The Effects of Pilates Exercise Training Combined with Walking on Cardiorespiratory Fitness, Functional Capacity, and Disease Activity in Patients with Non-Radiologically Confirmed Axial Spondylitis
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Design
2.3. Sample Size Estimation
2.4. Blood Analysis
- Less than 0.3 mg/dL: Normal.
- Between 0.3 and 1.0 mg/dL: Normal or minor elevation.
- Between 1.0 and 10.0 mg/dL: Moderate elevation.
- More than 10.0 mg/dL: Notable elevation.
- More than 50.0 mg/dL: Severe elevation.
2.5. Cardiorespiratory Exercise Testing
2.6. Timed up and Go Test
2.7. Five Times Sit-to-Stand Test
2.8. Sit-and-Reach Test
2.9. Back Scratch Test
2.10. Bath Ankylosing Spondylitis Disease Activity Index
2.11. Ankylosing Spondylitis Disease Activity Score
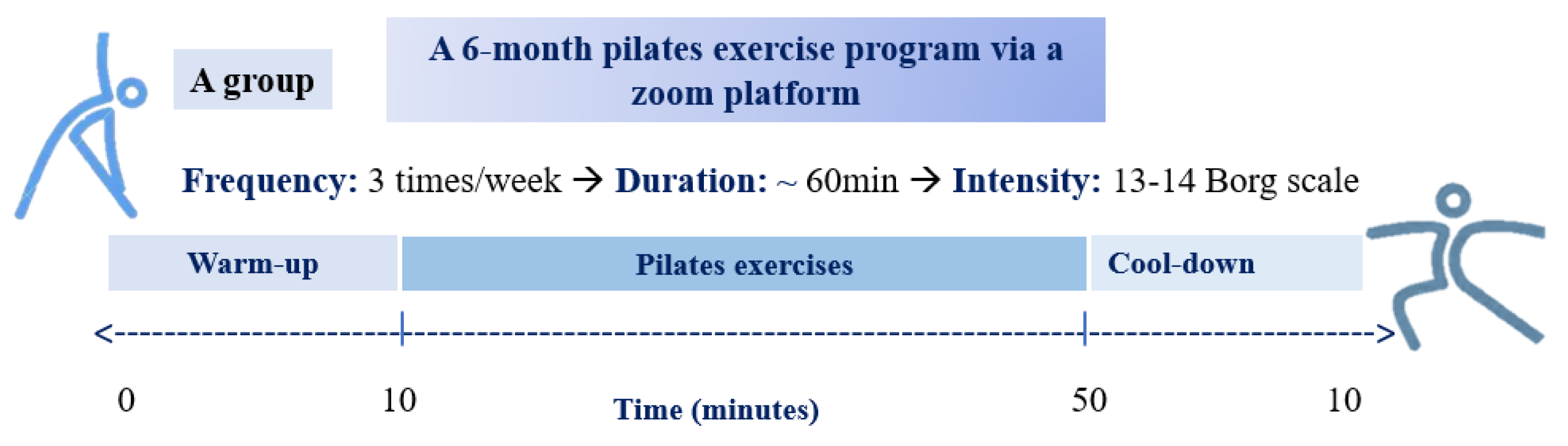
2.12. Pilates Exercise Training
- One Leg Stretch: Patients were asked to lie in a supine position on the mat and to pull one knee into their chest, then inhale and start to bend the out-stretched leg and straighten the bent leg. At the end of this exercise, patients exhale.
- Double Leg Stretch: Firstly, patients in the same supine position on the mat were asked to bend their legs, with their feet off the floor, and grab both knees. Secondly, they had to lift both shoulders off the floor, extend their arms toward their ears, and simultaneously raise both legs to a 45-degree angle from the floor. Thirdly, they had to bend their knees and bring their chin toward their chest while hugging them. Lastly, patients had to extend their upper and lower limbs and repeat until the set was completed.
- Shoulder Bridge: Firstly, patients were instructed to lay on their backs with their knees bent, heels lined with their bottom, and arms rested by their sides. Secondly, they were instructed to take a deep breath in; as they exhaled, they flattened their lower back to the floor as though they were lifting their tailbone to the ceiling. They had to visualize each vertebra leaving the floor one by one until they were weight-bearing through their shoulders.
- Chest Lift: In this exercise, patients were asked to lie down, keep their knees bent, their back and feet flat, and their hands supporting their heads. Then, they had to lift their shoulders and squeeze their abdominal muscles. They were instructed to hold this position for 1 to 2 s and then relax by returning to the initial position.
- Hundreds: In this exercise, patients were asked to lie on their backs with their knees bent and legs parallel to the floor, lift their shoulders off the mat, and extend their upper and lower limbs. Then, they were instructed to inhale for 5 s and exhale for 5 s while pumping arms. To achieve 100, they had to act 10 times.
- One Leg Circles: Patients were instructed to lie on their back, with their arms down and by their side, pelvis in a neutral position, and core engaged, to extend the right leg toward the ceiling and the left leg along the mat. They had to inhale to prepare, exhale, and circle the right leg away from the midline, keeping the leg extended.
- Spine Stretch: In this exercise, patients were instructed to sit up tall as if their back was against a wall. Legs should be out in front of them and opened about shoulder distance apart. Knees and toes will be pointed to the ceiling, and heels will reach away from them to create length and oppositional energy. They had to lift their arms in front of them, with fingertips reaching, palms down. Then, they had to roll their shoulder blades down their back to create space between the neck and ears. After a deep inhale, they were asked to exhale as they curled their head, neck, and upper spine down the imaginary wall while pulling their abs in. Moreover, they had to stretch forward as if bending over a round barrel toward their toes. Next, they had to inhale as they began rolling back up the “wall”, starting with their tailbone, lower back, upper back, neck, and head, returning to the starting position feeling taller than before.
