Biomechanical Analysis of the Unaffected Limb While Using a Hands-Free Crutch
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
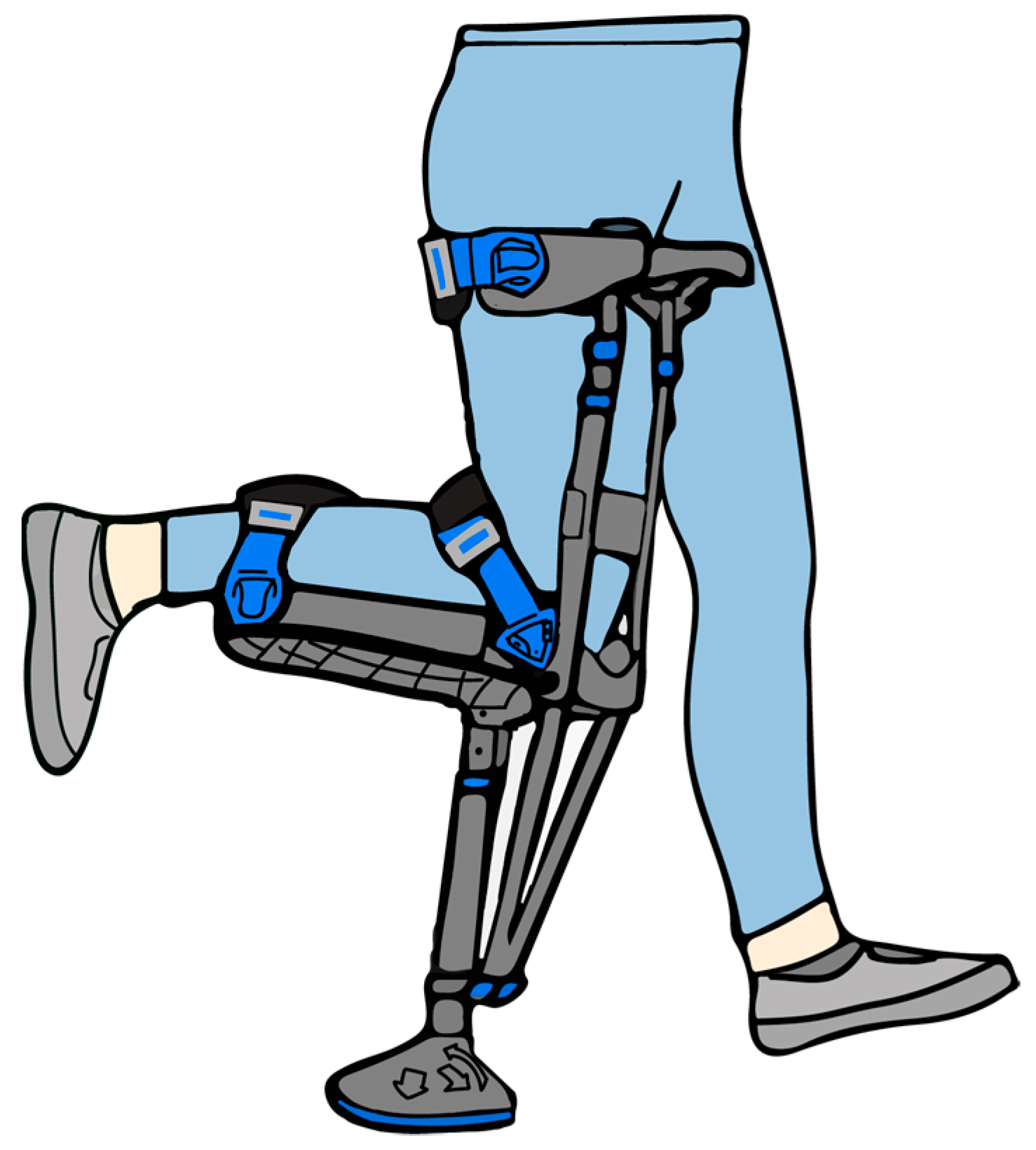
2.2. Experimental Protocol
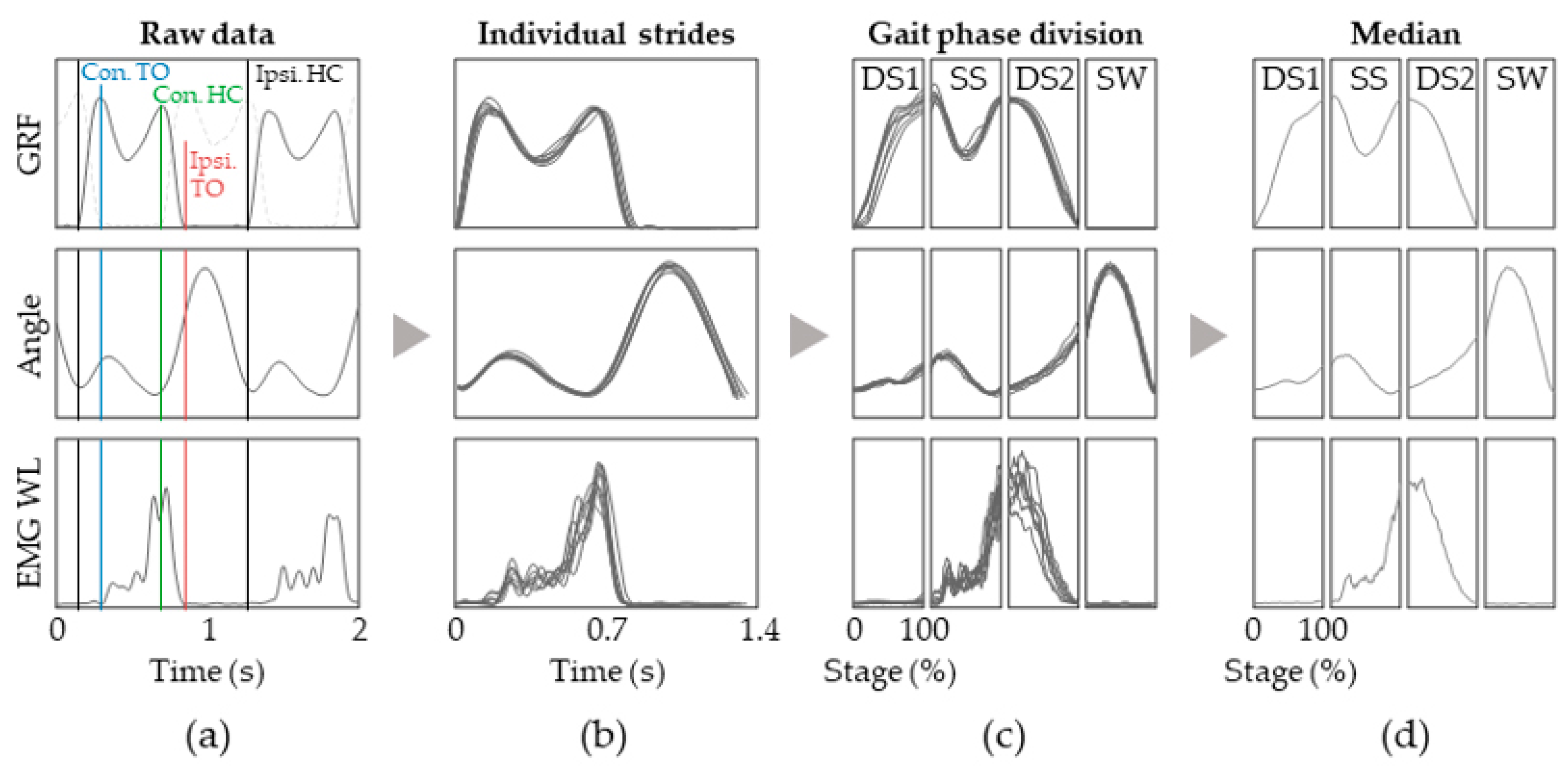
2.3. Signal Processing
2.4. Group Analysis
2.5. Statistical Analysis
3. Results
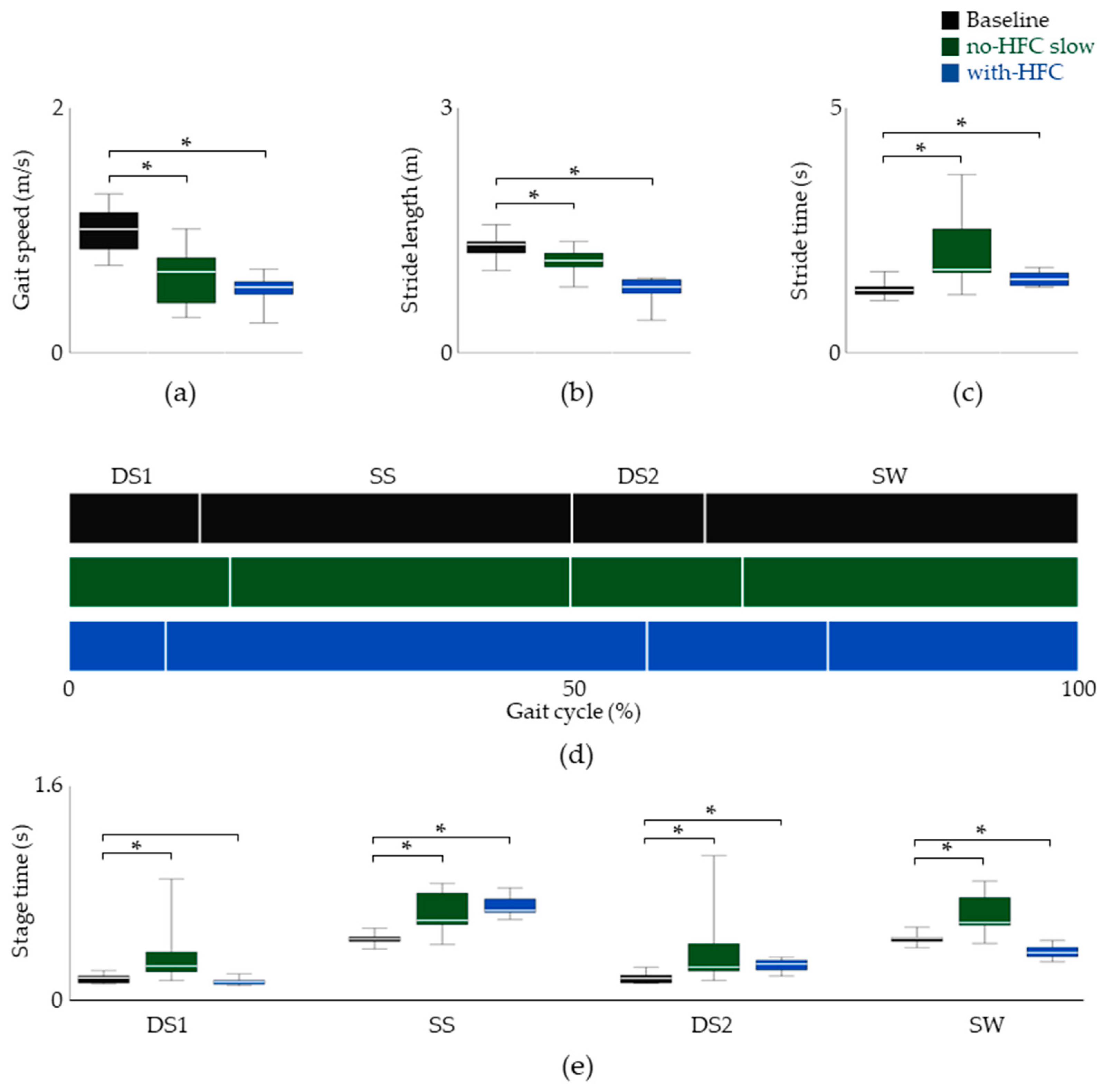
3.1. Temporal Parameters
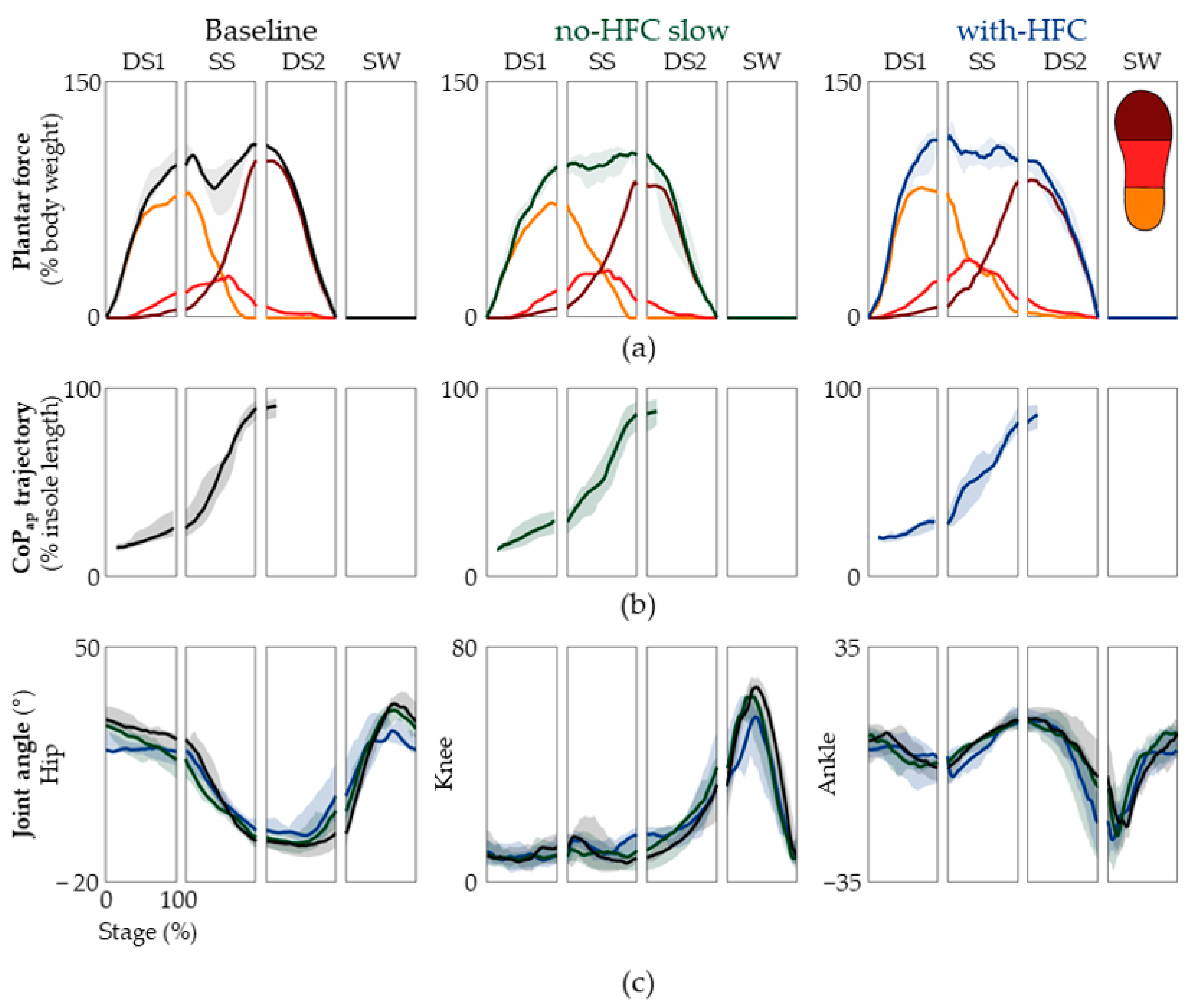
3.2. Plantar Force and Kinematics
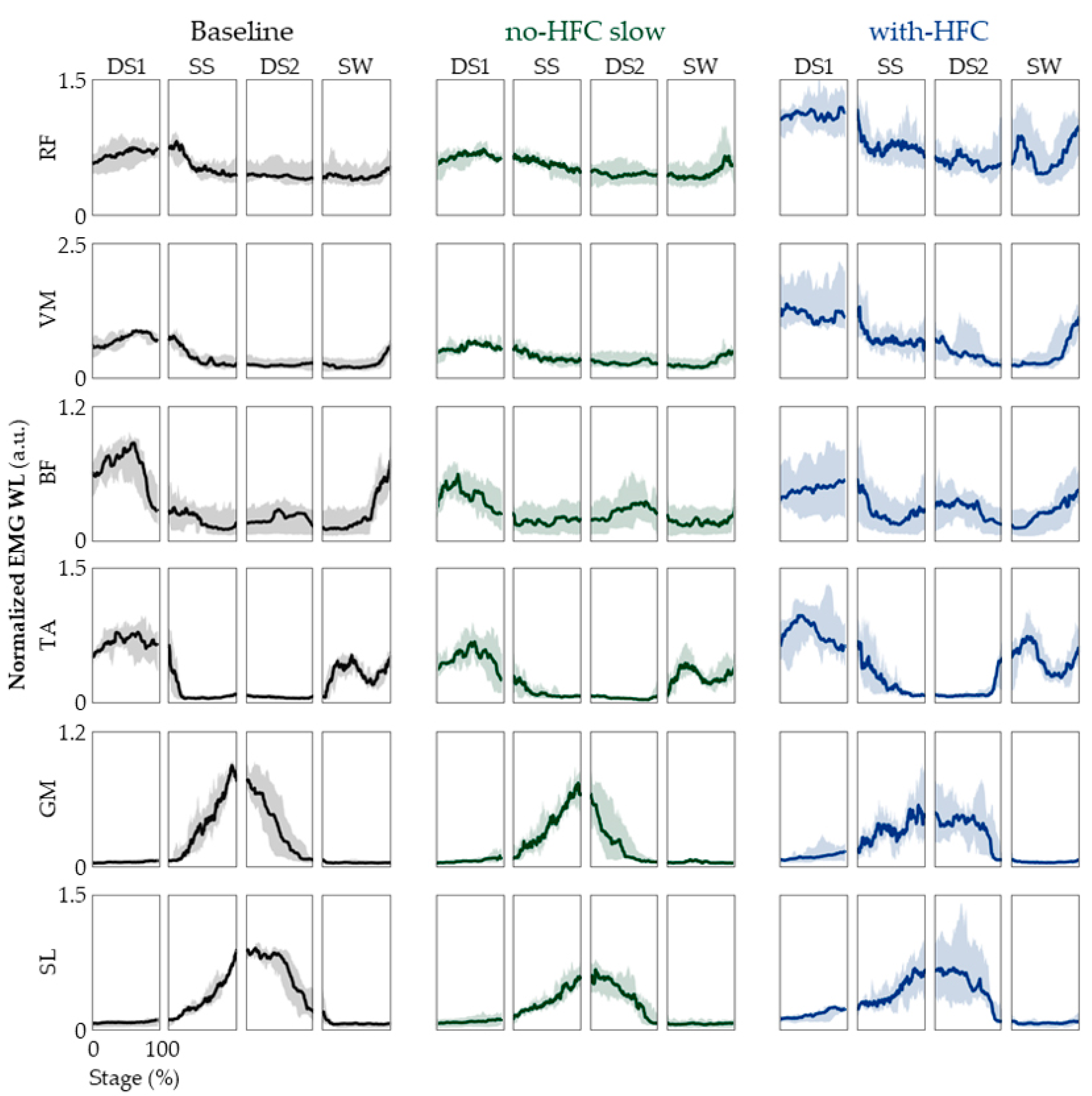
3.3. EMG
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.; Kim, T.H. The Effects of Balance and Gait Function on Quality of Life of Stroke Patients. NeuroRehabilitation 2019, 44, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Patterson, K.K.; Parafianowicz, I.; Danells, C.J.; Closson, V.; Verrier, M.C.; Staines, W.R.; Black, S.E.; McIlroy, W.E. Gait Asymmetry in Community-Ambulating Stroke Survivors. Arch. Phys. Med. Rehabil. 2008, 89, 304–310. [Google Scholar] [CrossRef]
- Eng, J.J.; Tang, P.F. Gait Training Strategies to Optimize Walking Ability in People with Stroke: A Synthesis of the Evidence. Expert. Rev. Neurother. 2007, 7, 1417–1436. [Google Scholar] [CrossRef] [PubMed]
- Perez-Lloret, S.; Negre-Pages, L.; Damier, P.; Delval, A.; Derkinderen, P.; Destée, A.; Meissner, W.G.; Schelosky, L.; Tison, F.; Rascol, O. Prevalence, Determinants, and Effect on Quality of Life of Freezing of Gait in Parkinson Disease. JAMA Neurol. 2014, 71, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Queralt, A.; Valls-Solé, J.; Castellote, J.M. Speeding up Gait Initiation and Gait-Pattern with a Startling Stimulus. Gait Posture 2010, 31, 185–190. [Google Scholar] [CrossRef]
- Swank, C.; Wang-Price, S.; Gao, F.; Almutairi, S. Walking with a Robotic Exoskeleton Does Not Mimic Natural Gait: A within-Subjects Study. JMIR Rehabil. Assist. Technol. 2019, 6, e11023. [Google Scholar] [CrossRef]
- Hutabarat, Y.; Owaki, D.; Hayashibe, M. Temporal Variation Quantification During Cognitive Dual-Task Gait Using Two IMU Sensors. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Glasgow, UK, 11–15 July 2022; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2022; Volume 2022, pp. 1121–1124. [Google Scholar]
