Beyond Mechanical Tension: A Review of Resistance Exercise-Induced Lactate Responses & Muscle Hypertrophy
Abstract
1. Introduction
- History of Lactate
2. Section I
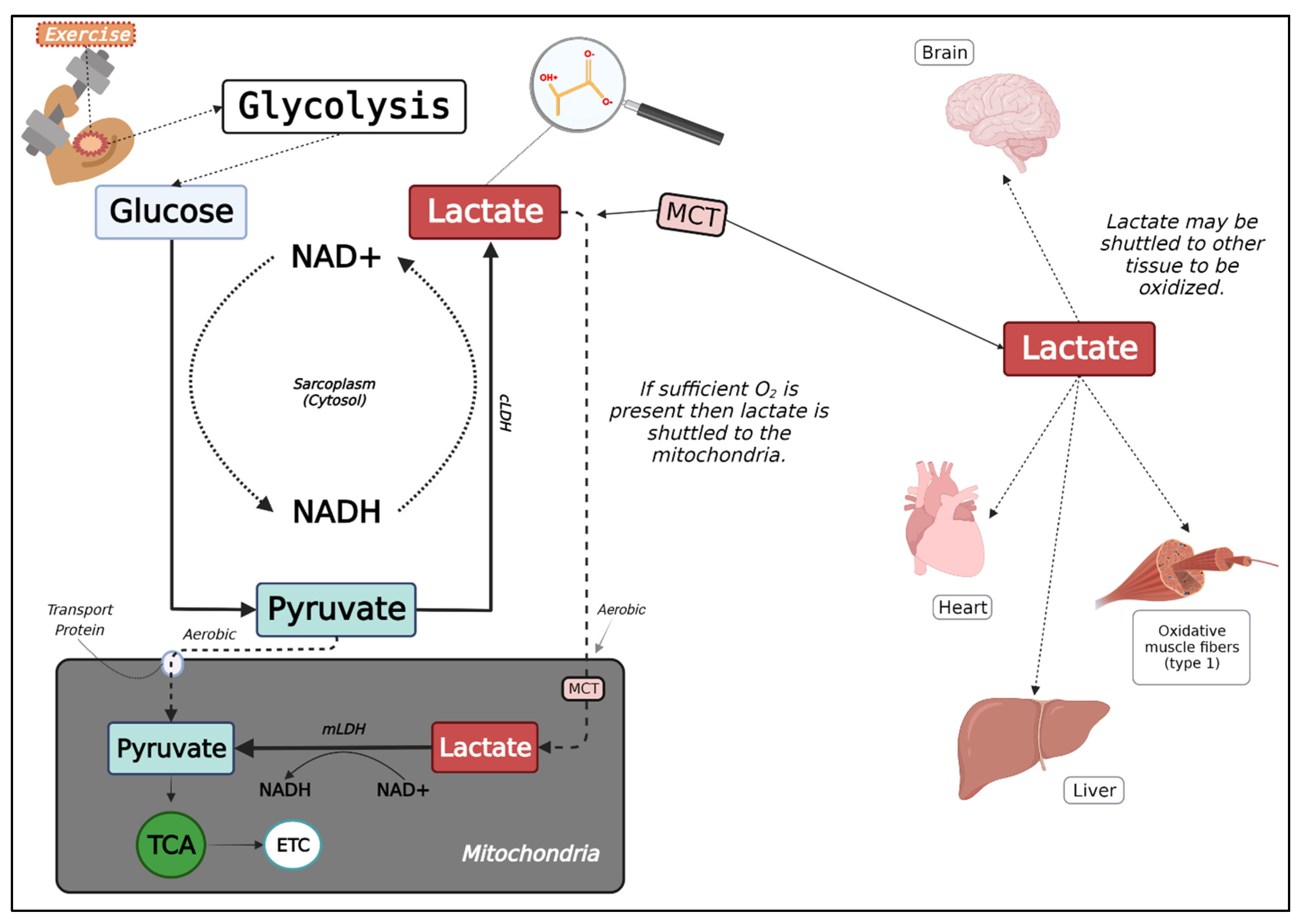
2.1. Lactate Metabolism
2.2. Measuring Lactate in the Blood versus Muscle
2.3. The Lactate Shuttle Hypothesis
3. Section II
3.1. Lactate-Stimulated Testosterone Production
3.2. Lactate-Related Epigenetic Modification
3.3. Anabolic Effects of Lactate
4. Section III
Blood Lactate Response to Various Training Protocols
5. Discussion
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gladden, L.B. Lactate Metabolism: A New Paradigm for the Third Millennium. J. Physiol. 2004, 558, 5–30. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B. Principles of Exercise Biochemistry, 3rd ed.; Karger: Basel, Switzerland , 2004. [Google Scholar]
- Benninga, H.A. A History of Lactic Acid Making; Kluwer Academic Publishers: Amsterdam, The Netherlands, 1990; Volume 1. [Google Scholar]
- Severinghaus, J.W. Eight Sages over Five Centuries Share Oxygen’s Discovery. Adv. Physiol. Educ. 2016, 40, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Gladden, L.B. The Metabolic Systems: Anaerobic Metabolism (Glycolytic and Phosphagen). In Exercise Physiology; American Psychological Association: Oxford, NY, USA, 2003; pp. 322–360. [Google Scholar]
- Fletcher, W.M.; Hopkins, F.G. Lactic Acid in Amphibian Muscle1. J. Physiol. 1907, 35, 247–309. [Google Scholar] [CrossRef] [PubMed]
- Kompanje, E.J.O.; Jansen, T.C.; van der Hoven, B.; Bakker, J. The First Demonstration of Lactic Acid in Human Blood in Shock by Johann Joseph Scherer (1814–1869) in January 1843. Intensive Care Med. 2007, 33, 1967–1971. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.M.; Rajasekaran, S.; Thomsen, T.W.; Peterson, A.R. Lactate: Friend or Foe. PMR 2016, 8, S8–S15. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.S.; Rogatzki, M.J.; Goodwin, M.L.; Kane, D.A.; Rightmire, Z.; Gladden, L.B. Lactate Metabolism: Historical Context, Prior Misinterpretations, and Current Understanding. Eur. J. Appl. Physiol. 2018, 118, 691–728. [Google Scholar] [CrossRef]
- Wasserman, K.; McIlroy, M.B. Detecting the Threshold of Anaerobic Metabolism in Cardiac Patients during Exercise. Am. J. Cardiol. 1964, 14, 844–852. [Google Scholar] [CrossRef]
- Wasserman, K.; Whipp, B.J.; Koyl, S.N.; Beaver, W.L. Anaerobic Threshold and Respiratory Gas Exchange during Exercise. J. Appl. Physiol. 1973, 35, 236–243. [Google Scholar] [CrossRef]
- Cheng, B.; Kuipers, H.; Snyder, A.; Keizer, H.; Jeukendrup, A.; Hesselink, M. A New Approach for the Determination of Ventilatory and Lactate Thresholds. Int. J. Sports Med. 1992, 13, 518–522. [Google Scholar] [CrossRef]
- Faude, O.; Kindermann, W.; Meyer, T. Lactate Threshold Concepts. Sports Med. 2009, 39, 469–490. [Google Scholar] [CrossRef] [PubMed]