- Spine Twist: Initially, patients were asked to sit up tall on their feet and pull their abdominals in to support their upper body. Then, they had to flex their feet, reach their heels, and extend their arms directly out to the sides, keeping them even with their shoulders so there was one long line from fingertip to fingertip. In addition, patients were asked to exhale as they imagined a line running straight up through the middle of their body, turning their torso and head on that central axis and getting taller as they twisted. The movement is a two-part pulse where they had to exhale to twist halfway and then exhale again to turn as far as possible.
- Hip Twist: Firstly, patients were asked to lie on their backs and bend their legs, keeping their knees and feet parallel at hip-width apart and their arms by their sides. Secondly, they had to exhale, rotate their hips, and slowly open one leg outwards with their knee reaching the mat. Thirdly, they had to inhale and bring their knee back in line with their hip (1 repetition with the same leg). Lastly, they were instructed to keep their knee at a consistent angle and in line with their opposite knee as they rotate in their hip joint. Also, they had to maintain a stable pelvis and use their adductors to return the knee to the starting position.
- Swimming: Patients were asked to lie prone with extended upper and lower limbs. Then, they had to raise both arms and legs off the mat and lift their head and chest. Lastly, they were instructed to flutter their arms and legs and keep alternating sides for the entire set.
- Standing Side Bend: Initially, patients were asked to stand with their feet shoulder-width apart and put their left hand behind their head and their right hand at their side. Secondly, they had to bend to the right and lower their right hand toward the floor. Lastly, they had to return, switch sides, and repeat.
- Cat Stretching: Firstly, patients were asked to start with four-point kneeling, with hands underneath their shoulders and knees underneath their hips. Secondly, they were instructed to gently flex their neck by dropping their chin toward their chest while arching the rest of their spine into a curve. Thirdly, they had to slowly move into the opposite position by lifting their head upwards and extending their neck while allowing the rest of their spine to drop down into an extended position.
2.13. Statistical Analysis
3. Results
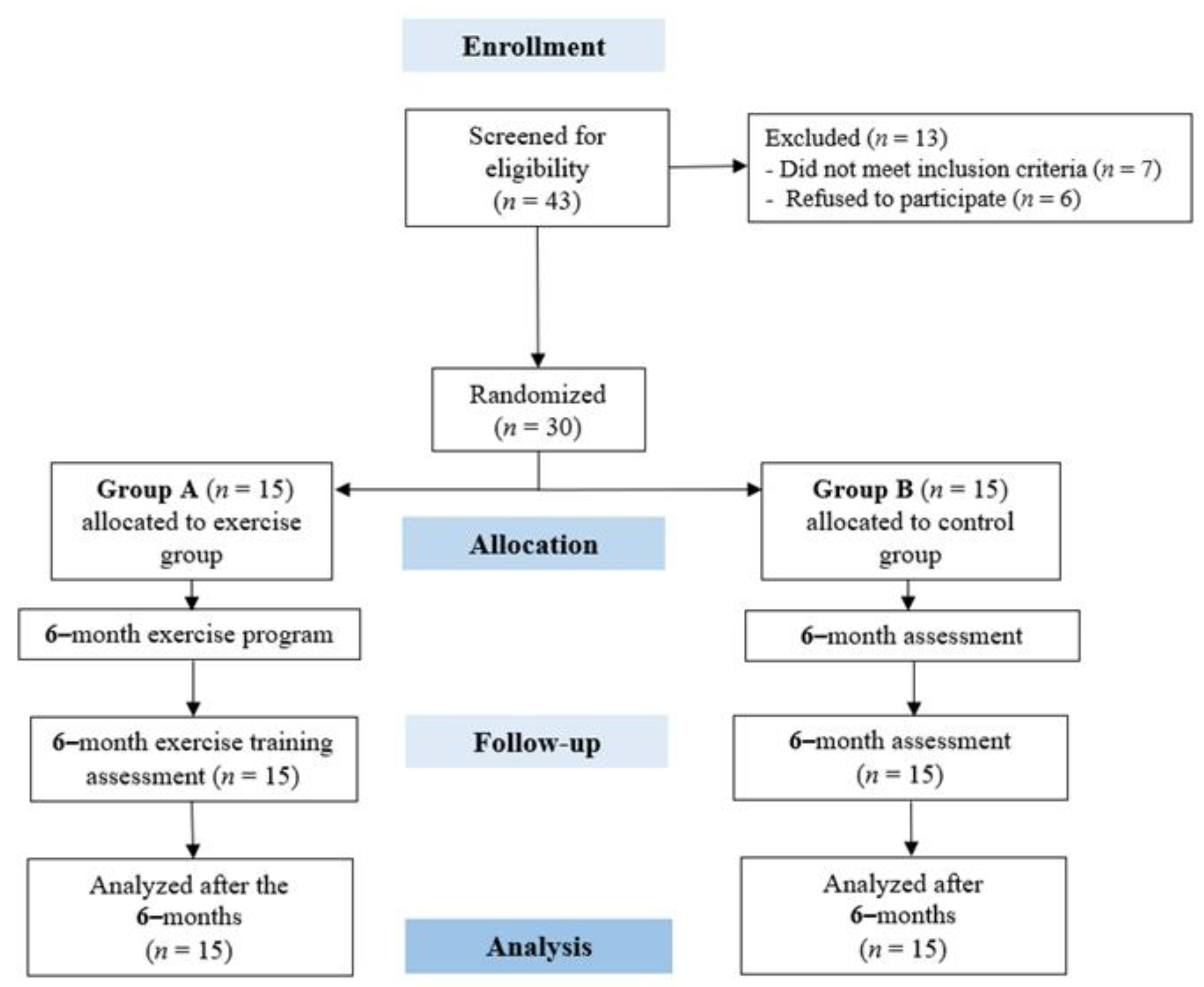
3.1. Study Population
3.2. Cardiopulmonary Exercise Testing
3.3. Functional Capacity and Disease Activity Results
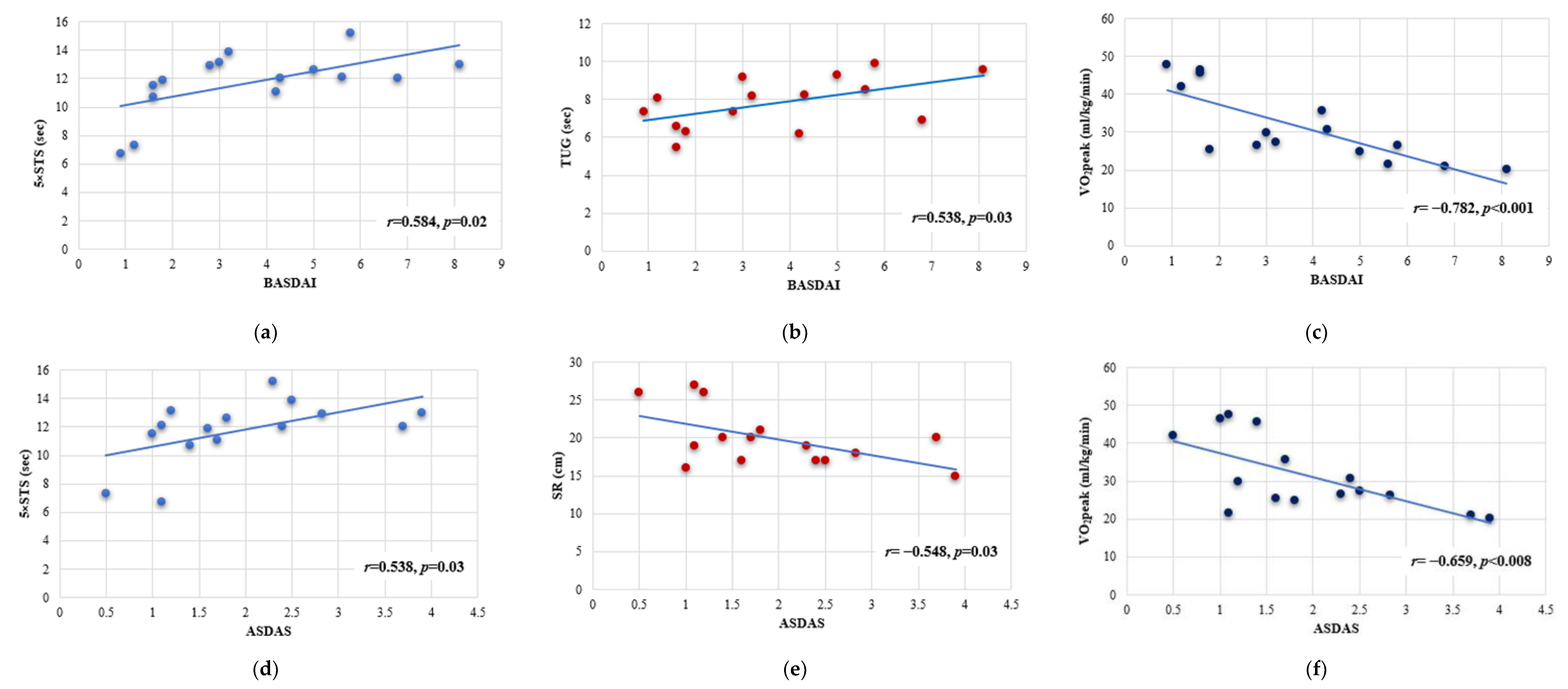
3.4. Linear Regression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deodhar, A.; Strand, V.; Kay, J.; Braun, J. The term ‘non-radiographic axial spondyloarthritis’ is much more important to classify than to diagnose patients with axial spondyloarthritis. Ann. Rheum. Dis. 2016, 75, 791–794. [Google Scholar] [CrossRef] [PubMed]
- De Koning, A.; Schoones, J.W.; van der Heijde, D.; van Gaalen, F.A. Pathophysiology of axial spondyloarthritis: Consensus and controversies. Eur. J. Clin. Investig. 2018, 48, e12913. [Google Scholar] [CrossRef] [PubMed]
- Mease, P.; Deodhar, A. Differentiating nonradiographic axial spondyloarthritis from its mimics: A narrative review. BMC Musculoskelet. Disord. 2022, 23, 240. [Google Scholar] [CrossRef] [PubMed]