- Beauchet, O.; Berrut, G. Gait and Dual-Task: Definition, Interest, and Perspectives in the Elderly. Psychol. Neuropsychiatr. Vieil. 2006, 4, 215–225. [Google Scholar] [PubMed]
- Paul, S.S.; Ada, L.; Canning, C.G. Automaticity of Walking–Implications for Physiotherapy Practice. Phys. Ther. Rev. 2005, 10, 15–23. [Google Scholar] [CrossRef]
- Rasouli, F.; Reed, K.B. Walking Assistance Using Crutches: A State of the Art Review. J. Biomech. 2020, 98, 109489. [Google Scholar] [CrossRef]
- Melis, E.; Torres-Moreno, R.; Barbeau, H.; Lemaire, E. Analysis of assisted-gait characteristics in persons with incomplete spinal cord injury. Spinal Cord 1999, 37, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Manocha, R.H.K.; MacGillivray, M.K.; Eshraghi, M.; Sawatzky, B.J. Injuries Associated with Crutch Use: A Narrative Review. PM&R 2021, 13, 1176–1192. [Google Scholar]
- Salminen, A.L.; Brandt, Å.; Samuelsson, K.; Töytäri, O.; Malmivaara, A. Mobility Devices to Promote Activity and Participation: A Systematic Review. J. Rehabil. Med. 2009, 41, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Reynders-Frederix, C.; Reynders-Frderix, P.; Bernardo, I.; Illes, T.; Berteanu, M. Monitoring Healing of Orthopedic Ailments with an Original Instrumented Crutch. Health Sport. Rehabil. Med. 2020, 21, 88–92. [Google Scholar] [CrossRef]
- Reynders-Frederix, C.; Reynders-Frederix, P.; Bernardo, I.; Mihai, B. Development of an Electronic Assistive Walking Device. Rom. Biotechnol. Lett. 2020, 25, 1992–1997. [Google Scholar] [CrossRef]
- Fisher, S.V.; Patterson, R.P. Energy Cost of Ambulation with Crutches. Arch. Phys. Med. Rehabil. 1981, 62, 250–256. [Google Scholar] [PubMed]
- Bellenfant, K.B.; Robbins, G.L.; Rogers, R.R.; Kopec, T.J.; Ballmann, C.G. Effects of Dominant and Nondominant Limb Immobilization on Muscle Activation and Physical Demand during Ambulation with Axillary Crutches. J. Funct. Morphol. Kinesiol. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.H.; Harris, J.M.; Minton, Y.M.; Motley, N.S.; Rowley, J.L.; Wadsworth, C.T. Energy cost, exercise intensity, and gait efficiency of standard versus rocker-bottom axillary crutch walking. Phys. Ther. 1990, 70, 487–493. [Google Scholar] [CrossRef]