- Gladden, L.B.; Yates, J.W.; Stremel, R.W.; Stamford, B.A. Gas Exchange and Lactate Anaerobic Thresholds: Inter- and Intraevaluator Agreement. J. Appl. Physiol. 1985, 58, 2082–2089. [Google Scholar] [CrossRef] [PubMed]
- Heck, H.; Mader, A.; Hess, G.; Mücke, S.; Müller, R.; Hollmann, W. Justification of the 4-Mmol/L Lactate Threshold. Int. J. Sports Med. 1985, 6, 117–130. [Google Scholar] [CrossRef] [PubMed]
- Tesch, P.A.; Daniels, W.L.; Sharp, D.S. Lactate Accumulation in Muscle and Blood during Submaximal Exercise. Acta Physiol. Scand. 1982, 114, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Connett, R.J.; Honig, C.R.; Gayeski, T.E.; Brooks, G.A. Defining Hypoxia: A Systems View of VO2, Glycolysis, Energetics, and Intracellular PO2. J. Appl. Physiol. 1990, 68, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Davis, J.A.; Rozenek, R.; DeCicco, D.M.; Carizzi, M.T.; Pham, P.H. Comparison of Three Methods for Detection of the Lactate Threshold. Clin. Physiol. Funct. Imaging 2007, 27, 381–384. [Google Scholar] [CrossRef] [PubMed]
- Dodd, S.; Powers, S.K.; Callender, T.; Brooks, E. Blood Lactate Disappearance at Various Intensities of Recovery Exercise. J. Appl. Physiol. 1984, 57, 1462–1465. [Google Scholar] [CrossRef]
- Svedahl, K.; MacIntosh, B.R. Anaerobic Threshold: The Concept and Methods of Measurement. Can. J. Appl. Physiol. 2003, 28, 299–323. [Google Scholar] [CrossRef]
- Jöbsis, F.F.; Stainsby, W.N. Oxidation of NADH during Contractions of Circulated Mammalian Skeletal Muscle. Respir. Physiol. 1968, 4, 292–300. [Google Scholar] [CrossRef]
- Connett, R.J.; Gayeski, T.E.J.; Honig, C.R. Lactate Production in a Pure Red Muscle in Absence of Anoxia: Mechanisms and Significance. Adv. Exp. Med. Biol. 1983, 159, 327–335. [Google Scholar]
- Connett, R.J.; Gayeski, T.E.; Honig, C.R. Lactate Accumulation in Fully Aerobic, Working, Dog Gracilis Muscle. Am. J. Physiol.-Heart Circ. Physiol. 1984, 246, H120–H128. [Google Scholar] [CrossRef]
- Connett, R.J.; Gayeski, T.E.; Honig, C.R. Lactate Efflux Is Unrelated to Intracellular PO2 in a Working Red Muscle in Situ. J. Appl. Physiol. 1986, 61, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate as a Fulcrum of Metabolism. Redox Biol. 2020, 35, 101454. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Hussien, R.; Oommen, S.; Gohil, K.; Brooks, G.A. Lactate Sensitive Transcription Factor Network in L6 Cells: Activation of MCT1 and Mitochondrial Biogenesis. FASEB J. 2007, 21, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Nalbandian, M.; Takeda, M. Lactate as a Signaling Molecule That Regulates Exercise-Induced Adaptations. Biology 2016, 5, 38. [Google Scholar] [CrossRef]
- Philp, A.; Macdonald, A.L.; Watt, P.W. Lactate—A Signal Coordinating Cell and Systemic Function. J. Exp. Biol. 2005, 208, 4561–4575. [Google Scholar] [CrossRef]
- Brooks, G.A.; Arevalo, J.A.; Osmond, A.D.; Leija, R.G.; Curl, C.C.; Tovar, A.P. Lactate in Contemporary Biology: A Phoenix Risen. J. Physiol. 2021, 600, 1229–1251. [Google Scholar] [CrossRef]