- Wright, G.C.; Kaine, J.; Deodhar, A. Understanding differences between men and women with axial spondyloarthritis. Semin. Arthritis Rheum. 2020, 50, 687–694. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.F.; Wei, J.C.C.; Lee, H.Y.; Chuang, C.C.; Jiang, J.S.; Chang, K.C. Aerobic capacity and its correlates in patients with ankylosing spondylitis. Int. J. Rheum. Dis. 2016, 19, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Swinnen, T.W.; Scheers, T.; Lefevre, J.; Dankaerts, W.; Westhovens, R.; de Vlam, K. Physical Activity Assessment in Patients with Axial Spondyloarthritis Compared to Healthy Controls: A Technology-Based Approach. PLoS ONE 2014, 9, e85309. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.M.; Lories, R.; Lubrano, E. To move or not to move: The paradoxical effect of physical exercise in axial spondyloarthritis. RMD Open 2021, 7, e001480. [Google Scholar] [CrossRef] [PubMed]
- Zochling, J. Assessment and treatment of ankylosing spondylitis: Current status and future directions. Curr. Opin. Rheumatol. 2008, 20, 398–403. [Google Scholar] [CrossRef]
- Perrotta, F.M.; Musto, A.; Lubrano, E. New Insights in Physical Therapy and Rehabilitation in Axial Spondyloarthritis: A Review. Rheumatol. Ther. 2019, 6, 479–486. [Google Scholar] [CrossRef]
- Oskay, D.; Tuna, Z.; Baglan-Yentur, S. Effect of Clinical Pilates training on the fear of movement in patients with ankylosing spondylitis. Int. J. Ther. Rehabil. 2018, 25, 597–601. [Google Scholar] [CrossRef]
- Dagfinrud, H.; Kvien, T.K.; Hagen, K.B. The Cochrane review of physiotherapy interventions for ankylosing spondylitis. J. Rheumatol. 2005, 32, 1899–1906. [Google Scholar] [PubMed]
- Ortolan, A.; Webers, C.; Sepriano, A.; Falzon, L.; Baraliakos, X.; Landewé, R.B.; Ramiro, S.; van der Heijde, D.; Nikiphorou, E. Efficacy and safety of non-pharmacological and non-biological interventions: A systematic literature review informing the 2022 update of the ASAS/EULAR recommendations for the management of axial spondyloarthritis. Ann. Rheum. Dis. 2023, 82, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Verep, U.; Çiçek, E.; Özyürek, S. The Role of Core Stability and Core Muscles in Ankylosing Spondylitis: A Review of Functional and Clinical Importance. J. Basic Clin. Health Sci. 2023, 7, 545–552. [Google Scholar] [CrossRef]
- Bağlan Yentür, S.; Saraç, D.C.; Sari, F.; Tore, G.; Bilici Salman, R.; Akif Öztürk, M.; Oskay, D. The effects of Pilates training on respiratory muscle strength in patients with ankylosing spondylitis. Physiother. Theory Pract. 2022, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Altan, L.; Korkmaz, N.; Dizdar, M.; Yurtkuran, M. Effect of Pilates training on people with ankylosing spondylitis. Rheumatol. Int. 2012, 32, 2093–2099. [Google Scholar] [CrossRef] [PubMed]
- Rosu, O.M.; Ancuta, C. McKenzie training in patients with early stages of ankylosing spondylitis: Results of a 24-week controlled study. Eur. J. Phys. Rehabil. Med. 2015, 51, 261–268. [Google Scholar] [PubMed]
- Oksüz, S.; Unal, E. Comparison of the effects of aerobic training alone versus aerobic training combined with clinical Pilates exercises on the functional and psychosocial status of patients with ankylosing spondylitis: A randomized controlled trial. Physiother. Theory Pract. 2023, 39, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Lukas, C.; Landewé, R.; Sieper, J.; Dougados, M.; Davis, J.; Braun, J.; van der Linden, S.; van der Heijde, D. Development of an ASAS-endorsed disease activity score (ASDAS) in patients with ankylosing spondylitis. Ann. Rheum. Dis. 2009, 68, 18–24. [Google Scholar] [CrossRef]
- Nuttall, F.Q. Body Mass Index. Nutr. Today 2015, 50, 117–128. [Google Scholar] [CrossRef]
- Yentür, S.B.; Ataş, N.; Öztürk, M.A.; Oskay, D. Comparison of the effectiveness of pilates exercises, aerobic exercises, and pilates with aerobic exercises in patients with rheumatoid arthritis. Ir. J. Med. Sci. (1971) 2021, 190, 1027–1034. [Google Scholar] [CrossRef]