- Hackney, K.J.; Bradley, A.P.; Roehl, A.S.; McGrath, R.; Smith, J. Energy Expenditure and Substrate Utilization with Hands-Free Crutches Compared to Conventional Lower-Extremity Injury Mobility Devices. Foot Ankle Orthop. 2022, 7, 1–8. [Google Scholar] [CrossRef]
- Holder, C.G.; Haskvitz, E.M.; Weltman, A. The effects of assistive devices on the oxygen cost, cardiovascular stress, and perception of nonweight-bearing ambulation. J. Orthop. Sports Phys. Ther. 1993, 18, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Sala, D.A.; Leva, L.M.; Kummer, F.J.; Grant, A.D. Crutch handle design: Effect on palmar loads during ambulation. Arch. Phys. Med. Rehabil. 1998, 79, 1473–1476. [Google Scholar] [CrossRef]
- Weiss, D.J.; Conliffe, T.; Tata, N. Low back pain caused by a duodenal ulcer. Arch. Phys. Med. Rehabil. 1998, 79, 1137–1139. [Google Scholar] [CrossRef]
- Hather, B.M.; Adams, G.R.; Tesch, P.A.; Dudley, G.A. Skeletal Muscle Responses to Lower Limb Suspension in Humans. J. Appl. Physiol. 1992, 72, 1493–1498. [Google Scholar] [CrossRef]
- Dewar, C.; Martin, K.D. Comparison of Lower Extremity EMG Muscle Testing With Hands-Free Single Crutch vs Standard Axillary Crutches. Foot Ankle Orthop. 2020, 5, 1–8. [Google Scholar] [CrossRef]
- Li, S.; Armstrong, C.W.; Cipriani, D. Three-Point Gait Crutch Walking: Variability in Ground Reaction Force during Weight Bearing. Arch. Phys. Med. Rehabil. 2001, 82, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Shoup, T.E.; Fletcher, L.S.; Merrill, B.R. Biomechanics of crutch locomotion. J. Biomech. 1974, 7, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Febrer-Nafría, M.; Pallarès-López, R.; Fregly, B.J.; Font-Llagunes, J.M. Prediction of Three-Dimensional Crutch Walking Patterns Using a Torque-Driven Model. Multibody Syst. Dyn. 2021, 51, 1–19. [Google Scholar] [CrossRef]
- Rambani, R.; Shahid, M.S.; Goyal, S. The use of a hands-free crutch in patients with musculoskeletal injuries: Randomized control trial. Int. J. Rehabil. Res. 2007, 30, 357–359. [Google Scholar] [CrossRef]
- Martin, K.D.; Unangst, A.M.; Huh, J.; Chisholm, J. Patient Preference and Physical Demand for Hands-Free Single Crutch vs Standard Axillary Crutches in Foot and Ankle Patients. Foot Ankle Int. 2019, 40, 1203–1208. [Google Scholar] [CrossRef] [PubMed]