- Rogatzki, M.J.; Ferguson, B.S.; Goodwin, M.L.; Gladden, L.B. Lactate Is Always the End Product of Glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Robergs, R.A.; McNulty, C.R.; Minett, G.M.; Holland, J.; Trajano, G. Lactate, Not Lactic Acid, Is Produced by Cellular Cytosolic Energy Catabolism. Physiology 2018, 33, 10–12. [Google Scholar] [CrossRef]
- Schurr, A.; Gozal, E. Glycolysis at 75: Is It Time to Tweak the First Elucidated Metabolic Pathway in History? Front. Neurosci. 2015, 9, 170. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry, 5th ed.; New York Freeman: New York, NY, USA, 2007. [Google Scholar]
- Forkasiewicz, A.; Dorociak, M.; Stach, K.; Szelachowski, P.; Tabola, R.; Augoff, K. The Usefulness of Lactate Dehydrogenase Measurements in Current Oncological Practice. Cell. Mol. Biol. Lett. 2020, 25, 35. [Google Scholar] [CrossRef]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, C.E. Role of Mitochondrial Lactate Dehydrogenase and Lactate Oxidation in the Intracellular Lactate Shuttle. Proc. Natl. Acad. Sci. USA 1999, 96, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Skinner, J.S.; Mclellan, T.H. The Transition from Aerobic to Anaerobic Metabolism. Res. Q. Exerc. Sport 1980, 51, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Stainsby, W.N.; Sumners, C.; Andrew, G.M. Plasma Catecholamines and Their Effect on Blood Lactate and Muscle Lactate Output. J. Appl. Physiol. 1984, 57, 321–325. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate:Glycolytic End Product and Oxidative Substrate during Sustained Exercise in Mammals—The “Lactate Shuttle”; Springer: Berlin/Heidelberg, Germany, 1985; pp. 208–218. [Google Scholar]
- Brooks, G.A. Lactate Production under Fully Aerobic Conditions: The Lactate Shuttle during Rest and Exercise. Fed. Proc. 1986, 45, 2924–2929. [Google Scholar] [PubMed]
- Gladden, L.B. 200th Anniversary of Lactate Research in Muscle. Exerc. Sport Sci. Rev. 2008, 36, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Van Hall, G. Lactate as a Fuel for Mitochondrial Respiration. Acta Physiol. Scand. 2000, 168, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Takeda, R.; Nonaka, Y.; Kakinoki, K.; Miura, S.; Kano, Y.; Hoshino, D. Effect of Endurance Training and PGC-1α Overexpression on Calculated Lactate Production Volume during Exercise Based on Blood Lactate Concentration. Sci. Rep. 2022, 12, 1635. [Google Scholar] [CrossRef]
- Karlsson, J.; Jacobs, I.; Sjödin, B.; Tesch, P.; Kaiser, P.; Sahl, O.; Karlberg, B. Semi-Automatic Blood Lactate Assay: Experiences from an Exercise Laboratory. Int. J. Sports Med. 1983, 4, 52–55. [Google Scholar] [CrossRef]
- Maughan, R.J. A Simple, Rapid Method for the Determination of Glucose, Lactate, Pyruvate, Alanine, 3-Hydroxybutyrate and Acetoacetate on a Single 20-Μl Blood Sample. Clin. Chim. Acta 1982, 122, 231–240. [Google Scholar] [CrossRef]