- Plebani, M.; Piva, E. Erythrocyte Sedimentation Rate. Am. J. Clin. Pathol. 2002, 117, 621–626. [Google Scholar] [CrossRef]
- Lee, Y.; McKechnie, T.; Doumouras, A.G.; Handler, C.; Eskicioglu, C.; Gmora, S.; Anvari, M.; Hong, D. Diagnostic Value of C-Reactive Protein Levels in Postoperative Infectious Complications After Bariatric Surgery: A Systematic Review and Meta-Analysis. Obes. Surg. 2019, 29, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Aspenes, S.T.; Nilsen, T.I.L.; Skaug, E.A.; Bertheussen, G.F.; Ellingsen, Ø.; Vatten, L.; Wisloff, U. Peak Oxygen Uptake and Cardiovascular Risk Factors in 4631 Healthy Women and Men. Med. Sci. Sports Exerc. 2011, 43, 1465–1473. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.L.; Buskirk, E.; Henschel, A. Maximal oxygen intake as an objective measure of cardio-respiratory performance. J. Appl. Physiol. 1955, 8, 73–80. [Google Scholar] [CrossRef]
- Niemeyer, M.; Knaier, R.; Beneke, R. The Oxygen Uptake Plateau-A Critical Review of the Frequently Misunderstood Phenomenon. Sports Med. 2021, 51, 1815–1834. [Google Scholar] [CrossRef] [PubMed]
- Kear, B.M.; Guck, T.P.; McGaha, A.L. Timed Up and Go (TUG) Test. J. Prim. Care Community Health 2017, 8, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Rose, D.J.; Jones, C.J.; Lucchese, N. Predicting the Probability of Falls in Community-Residing Older Adults Using the 8-Foot Up-and-Go: A New Measure of Functional Mobility. J. Aging Phys. Act. 2002, 10, 466–475. [Google Scholar] [CrossRef]
- Muñoz-Bermejo, L.; Adsuar, J.C.; Mendoza-Muñoz, M.; Barrios-Fernández, S.; Garcia-Gordillo, M.A.; Pérez-Gómez, J. Test-Retest Reliability of Five Times Sit to Stand Test (FTSST) in Adults: A Systematic Review and Meta-Analysis. Biology 2021, 10, 510. [Google Scholar] [CrossRef]
- Bohannon, R.W.; Bubela, D.J.; Magasi, S.R.; Wang, Y.C.; Gershon, R.C. Sit-to-stand test: Performance and determinants across the age-span. Isokinet Exerc. Sci. 2010, 18, 235–240. [Google Scholar] [CrossRef]
- Mayorga-Vega, D.; Merino-Marban, R.; Viciana, J. Criterion-Related Validity of Sit-and-Reach Tests for Estimating Hamstring and Lumbar Extensibility: A Meta-Analysis. J. Sports Sci. Med. 2014, 13, 1–14. [Google Scholar]
- Jones, C.J.; Rikli, R.E.; Max, J.; Noffal, G. The Reliability and Validity of a Chair Sit-and-Reach Test as a Measure of Hamstring Flexibility in Older Adults. Res. Q. Exerc. Sport. 1998, 69, 338–343. [Google Scholar] [CrossRef] [PubMed]
- Rikli, R.E.; Jones, C.J. Development and Validation of a Functional Fitness Test for Community-Residing Older Adults. J. Aging Phys. Act. 1999, 7, 129–161. [Google Scholar] [CrossRef]
- Lavín-Pérez, A.M.; León-Llamas, J.L.; Salas Costilla, F.J.; Collado-Mateo, D.; López de las Heras, R.; Gasque Celma, P.; Villafaina, S. Validity of On-Line Supervised Fitness Tests in People with Low Back Pain. Healthcare 2023, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Garrett, S.; Jenkinson, T.; Kennedy, L.G.; Whitelock, H.; Gaisford, P.; Calin, A. A new approach to defining disease status in ankylosing spondylitis: The Bath Ankylosing Spondylitis Disease Activity Index. J. Rheumatol. 1994, 21, 2286–2291. [Google Scholar] [PubMed]
- Fagerli, K.M.; Lie, E.; van der Heijde, D.; Heiberg, M.S.; Kaufmann, C.; Rodevand, E.; Mikkelsen, K.; Kalstad, S.; Kvien, T.K. Selecting patients with ankylosing spondylitis for TNF inhibitor therapy: Comparison of ASDAS and BASDAI eligibility criteria. Rheumatology 2012, 51, 1479–1483. [Google Scholar] [CrossRef]