- Raja, B.; Neptune, R.R.; Kautz, S.A. Coordination of the Non-Paretic Leg during Hemiparetic Gait: Expected and Novel Compensatory Patterns. Clin. Biomech. 2012, 27, 1023–1030. [Google Scholar] [CrossRef]
- Tabard-Fougère, A.; Rose-Dulcina, K.; Pittet, V.; Dayer, R.; Vuillerme, N.; Armand, S. EMG Normalization Method Based on Grade 3 of Manual Muscle Testing: Within- and between-Day Reliability of Normalization Tasks and Application to Gait Analysis. Gait Posture 2018, 60, 6–12. [Google Scholar] [CrossRef]
- Saito, A.; Kizawa, S.; Kobayashi, Y.; Miyawaki, K. Pose Estimation by Extended Kalman Filter Using Noise Covariance Matrices Based on Sensor Output. Robomech J. 2020, 7, 36. [Google Scholar] [CrossRef]
- Bakhshi, S.; Mahoor, M.H.; Davidson, B.S. Development of a Body Joint Angle Measurement System Using IMU Sensors. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Boston, MA, USA, 30 August–3 September 2011; pp. 6923–6926. [Google Scholar]
- Kim, J.; Kim, Y.; Kang, S.; Kim, S.J. Biomechanical Analysis Suggests Myosuit Reduces Knee Extensor Demand during Level and Incline Gait. Sensors 2022, 22, 6127. [Google Scholar] [CrossRef]
- Mickle, K.J.; Munro, B.J.; Lord, S.R.; Menz, H.B.; Steele, J.R. Gait, balance and plantar pressures in older people with toe deformities. Gait Posture 2011, 34, 347–351. [Google Scholar] [CrossRef]
- Alfuth, M.; Rosenbaum, D. Effects of Changes in Plantar Sensory Feedback on Human Gait Characteristics: A Systematic Review. Footwear Sci. 2012, 4, 1–22. [Google Scholar] [CrossRef]
- Maggioni, M.A.; Veicsteinas, A.; Rampichini, S.; Cé, E.; Nemni, R.; Riboldazzi, G.; Merati, G. Energy Cost of Spontaneous Walking in Parkinson’s Disease Patients. Neurol. Sci. 2012, 33, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Selinger, J.C.; O’Connor, S.M.; Wong, J.D.; Donelan, J.M. Humans Can Continuously Optimize Energetic Cost during Walking. Curr. Biol. 2015, 25, 2452–2456. [Google Scholar] [CrossRef]
- Donelan, J.M.; Kram, R.; Kuo, A.D. Mechanical and Metabolic Determinants of the Preferred Step Width in Human Walking. Proc. R. Soc. B: Biol. Sci. 2001, 268, 1985–1992. [Google Scholar] [CrossRef]
- Winter, D.A.; Quanbury, A.O.; Reimer, G.D. Analysis of instantaneous energy of normal gait. J. Biomech. 1976, 9, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Miller, C.A.; Verstraete, M.C. A mechanical energy analysis of gait initiation. Gait Posture 1999, 9, 158–166. [Google Scholar] [CrossRef]