- Tanner, R.K.; Fuller, K.L.; Ross, M.L.R. Evaluation of Three Portable Blood Lactate Analysers: Lactate Pro, Lactate Scout and Lactate Plus. Eur. J. Appl. Physiol. 2010, 109, 551–559. [Google Scholar] [CrossRef]
- Jacobs, I. Blood Lactate. Sports Med. 1986, 3, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Bergman, B.C.; Wolfel, E.E.; Butterfield, G.E.; Lopaschuk, G.D.; Casazza, G.A.; Horning, M.A.; Brooks, G.A. Active Muscle and Whole Body Lactate Kinetics after Endurance Training in Men. J. Appl. Physiol. 1999, 87, 1684–1696. [Google Scholar] [CrossRef] [PubMed]
- Gorostiaga, E.M.; Navarro-Amézqueta, I.; Calbet, J.A.L.; Sánchez-Medina, L.; Cusso, R.; Guerrero, M.; Granados, C.; González-Izal, M.; Ibáñez, J.; Izquierdo, M. Blood Ammonia and Lactate as Markers of Muscle Metabolites during Leg Press Exercise. J. Strength Cond. Res. 2014, 28, 2775–2785. [Google Scholar] [CrossRef]
- Jorfeldt, L.; Juhlin-Dannfelt, A.; Karlsson, J. Lactate Release in Relation to Tissue Lactate in Human Skeletal Muscle during Exercise. J. Appl. Physiol. 1978, 44, 350–352. [Google Scholar] [CrossRef]
- Liegnell, R.; Apró, W.; Danielsson, S.; Ekblom, B.; van Hall, G.; Holmberg, H.-C.; Moberg, M. Elevated Plasma Lactate Levels via Exogenous Lactate Infusion Do Not Alter Resistance Exercise-Induced Signaling or Protein Synthesis in Human Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2020, 319, E792–E804. [Google Scholar] [CrossRef]
- MacRae, H.S.; Dennis, S.C.; Bosch, A.N.; Noakes, T.D. Effects of Training on Lactate Production and Removal during Progressive Exercise in Humans. J. Appl. Physiol. 1992, 72, 1649–1656. [Google Scholar] [CrossRef]
- Stanley, W.C.; Gertz, E.W.; Wisneski, J.A.; Morris, D.L.; Neese, R.A.; Brooks, G.A. Systemic Lactate Kinetics during Graded Exercise in Man. Am. J. Physiol.-Endocrinol. Metab. 1985, 249, E595–E602. [Google Scholar] [CrossRef] [PubMed]
- Stellingwerff, T.; LeBlanc, P.J.; Hollidge, M.G.; Heigenhauser, G.J.F.; Spriet, L.L. Hyperoxia Decreases Muscle Glycogenolysis, Lactate Production, and Lactate Efflux during Steady-State Exercise. Am. J. Physiol.-Endocrinol. Metab. 2006, 290, E1180–E1190. [Google Scholar] [CrossRef] [PubMed]
- Sjogaard, G.; Adams, R.P.; Saltin, B. Water and Ion Shifts in Skeletal Muscle of Humans with Intense Dynamic Knee Extension. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1985, 248, R190–R196. [Google Scholar] [CrossRef] [PubMed]
- Chwalbinska-Moneta, J.; Robergs, R.A.; Costill, D.L.; Fink, W.J. Threshold for Muscle Lactate Accumulation during Progressive Exercise. J. Appl. Physiol. 1989, 66, 2710–2716. [Google Scholar] [CrossRef]
- Kubera, B.; Hubold, C.; Otte, S.; Lindenberg, A.-S.; Zeiß, I.; Krause, R.; Steinkamp, M.; Klement, J.; Entringer, S.; Pellerin, L.; et al. Rise in Plasma Lactate Concentrations with Psychosocial Stress: A Possible Sign of Cerebral Energy Demand. Obes. Facts 2012, 5, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Feliu, J.; Ventura, J.L.; Segura, R.; Rodas, G.; Riera, J.; Estruch, A.; Zamora, A.; Capdevila, L. Differences between lactate concentration of samples from ear lobe and the finger tip. J Physiol. Biochem. 1999, 55, 333–339. [Google Scholar]