- Machado, P.; Landewe, R.; Lie, E.; Kvien, T.K.; Braun, J.; Baker, D.; van der Heijde, D. Ankylosing Spondylitis Disease Activity Score (ASDAS): Defining cut-off values for disease activity states and improvement scores. Ann. Rheum. Dis. 2011, 70, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Rudwaleit, M.; van der Heijde, D.; Landewe, R.; Listing, J.; Akkoc, N.; Brandt, J.; Braun, J.; Chou, C.T.; Collantes-Estevez, E.; Dougados, M.; et al. The development of Assessment of SpondyloArthritis international Society classification criteria for axial spondyloarthritis (part II): Validation and final selection. Ann. Rheum. Dis. 2009, 68, 777–783. [Google Scholar] [CrossRef] [PubMed]
- Metsios, G.S.; Brodin, N.; Vlieland, T.P.M.V.; Van den Ende, C.H.M.; Stavropoulos-Kalinoglou, A.; Fatouros, I.; van der Esch, M.; Fenton, S.A.M.; Tzika, K.; Moe, R.H.; et al. Position Statement on Exercise Dosage in Rheumatic and Musculoskeletal Diseases: The Role of the IMPACT-RMD Toolkit. Mediterr. J. Rheumatol. 2021, 32, 378–385. [Google Scholar] [CrossRef]
- Fenton, S.A.M.; Duda, J.L.; Veldhuijzen van Zanten, J.J.C.S.; Metsios, G.S.; Kitas, G.D. Theory-informed interventions to promote physical activity and reduce sedentary behaviour in rheumatoid arthritis: A critical review of the literature. Mediterr. J. Rheumatol. 2019, 31, 19. [Google Scholar] [CrossRef]
- Akinci, A.; Kiliç, G. Future of Rehabilitation Interventions for Rheumatic Patients in the Mediterranean Region. Mediterr. J. Rheumatol. 2017, 28, 70–74. [Google Scholar] [CrossRef][Green Version]
- Ward, M.M.; Deodhar, A.; Akl, E.A.; Lui, A.; Ermann, J.; Gensler, L.S.; Smith, J.A.; Borenstein, D.; Hiratzka, J.; Weiss, P.F.; et al. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2016, 68, 282–298. [Google Scholar] [CrossRef] [PubMed]
- Masiero, S.; Bonaldo, L.; Pigatto, M.; LONigro, A.; Ramonda, R.; Punzi, L. Rehabilitation Treatment in Patients with Ankylosing Spondylitis Stabilized with Tumor Necrosis Factor Inhibitor Therapy. A Randomized Controlled Trial. J. Rheumatol. 2011, 38, 1335–1342. [Google Scholar] [CrossRef] [PubMed]
- Sveaas, S.H.; Berg, I.J.; Provan, S.A.; Semb, A.G.; Hagen, K.B.; Vøllestad, N.; Fongen, C.; Olsen, I.C.; Michelsen, A.; Ueland, T.; et al. Efficacy of High Intensity Exercise on Disease Activity and Cardiovascular Risk in Active Axial Spondyloarthritis: A Randomized Controlled Pilot Study. PLoS ONE 2014, 9, e108688. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.; Wu, P.J.; Whillier, S. Is Pilates an effective rehabilitation tool? A systematic review. J. Bodyw. Mov. Ther. 2018, 22, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Levitova, A.; Hulejova, H.; Spiritovic, M.; Pavelka, K.; Senolt, L.; Husakova, M. Clinical improvement and reduction in serum calprotectin levels after an intensive exercise programme for patients with ankylosing spondylitis and non-radiographic axial spondyloarthritis. Arthritis Res. Ther. 2016, 18, 275. [Google Scholar] [CrossRef] [PubMed]
- Husakova, M.; Siebuhr, A.S.; Pavelka, K.; Spiritovic, M.; Bay-Jensen, A.C.; Levitova, A. Changes of patient-reported outcomes and protein fingerprint biomarkers after exercise therapy for axial spondyloarthritis. Clin. Rheumatol. 2019, 38, 173–179. [Google Scholar] [CrossRef]
- Escalas, C.; Dalichampt, M.; Dougados, M.; Poiraudeau, S. Evaluation of physiotherapy in a prospective cohort of early axial spondyloarthritis. Data from the DESIR cohort. Jt. Bone Spine 2016, 83, 185–190. [Google Scholar] [CrossRef]
- Sveaas, S.; Berg, I.; Fongen, C.; Provan, S.; Dagfinrud, H. High-intensity cardiorespiratory and strength exercises reduced emotional distress and fatigue in patients with axial spondyloarthritis: A randomized controlled pilot study. Scand. J. Rheumatol. 2018, 47, 117–121. [Google Scholar] [CrossRef]