- Bennett, B.C.; Abel, M.F.; Wolovick, A.; Franklin, T.; Allaire, P.E.; Kerrigan, D.C. Center of Mass Movement and Energy Transfer during Walking in Children with Cerebral Palsy. Arch. Phys. Med. Rehabil. 2005, 86, 2189–2194. [Google Scholar] [CrossRef]
- Sobhani, S.; van den Heuvel, E.R.; Dekker, R.; Postema, K.; Kluitenberg, B.; Bredeweg, S.W.; Hijmans, J.M. Biomechanics of Running with Rocker Shoes. J. Sci. Med. Sport 2017, 20, 38–44. [Google Scholar] [CrossRef]
- Mariani, B.; Rochat, S.; Büla, C.J.; Aminian, K. Heel and Toe Clearance Estimation for Gait Analysis Using Wireless Inertial Sensors. IEEE Trans. Biomed. Eng. 2012, 59, 3162–3168. [Google Scholar] [CrossRef] [PubMed]
- Kharb, A.; Saini, V.; Jain, Y.; Dhiman, S. A review of gait cycle and its parameters. IJCEM Int. J. Comput. Eng. Manag. 2011, 13, 78–83. [Google Scholar]
- Dewar, C.; Grindstaff, T.L.; Farmer, B.; Sainsbury, M.; Gay, S.; Kroes, W.; Martin, K.D. EMG Activity With Use of a Hands-Free Single Crutch vs a Knee Scooter. Foot Ankle Orthop. 2021, 6, 1–8. [Google Scholar] [CrossRef]
- Androwis, G.J.; Pilkar, R.; Ramanujam, A.; Nolan, K.J. Electromyography Assessment during Gait in a Robotic Exoskeleton for Acute Stroke. Front. Neurol. 2018, 9, 630. [Google Scholar] [CrossRef]
- Kim, J.J.; Cho, H.; Park, Y.; Jang, J.; Kim, J.W.; Ryu, J.S. Biomechanical Influences of Gait Patterns on Knee Joint: Kinematic & EMG Analysis. PLoS ONE 2020, 15, e0233593. [Google Scholar] [CrossRef]
- Pellegrini, B.; Peyré-Tartaruga, L.A.; Zoppirolli, C.; Bortolan, L.; Bacchi, E.; Figard-Fabre, H.; Schena, F. Exploring Muscle Activation during Nordic Walking: A Comparison between Conventional and Uphill Walking. PLoS ONE 2015, 10, e0138906. [Google Scholar] [CrossRef]
- Calabrò, R.S.; Billeri, L.; Andronaco, V.A.; Accorinti, M.; Milardi, D.; Cannavò, A.; Aliberti, E.; Militi, A.; Bramanti, P.; Naro, A. Walking on the Moon: A Randomized Clinical Trial on the Role of Lower Body Positive Pressure Treadmill Training in Post-Stroke Gait Impairment. J. Adv. Res. 2020, 21, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Von Schroeder, H.; Coutts, R.; Lyden, P.; Billings, E.; Nickel, V. Gait parameters following stroke: A practical assessment. J. Rehabil. Res. Dev. 1995, 32, 25–31. [Google Scholar]