- Forsyth, J.J.; Farrally, M.R. A Comparison of Lactate Concentration in Plasma Collected from the Toe, Ear, and Fingertip after a Simulated Rowing Exercise. Br. J. Sports Med. 2000, 34, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Moran, P.; Prichard, J.G.; Ansley, L.; Howatson, G. The Influence of Blood Lactate Sample Site on Exercise Prescription. J. Strength Cond. Res. 2012, 26, 563–567. [Google Scholar] [CrossRef] [PubMed]
- Yamagata, T.; Kume, D.; Wakimoto, T.; Nagao, N. The Influence of Sampling Site on Blood Lactate Response to Steady State Exercise. J. Sports Sci. 2018, 6, 276–284. [Google Scholar] [CrossRef]
- Dassonville, J.; Beillot, J.; Lessard, Y.; Jan, J.; André, A.; Pourcelet, L.; Rochcongar, P.; Carré, F. Blood Lactate Concentrations during Exercise: Effect of Sampling Site and Exercise Mode. J. Sports Med. Phys. Fit. 1998, 38, 39–46. [Google Scholar]
- Foxdal, P.; Sjödin, B.; Rudstam, H.; Östman, C.; Östman, B.; Hedenstierna, G.C. Lactate Concentration Differences in Plasma, Whole Blood, Capillary Finger Blood and Erythrocytes during Submaximal Graded Exercise in Humans. Eur. J. Appl. Physiol. Occup. Physiol. 1990, 61, 218–222. [Google Scholar] [CrossRef]
- Harris, R.T.; Dudley, G.A. Exercise Alters the Distribution of Ammonia and Lactate in Blood. J. Appl. Physiol. 1989, 66, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Cell-Cell and Intracellular Lactate Shuttles. J. Physiol. 2009, 587, 5591–5600. [Google Scholar] [CrossRef] [PubMed]
- Bonen, A. The Expression of Lactate Transporters (MCT1 and MCT4) in Heart and Muscle. Eur. J. Appl. Physiol. 2001, 86, 6–11. [Google Scholar] [CrossRef]
- Dubouchaud, H.; Butterfield, G.E.; Wolfel, E.E.; Bergman, B.C.; Brooks, G.A. Endurance Training, Expression, and Physiology of LDH, MCT1, and MCT4 in Human Skeletal Muscle. Am. J. Physiol.-Endocrinol. Metab. 2000, 278, E571–E579. [Google Scholar] [CrossRef] [PubMed]
- Halestrap, A.P.; Price, N.T. The Proton-Linked Monocarboxylate Transporter (MCT) Family: Structure, Function and Regulation. Biochem. J. 1999, 343, 281–299. [Google Scholar] [CrossRef] [PubMed]
- Juel, C. Current Aspects of Lactate Exchange: Lactate/H+ Transport in Human Skeletal Muscle. Eur. J. Appl. Physiol. 2001, 86, 12–16. [Google Scholar] [CrossRef]
- Juel, C.; Halestrap, A.P. Lactate Transport in Skeletal Muscle—Role and Regulation of the Monocarboxylate Transporter. J. Physiol. 1999, 517, 633–642. [Google Scholar] [CrossRef]
- Fagnété, S.; Philippe, C.; Olivier, H.; Mona, M.-H.; Maryse, E.-J.; Marie-Dominique, H.-D. Faster Lactate Transport across Red Blood Cell Membrane in Sickle Cell Trait Carriers. J. Appl. Physiol. 2006, 100, 427–432. [Google Scholar] [CrossRef]