- Niedermann, K.; Sidelnikov, E.; Muggli, C.; Dagfinrud, H.; Hermann, M.; Tamborrini, G.; Ciurea, A.; Bischoff-Ferrari, H. Effect of Cardiovascular Training on Fitness and Perceived Disease Activity in People With Ankylosing Spondylitis. Arthritis Care Res. 2013, 65, 1844–1852. [Google Scholar] [CrossRef]
- Nolte, K.; van Rensburg, D.C.J.; Fletcher, L. Effects of a 6-month exercise programme on disease activity, physical and functional parameters in patients with ankylosing spondylitis: Randomised controlled trial. S. Afr. J. Physiother. 2021, 77, 8. [Google Scholar] [CrossRef] [PubMed]
- Jennings, F.; Oliveira, H.A.; de Souza, M.C.; Cruz V da, G.; Natour, J. Effects of Aerobic Training in Patients with Ankylosing Spondylitis. J. Rheumatol. 2015, 42, 2347–2353. [Google Scholar] [CrossRef] [PubMed]
- Taskin, H.; Telli Atalay, O.; Pekesen Kurtca, M.; Gür Kabul, E.; Basakci Calik, B.; Yalman, A.; Yigit, M.; Tasci, M.; Cobankara, V. The effects of aerobic training on respiratory muscle strength and exercise capacity in ankylosing spondylitis patients. In Physiotherapists; European Respiratory Society: Lausanne, Switzerland, 2018; p. PA1444. [Google Scholar]
- Kloubec, J. Pilates: How does it work and who needs it? Muscles Ligaments Tendons J. 2011, 1, 61–66. [Google Scholar] [PubMed]
- Nickels, M.; Mastana, S.; Denniff, M.; Codd, V.; Akam, E. Pilates and telomere dynamics: A 12-month longitudinal study. J. Bodyw. Mov. Ther. 2022, 30, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Calik, B.B.; Kurtca, M.P.; Kabul, E.G.; Atalay, O.T.; Taskin, H.; Yigit, M.; Tasci, M.; Cobankara, V. Investigation of the effectiveness of aerobic exercise training in individuals with ankylosing spondylitis: Randomized controlled study. Mod. Rheumatol. 2021, 31, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Regnaux, J.P.; Davergne, T.; Palazzo, C.; Roren, A.; Rannou, F.; Boutron, I.; Lefevre-Colau, M.-M. Exercise programmes for ankylosing spondylitis. Cochrane Database Syst. Rev. 2019, 2019, CD011321. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, G.C.; Costa, L.O.P.; Galvanin, T.; Cabral, C.M.N. Efficacy of the Addition of Modified Pilates Exercises to a Minimal Intervention in Patients with Chronic Low Back Pain: A Randomized Controlled Trial. Phys. Ther. 2013, 93, 310–320. [Google Scholar] [CrossRef] [PubMed]
- Acar, Y.; İlçin, N.; Gürpınar, B.; Can, G. The effects of clinical pilates training on disease-specific indices, core stability, and balance in patients with ankylosing spondylitis. J. Bodyw. Mov. Ther. 2023, 33, 69–75. [Google Scholar] [CrossRef]
- Rodríguez-López, E.S.; Garnacho-Garnacho, V.E.; Guodemar-Pérez, J.; García-Fernández, P.; Ruiz-López, M. One Year of Pilates Training for Ankylosing Spondylitis: A Pilot Study. J. Altern. Complement. Med. 2019, 25, 1054–1061. [Google Scholar] [CrossRef]
- Gandomi, F.; Soufivand, P.; Ezati, M.; Salimi, M.; Assar, S.; Pournazari, M.; Abbasi, H. The effect of Aqua Stretching exercises and Pilates on pain, function and spine posture in patients with ankylosing spondylitis: A randomized controlled trial. BMC Sports Sci. Med. Rehabil. 2022, 14, 183. [Google Scholar] [CrossRef]
- Ingram, T.; Sengupta, R.; Standage, M.; Barnett, R.; Rouse, P. Correlates of physical activity in adults with spondyloarthritis and rheumatoid arthritis: A systematic review. Rheumatol. Int. 2022, 42, 1693–1713. [Google Scholar] [CrossRef]
- Arends, S.; Hofman, M.; Kamsma, Y.P.; der Veer E van Houtman, P.M.; Kallenberg, C.G.; Spoorenberg, A.; Brouwer, E. Daily physical activity in ankylosing spondylitis: Validity and reliability of the IPAQ and SQUASH and the relation with clinical assessments. Arthritis Res. Ther. 2013, 15, R99. [Google Scholar] [CrossRef]