- Kim, J.; Chung, S.H.; Choi, J.; Lee, J.M.; Kim, S.J. Practical Method for Predicting Intended Gait Speed via Soleus Surface EMG Signals. Electron. Lett. 2020, 56, 528–531. [Google Scholar] [CrossRef]
| Stage | Condition | Full (%) | Hindfoot (%) | Midfoot (%) | Forefoot (%) | CoPap (%) |
|---|---|---|---|---|---|---|
| DS1 | Baseline | 67.55 (63.31, 79.60) | 62.96 (54.40, 79.14) | 4.10 (2.38, 5.93) | 0.56 (0.00, 1.31) | 18.02 (16.60, 24.14) |
| No HFC, slow | 63.55 (55.73, 70.96) | 53.23 (49.08, 59.23) | 2.98 (0.88, 8.23) | 1.10 (0.05, 1.78) | 19.81 (15.53, 27.22) | |
| with HFC | 81.65 (77.13, 85.87) | 69.74 (57.68, 79.72) | 6.30 (4.98, 11.54) | 1.56 (0.89, 3.54) | 23.86 (21.07, 26.65) | |
| SS | Baseline | 95.87 (83.30, 100.39) | 28.90 (14.85, 38.50) | 22.75 (16.80, 25.39) | 31.72 (27.17, 39.42) | 57.22 (44.33, 61.50) |
| No HFC, slow | 98.41 (89.22, 105.49) | 31.45 (21.58, 43.23) | 26.90 (24.70, 29.10) | 32.15 (24.22, 39.62) | 50.62 (41.34, 62.82) | |
| with HFC | 104.78 * (95.43, 110.48) | 33.00 (23.56, 44.33) | 30.22 (24.06, 36.33) | 34.91 (22.91, 47.39) | 54.74 (45.29, 65.38) | |
| DS2 | Baseline | 70.32 (62.91, 72.62) | 0.00 (0.00, 0.00) | 2.50 (0.77, 7.95) | 65.05 (54.76, 69.42) | 89.84 (83.60, 93.55) |
| No HFC, slow | 55.78 (35.96, 71.02) | 0.00 (0.00, 0.00) | 1.24 (0.00, 5.14) | 51.44 (35.26, 66.38) | 87.02 (77.85, 93.49) | |
| with HFC | 72.21 (57.21, 83.46) | 0.57 * (0.00, 2.24) | 3.08 (0.54, 5.95) | 66.00 (54.65, 74.27) | 83.93 (77.33, 90.61) |
| Stage | Condition | Hip (°) | Knee (°) | Ankle (°) |
|---|---|---|---|---|
| DS1 | Baseline | 5.77 (5.15, 7.86) | 8.80 (6.31, 9.96) | 9.20 (7.77, 11.21) |
| No HFC, slow | 10.35 * (7.74, 14.08) | 5.39 * (4.17, 6.74) | 11.21 (9.73, 15.17) | |
| with HFC | 4.07 * (3.29, 5.08) | 7.42 (5.89, 7.96) | 9.40 (6.12, 10.83) | |
| SS | Baseline | 31.78 (25.35, 34.47) | 9.85 (5.81, 12.31) | 16.58 (15.83, 18.25) |
| No HFC, slow | 23.80 * (18.20, 27.03) | 6.37 (5.02, 10.04) | 13.54 (8.98, 18.95) | |
| with HFC | 23.64 * (21.66, 24.86) | 7.75 (5.50, 10.31) | 15.07 (11.59, 22.58) | |
| DS2 | Baseline | 6.60 (3.01, 10.17) | 25.31 (13.67, 28.03) | 13.01 (9.54, 26.93) |