- Skelton, M.; Kremer, D.; Smith, E.; Gladden, L. Lactate Influx into Red Blood Cells from Trained and Untrained Human Subjects. Med. Sci. Sports Exerc. 1998, 30, 536–542. [Google Scholar] [CrossRef]
- Ide, K.; Secher, N.H. Cerebral Blood Flow and Metabolism during Exercise. Prog. Neurobiol. 2000, 61, 397–414. [Google Scholar] [CrossRef]
- Miller, B.F.; Fattor, J.A.; Jacobs, K.A.; Horning, M.A.; Navazio, F.; Lindinger, M.I.; Brooks, G.A. Lactate and Glucose Interactions during Rest and Exercise in Men: Effect of Exogenous Lactate Infusion. J. Physiol. 2002, 544, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Mammalian Fuel Utilization during Sustained Exercise. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 1998, 120, 89–107. [Google Scholar] [CrossRef]
- Passarella, S.; Paventi, G.; Pizzuto, R. The Mitochondrial L-Lactate Dehydrogenase Affair. Front. Neurosci. 2014, 8, 407. [Google Scholar] [CrossRef]
- Figley, C.R. Lactate Transport and Metabolism in the Human Brain: Implications for the Astrocyte-Neuron Lactate Shuttle Hypothesis. J. Neurosci. 2011, 31, 4768–4770. [Google Scholar] [CrossRef] [PubMed]
- Pellerin, L.; Pellegri, G.; Bittar, P.G.; Charnay, Y.; Bouras, C.; Martin, J.-L.; Stella, N.; Magistretti, P.J. Evidence Supporting the Existence of an Activity-Dependent Astrocyte-Neuron Lactate Shuttle. Dev. Neurosci. 1998, 20, 291–299. [Google Scholar] [CrossRef]
- Chih, C.-P.; Lipton, P.; Roberts, E.L. Do Active Cerebral Neurons Really Use Lactate rather than Glucose? Trends Neurosci. 2001, 24, 573–578. [Google Scholar] [CrossRef]
- Cumming, D.C.; Brunsting, L.A.; Strich, G.I.; Ries, A.L.; Rebar, R.W. Reproductive Hormone Increases in Response to Acute Exercise in Men. Med. Sci. Sports Exerc. 1986, 18, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Dessypris, A.; Kuoppasalmi, K.; Adlercreutz, H. Plasma Cortisol, Testosterone, Androstenedione and Luteinizing Hormone (LH) in a Non-Competitive Marathon Run. J. Steroid Biochem. 1976, 7, 33–37. [Google Scholar] [CrossRef]
- Gawel, M.J.; Park, D.M.; Alaghband-Zadeh, J.; Rose, F.C. Exercise and Hormonal Secretion. Postgrad. Med. J. 1979, 55, 373–376. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lu, S.; Lau, C.; Tung, Y.; Huang, S.; Chen, Y.; Shih, H.; Tsai, S.; Lu, C.; Wang, S.; Chen, J.; et al. Lactate and the Effects of Exercise on Testosterone Secretion: Evidence for the Involvement of a CAMPmediated Mechanism. Med. Sci. Sports Exerc. 1997, 29, 1048–1054. [Google Scholar] [CrossRef]
- Lin, H.; Wang, S.-W.; Wang, R.-Y.; Wang, P.S. Stimulatory Effect of Lactate on Testosterone Production by Rat Leydig Cells. J. Cell. Biochem. 2001, 83, 147–154. [Google Scholar] [CrossRef]
- Wang, X.; Proud, C.G. The MTOR Pathway in the Control of Protein Synthesis. Physiology 2006, 21, 362–369. [Google Scholar] [CrossRef]
- White, J.P.; Gao, S.; Puppa, M.J.; Sato, S.; Welle, S.L.; Carson, J.A. Testosterone Regulation of Akt/MTORC1/FoxO3a Signaling in Skeletal Muscle. Mol. Cell. Endocrinol. 2013, 365, 174–186. [Google Scholar] [CrossRef]