- Lubrano, E.; D’Angelo, S.; Parsons, W.J.; Serino, F.; Tanzillo, A.T.; Olivieri, I.; Pappone, N. Effects of a combination treatment of an intensive rehabilitation program and etanercept in patients with ankylosing spondylitis: A pilot study. J. Rheumatol. 2006, 33, 2029–2034. [Google Scholar]
| Group A (nA = 15) | Group B (nB = 15) | A vs. B Group | ||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value | Baseline | Follow-Up | p-Value | Pre | Post | |
| Sex (female/male) | 14/1 | - | 13/2 | - | p = 0.29 | |||
| Age (years) | 43.73 ± 9.81 | - | 49.33 ± 10.01 | - | p = 0.13 | |||
| Height (cm) | 1.63 ± 0.05 | - | 1.63 ± 0.06 | - | p = 0.95 | |||
| Weight (kg) | 76.72 ± 21.92 | 76.48 ± 21.05 | p = 0.81 | 79.10 ± 16.92 | 78.03 ± 16.54 | p = 0.11 | p = 0.74 | p = 0.28 |
| BMI (kg/m2) | 28.54 ± 7.88 | 28.44 ± 7.50 | p = 0.80 | 29.42 ± 6.00 | 29.00 ± 5.73 | p = 0.09 | p = 0.95 | p = 0.24 |
| CRP (mg/L) | 2.55 ± 2.51 | 2.30 ± 2.15 | p = 0.73 | 2.59 ± 1.92 | 2.44 ± 1.56 | p = 0.58 | p = 0.96 | p = 0.84 |
| ESR (mm/h) | 20.46 ± 13.02 | 18.80 ± 3.36 | p = 0.60 | 19.93 ± 7.62 | 20.26 ± 7.61 | p = 0.67 | p = 0.89 | p = 0.27 |
| Group A | Group B | A vs. B Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value | Intra-Observer Variability ICC (95% CI) | Baseline | Follow-Up | p-Value | Intra-Observer Variability ICC (95% CI) | Pre | Inter-Observer Variability ICC (95% CI) | Post | Inter-Observer Variability ICC (95% CI) | |
| HRrest (bpm) | 83.66 ± 12.82 | 80.00 ± 11.62 | p = 0.02 | 0.78 (0.34/0.92) | 83.80 ± 10.26 | 84.40 ± 9.61 | p = 0.73 | −0.36 (−0.73/0.15) | p = 0.97 | 0.28 (−1.14/0.75) | p = 0.001 | 0.76 (0.33/0.91) |
| SBPrest (mmHg) | 119.66 ± 14.45 | 117.13 ± 8.55 | p = 0.74 | 0.45 (−0.61/0.81) | 120.33 ± 9.53 | 121.00 ± 9.29 | p = 0.81 | 0.10 (−0.41/0.57) | p = 0.88 | −0.18 (−2.51/0.60) | p = 0.15 | 0.01 (−1.96/0.66) |
| DBPrest (mmHg) | 77.00 ± 8.40 | 73.33 ± 7.94 | p = 0.03 | 0.70 (0.21/0.85) | 76.33 ± 7.89 | 76.00 ± 8.06 | p = 0.83 | 0.14 (−0.38/0.59) | p = 0.79 | 0.46 (−0.58/0.82) | p = 0.31 | 0.54 (−0.37/0.84) |
| Time (min) | 7.81 ± 1.85 | 9.88 ± 2.61 | p = 0.001 | 0.76 (0.31/0.92) | 7.22 ± 1.19 | 7.19 ± 1.41 | p = 0.92 | 0.17 (−0.34/0.62) | p = 0.21 | 0.54 (−0.35/0.84) | p = 0.001 | 0.72 (0.16/0.90) |
| METs | 7.46 ± 2.88 | 9.16 ± 2.73 | p = 0.02 | 0.74 (0.23/0.91) | 7.44 ± 1.28 | 7.12 ± 1.07 | p = 0.06 | 0.22 (−0.30/0.65) | p = 0.97 | 0.25 (−1.23/0.74) | p = 0.008 | 0.56 (0.10/0.82) |
| VO2peak (mL/kg/min) | 26.33 ± 9.87 | 31.46 ± 9.63 | p = 0.04 | 0.74 (0.23/0.91) | 26.13 ± 4.43 | 25.08 ± 2.75 | p = 0.13 | 0.31 (−0.22/0.69) | p = 0.94 | 0.19 (−1.41/0.72) | p = 0.01 | 0.77 (0.32/0.92) |
| VO2/HRmax | 12.68 ± 2.73 | 13.93 ± 2.76 | p = 0.04 | 0.64 (0.47/0.88) | 12.66 ± 2.02 | 12.13 ± 2.06 | p = 0.06 | 0.16 (−0.36/0.61) | p = 0.99 | −1.17 (−5.46/0.27) | p = 0.04 | 0.92 (0.78/0.97) |
| VE/VO2max | 29.60 ± 4.11 | 28.80 ± 6.48 | p = 0.73 | −1.03 (−5.07/0.31) | 29.53 ± 4.47 | 30.26 ± 4.97 | p = 0.46 | 0.42 (−0.09/0.76) | p = 0.96 | 0.11 (−1.62/0.70) | p = 0.75 | 0.29 (−1.10/0.76) |
| VE/VCO2max | 27.73 ± 3.28 | 26.33 ± 2.09 | p = 0.18 | −0.15 (−2.44/0.61) | 27.33 ± 4.46 | 27.46 ± 3.62 | p = 0.92 | 0.06 (−0.04/0.54) | p = 0.71 | 0.59 (−0.20/0.86) | p = 0.35 | 0.40 (−0.77/0.80) |
| HRmax (bpm) | 158.60 ± 21.65 | 171.06 ± 19.41 | p = 0.03 | 0.68 (0.05/0.89) | 157.93 ± 18.26 | 157.60 ± 19.74 | p = 0.89 | 0.37 (−0.14/0.73) | p = 0.87 | 0.30 (−0.22/0.69) | p = 0.05 | 0.36 (−0.15/0.73) |
| SBPmax (mmHg) | 155.33 ± 21.99 | 147.33 ± 16.02 | p = 0.04 | 0.86 (0.60/0.95) | 155.66 ± 18.82 | 155.60 ± 18.11 | p = 0.97 | 0.1 (−0.31/0.60) | p = 0.96 | 0.30 (−1.07/0.76) | p = 0.18 | 0.40 (−0.78/0.79) |