| No HFC, slow | 9.45 (6.47, 12.53) | 26.96 (21.78, 30.28) | 21.97 (16.79, 38.24) | |
| with HFC | 12.14 (8.51, 17.13) | 21.25 (13.85, 30.84) | 31.48 * (23.61, 32.71) | |
| SW | Baseline | 40.31 (30.25, 44.45) | 56.33 (50.41, 61.50) | 31.02 (24.79, 39.83) |
| No HFC, slow | 33.02 (25.53, 36.92) | 53.97 (51.87, 57.14) | 29.62 (23.22, 40.67) | |
| with HFC | 19.34 * (14.30, 28.18) | 41.68 * (34.67, 55.01) | 29.22 (23.00, 31.36) |
| DS1 (%) | SS (%) | DS2 (%) | SW (%) | |||||
|---|---|---|---|---|---|---|---|---|
| No HFC, Slow | with HFC | No HFC, Slow | with HFC | No HFC, Slow | with HFC | No HFC, Slow | with HFC | |
| RF | −3.57 (−11.04, 10.38) | 76.22 * (41.39, 112.1) | 0.16 (−5.37, 6.07) | 47.84 * (15.51, 70.38) | 2.08 (−9.55, 7.4) | 33.32 * (14.6, 54.66) | 3.19 (−7.91, 21.03) | 66.57 * (17.66, 112.2) |
| VM | −14.84 (−22.7, 15.34) | 98.85 * (43.22, 178.53) | −0.15 (−18.84, 32.59) | 87.99 * (20, 157.19) | 13.7 (3.1, 58.74) | 93.35 (52.38, 114.13) | 5.98 (−3.36, 28.44) | 49.02 (22.75, 139.54) |
| BF | −32.08 * (−49.4, −22.39) | −11.38 (−49.64, 8.06) | −7.74 (−25.4, 13.15) | 23.45 (8.31, 51.35) | 20.3 (9.85, 43.61) | 29.55 (1.93, 112.34) | −19.99 (−51.77, −5.77) | 0.23 (−45.57, 18.58) |
| TA | −18.08 (−32.69, −1.67) | 23.84 (−10.61, 61.78) | 29.19 (−6.05, 49.45) | 147.7 * (62.08, 193.44) | −9.06 (−16.19, 21.33) | 76.37 * (35.02, 127.76) | −8.41 (−22.79, 12.65) | 54.62 * (17.48, 112.29) |
| GM | 7.78 (1.52, 83.69) | 116.7 * (30.76, 234.81) | 0.08 (−26.9, 14.34) | −5.84 (−37.53, 8.23) | −33.81 (−55.31, −17.45) | 27.95 (−56.73, 53.77) | 0.67 (−29.49, 7.67) | 6.56 (−23.05, 13.71) |
| SL | 6.34 (−1.71, 29.98) | 100.46 (64.59, 115) | −12.92 (−26.34, −4.35) | 10.98 (−14.7, 38.69) | −28.1 * (−48.92, −26.36) | −21.12 (−43.49, 53.99) | −7.15 (−16.41, 3.18) | 15.7 (7.62, 30.85) |
| Stage | Condition | RF (%) | VM (%) | BF (%) | TA (%) | GM (%) | SL (%) |
|---|---|---|---|---|---|---|---|
| DS1 | Baseline | 0.50 (0.43, 0.62) | 0.54 (0.45, 0.66) | 0.82 (0.57, 0.91) | 0.64 (0.57, 0.77) | 0.02 (0.01, 0.04) | 0.03 (0.02, 0.09) |
| No HFC, slow | 0.68 (0.46, 0.76) | 0.70 (0.57, 0.86) | 0.86 (0.57, 0.97) | 0.82 (0.63, 0.97) | 0.05 (0.02, 0.26) | 0.10 * (0.05, 0.21) | |
| with HFC | 0.38 * (0.19, 0.46) | 0.48 (0.40, 0.54) | 0.55 * (0.48, 0.60) | 0.57 (0.40, 0.65) | 0.27 * (0.05, 0.40) | 0.10 * (0.07, 0.25) | |
