of these medications before exercise to prevent EIB is a subject of controversy and remains an experimental approach |
| Long-Acting Anti-Muscarinic | Tiotropium, Umeclidinium, Glycopyrronium | Maintenance treatment for bronchoconstriction | There is no existing evidence regarding the use of this class of medications in athletes as monotherapy |
| ICS/LABA | Combination therapies that include Inhaled Corticosteroids (ICS) and Long-Acting Beta 2 Agonists (LABA) | Management of asthma in athletes | Used both as needed and for maintenance therapy and typically considered the first-line treatment for mild-to-moderate asthma |
| Biologic Agents | Omalizumab, Mepolizumab, Benralizumab, Dupilumab | Treatment for severe asthma in athletes and allergic reactions | Used in severe asthma, and they are not contraindicated in asthmatic athletes |
| Leukotriene Modifier | Montelukast, Zafirlukast, Pranlukast | Management of asthma in athletes | Reduces exercise-induced bronchoconstriction and provides protection against bronchoconstriction triggered by exposure to pollutants |
| Cromones | Cromolyn sodium, Nedocromil sodium | Prophylactic treatment for asthma, especially in athletes | Permitted for use by athletes but may not be accessible in many markets |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ora, J.; De Marco, P.; Gabriele, M.; Cazzola, M.; Rogliani, P. Exercise-Induced Asthma: Managing Respiratory Issues in Athletes. J. Funct. Morphol. Kinesiol. 2024, 9, 15. https://doi.org/10.3390/jfmk9010015
Ora J, De Marco P, Gabriele M, Cazzola M, Rogliani P. Exercise-Induced Asthma: Managing Respiratory Issues in Athletes. Journal of Functional Morphology and Kinesiology. 2024; 9(1):15. https://doi.org/10.3390/jfmk9010015
Chicago/Turabian StyleOra, Josuel, Patrizia De Marco, Mariachiara Gabriele, Mario Cazzola, and Paola Rogliani. 2024. "Exercise-Induced Asthma: Managing Respiratory Issues in Athletes" Journal of Functional Morphology and Kinesiology 9, no. 1: 15. https://doi.org/10.3390/jfmk9010015
APA StyleOra, J., De Marco, P., Gabriele, M., Cazzola, M., & Rogliani, P. (2024). Exercise-Induced Asthma: Managing Respiratory Issues in Athletes. Journal of Functional Morphology and Kinesiology, 9(1), 15. https://doi.org/10.3390/jfmk9010015