| DBPmax (mmHg) | 76.33 ± 9.34 | 73.33 ± 7.94 | p = 0.24 | 0.55 (−0.31/0.85) | 76.66 ± 8.99 | 77.00 ± 8.82 | p = 0.86 | 0.30 (−0.23/0.70) | p = 0.97 | 0.36 (−0.87/0.78) | p = 0.19 | 0.25 (−0.28/0.66) |
| Group A | Group B | A vs. B Group | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Follow-Up | p-Value | Intra-Observer Variability ICC (95% CI) | Baseline | Follow-Up | p-Value | Intra-Observer Variability ICC (95% CI) | Pre | Inter-Observer Variability ICC (95% CI) | Post | Inter-Observer Variability ICC (95% CI) | |
| TUG (s) | 9.35 ± 1.44 | 7.81 ± 1.34 | p = 0.001 | 0.71 (0.15/0.90) | 9.17 ± 1.08 | 9.71 ± 1.40 | p = 0.14 | 0.19 (−1.40/0.72) | p = 0.69 | 0.27 (−1.47/0.71) | p = 0.001 | 0.57 (0.11/0.83) |
| SR (cm) | 17.20 ± 3.93 | 19.86 ± 3.73 | p = 0.001 | 0.98 (0.95/0.99) | 17.20 ± 5.22 | 16.73 ± 4.65 | p = 0.25 | 0.10 (−0.41/0.57) | p = 0.99 | 0.59 (−0.20/0.86) | p = 0.007 | 0.73 (0.20/0.91) |
| BSR (cm) | −1.93 ± 5.61 | −0.73 ± 3.78 | p = 0.03 | 0.89 (0.68/0.96) | −2.00 ± 5.96 | −2.13 ± 5.71 | p = 0.86 | −0.05 (−0.53/0.45) | p = 0.97 | −0.25 (−2.73/0.57) | p = 0.49 | −0.70 (−4.06/0.42) |
| BSL (cm) | −3.20 ± 7.10 | −2.26 ± 6.09 | p = 0.04 | 0.98 (0.94/0.99) | −3.46 ± 10.64 | −3.40 ± 9.47 | p = 0.88 | −0.26 (−0.67/0.27) | p = 0.06 | −0.25 (−2.75/0.57) | p = 0.71 | −0.10 (−2.30/0.62) |
| 5×STS (s) | 13.99 ± 2.59 | 11.73 ± 2.21 | p < 0.001 | 0.88 (0.64/0.96) | 13.43 ± 3.16 | 13.35 ± 4.08 | p = 0.89 | 0.13 (−0.48/0.50) | p = 0.58 | 0.02 (−1.90/0.67) | p = 0.001 | 0.86 (0.61/0.95) |
| BASDAI | 4.87 ± 2.32 | 3.72 ± 2.19 | p = 0.04 | 0.62 (0.43/0.86) | 4.29 ± 2.31 | 4.65 ± 2.34 | p = 0.10 | 0.30 (−1.06/0.76) | p = 0.51 | −0.06 (−2.17/0.64) | p = 0.04 | 0.73 (0.20/0.84) |
| ASDAS | 2.66 ± 0.97 | 1.93 ± 0.99 | p = 0.04 | 0.97 (0.91/0.99) | 2.45 ± 1.15 | 2.52 ± 1.23 | p = 0.19 | 0.18 (−0.34/0.62) | p = 0.63 | −0.47 (−3.39/0.50) | p = 0.03 | 0.88 (0.67/0.91) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaggelidou, E.; Theodoridou, A.; Michou, V.; Gika, H.; Panayiotou, G.; Dimitroulas, T.; Kouidi, E. The Effects of Pilates Exercise Training Combined with Walking on Cardiorespiratory Fitness, Functional Capacity, and Disease Activity in Patients with Non-Radiologically Confirmed Axial Spondylitis. J. Funct. Morphol. Kinesiol. 2023, 8, 140. https://doi.org/10.3390/jfmk8040140
Zaggelidou E, Theodoridou A, Michou V, Gika H, Panayiotou G, Dimitroulas T, Kouidi E. The Effects of Pilates Exercise Training Combined with Walking on Cardiorespiratory Fitness, Functional Capacity, and Disease Activity in Patients with Non-Radiologically Confirmed Axial Spondylitis. Journal of Functional Morphology and Kinesiology. 2023; 8(4):140. https://doi.org/10.3390/jfmk8040140
Chicago/Turabian StyleZaggelidou, Eleni, Athina Theodoridou, Vassiliki Michou, Helen Gika, George Panayiotou, Theodoros Dimitroulas, and Evangelia Kouidi. 2023. "The Effects of Pilates Exercise Training Combined with Walking on Cardiorespiratory Fitness, Functional Capacity, and Disease Activity in Patients with Non-Radiologically Confirmed Axial Spondylitis" Journal of Functional Morphology and Kinesiology 8, no. 4: 140. https://doi.org/10.3390/jfmk8040140
APA StyleZaggelidou, E., Theodoridou, A., Michou, V., Gika, H., Panayiotou, G., Dimitroulas, T., & Kouidi, E. (2023). The Effects of Pilates Exercise Training Combined with Walking on Cardiorespiratory Fitness, Functional Capacity, and Disease Activity in Patients with Non-Radiologically Confirmed Axial Spondylitis. Journal of Functional Morphology and Kinesiology, 8(4), 140. https://doi.org/10.3390/jfmk8040140