| SS | Baseline | 0.88 (0.70, 0.97) | 0.94 (0.77, 0.97) | 0.52 (0.23, 0.74) | 0.84 (0.47, 0.99) | 0.98 (0.92, 0.99) | 0.84 (0.78, 0.90) |
| No HFC, slow | 0.8 (0.62, 0.90) | 0.83 (0.69, 0.95) | 0.54 (0.28, 0.83) | 0.77 (0.55, 0.93) | 0.94 (0.78, 0.98) | 0.84 (0.75, 0.90) | |
| with HFC | 0.7 (0.46, 0.88) | 0.81 (0.75, 0.89) | 0.87 (0.50, 0.97) | 0.74 (0.59, 0.99) | 0.91 * (0.52, 0.97) | 0.61 * (0.40, 0.67) | |
| DS2 | Baseline | 0.29 (0.11, 0.41) | 0.14 (0.08, 0.34) | 0.15 (0.06, 0.37) | 0.08 (0.06, 0.13) | 0.76 (0.65, 0.93) | 0.88 (0.58, 0.92) |
| No HFC, slow | 0.28 (0.18, 0.45) | 0.39 (0.20, 0.46) | 0.87 * (0.45, 0.98) | 0.13 (0.08, 0.47) | 0.79 (0.54, 0.91) | 0.87 (0.77, 0.98) | |
| with HFC | 0.36 (0.25, 0.62) | 0.38 * (0.28, 0.55) | 0.47 (0.27, 0.67) | 0.56 * (0.09, 0.76) | 0.79 (0.66, 0.90) | 0.90 (0.61, 0.99) | |
| SW | Baseline | 0.69 (0.21, 1.00) | 0.76 (0.34, 1.00) | 1.00 (0.99, 1.00) | 0.86 (0.67, 1.00) | 0.05 (0.01, 0.40) | 0.15 (0.05, 0.42) |
| No HFC, slow | 0.93 (0.33, 1.00) | 0.78 (0.47, 1.00) | 0.76 (0.43, 0.86) | 0.87 (0.79, 1.00) | 0.02 (0.01, 0.08) | 0.08 (0.02, 0.10) | |
| with HFC | 0.74 (0.45, 1.00) | 0.66 (0.56, 1.00) | 0.88 (0.74, 1.00) | 0.74 (0.64, 0.87) | 0.15 (0.08, 0.17) | 0.10 (0.05, 0.24) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.; Kim, Y.; Moon, J.; Kong, J.; Kim, S.-J. Biomechanical Analysis of the Unaffected Limb While Using a Hands-Free Crutch. J. Funct. Morphol. Kinesiol. 2023, 8, 56. https://doi.org/10.3390/jfmk8020056
Kim J, Kim Y, Moon J, Kong J, Kim S-J. Biomechanical Analysis of the Unaffected Limb While Using a Hands-Free Crutch. Journal of Functional Morphology and Kinesiology. 2023; 8(2):56. https://doi.org/10.3390/jfmk8020056
Chicago/Turabian StyleKim, Jaewook, Yekwang Kim, Juhui Moon, Joo Kong, and Seung-Jong Kim. 2023. "Biomechanical Analysis of the Unaffected Limb While Using a Hands-Free Crutch" Journal of Functional Morphology and Kinesiology 8, no. 2: 56. https://doi.org/10.3390/jfmk8020056
APA StyleKim, J., Kim, Y., Moon, J., Kong, J., & Kim, S.-J. (2023). Biomechanical Analysis of the Unaffected Limb While Using a Hands-Free Crutch. Journal of Functional Morphology and Kinesiology, 8(2), 56. https://doi.org/10.3390/jfmk8020056