- Morton, R.W.; Sato, K.; Gallaugher, M.P.B.; Oikawa, S.Y.; McNicholas, P.D.; Fujita, S.; Phillips, S.M. Muscle Androgen Receptor Content but Not Systemic Hormones Is Associated with Resistance Training-Induced Skeletal Muscle Hypertrophy in Healthy, Young Men. Front. Physiol. 2018, 9, 1373. [Google Scholar] [CrossRef] [PubMed]
- Spiering, B.A.; Kraemer, W.J.; Anderson, J.M.; Armstrong, L.E.; Nindl, B.C.; Volek, J.S.; Judelson, D.A.; Joseph, M.; Vingren, J.L.; Hatfield, D.L.; et al. Effects of Elevated Circulating Hormones on Resistance Exercise-Induced Akt Signaling. Med. Sci. Sports Exerc. 2008, 40, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.D.; Kujbida, G.W.; Moore, D.R.; Atherton, P.; Burd, N.A.; Padzik, J.P.; De Lisio, M.; Tang, J.E.; Parise, G.; Rennie, M.J.; et al. Resistance Exercise-Induced Increases in Putative Anabolic Hormones Do Not Enhance Muscle Protein Synthesis or Intracellular Signalling in Young Men. J. Physiol. 2009, 587, 5239–5247. [Google Scholar] [CrossRef] [PubMed]
- West, D.W.D.; Phillips, S.M. Associations of Exercise-Induced Hormone Profiles and Gains in Strength and Hypertrophy in a Large Cohort after Weight Training. Eur. J. Appl. Physiol. 2011, 112, 2693–2702. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, S.B.; Tarnopolsky, M.A.; Grant, E.J.; Correia, C.E.; Phillips, S.M. Hypertrophy with Unilateral Resistance Exercise Occurs without Increases in Endogenous Anabolic Hormone Concentration. Eur. J. Appl. Physiol. 2006, 98, 546–555. [Google Scholar] [CrossRef] [PubMed]
- Basaria, S.; Travison, T.G.; Alford, D.; Knapp, P.E.; Teeter, K.; Cahalan, C.; Eder, R.; Lakshman, K.; Bachman, E.; Mensing, G.; et al. Effects of Testosterone Replacement in Men with Opioid-Induced Androgen Deficiency. Pain 2015, 156, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Bhasin, S.; Storer, T.W.; Berman, N.; Yarasheski, K.E.; Clevenger, B.; Phillips, J.; Lee, W.P.; Bunnell, T.J.; Casaburi, R. Testosterone Replacement Increases Fat-Free Mass and Muscle Size in Hypogonadal Men*. J. Clin. Endocrinol. Metab. 1997, 82, 407–413. [Google Scholar] [CrossRef]
- Brodsky, I.G.; Balagopal, P.; Nair, K.S. Effects of Testosterone Replacement on Muscle Mass and Muscle Protein Synthesis in Hypogonadal Men—A Clinical Research Center Study. J. Clin. Endocrinol. Metab. 1996, 81, 3469–3475. [Google Scholar]
- Sullivan, D.H.; Roberson, P.K.; Johnson, L.E.; Bishara, O.; Evans, W.J.; Smith, E.S.; Price, J.A. Effects of Muscle Strength Training and Testosterone in Frail Elderly Males. Med. Sci. Sports Exerc. 2005, 37, 1664–1672. [Google Scholar] [CrossRef]
- Urban, R.J.; Bodenburg, Y.H.; Gilkison, C.; Foxworth, J.; Coggan, A.R.; Wolfe, R.R.; Ferrando, A. Testosterone Administration to Elderly Men Increases Skeletal Muscle Strength and Protein Synthesis. Am. J. Physiol.-Endocrinol. Metab. 1995, 269, E820–E826. [Google Scholar] [CrossRef]
- Wang, C.; Swerdloff, R.S.; Iranmanesh, A.; Dobs, A.; Snyder, P.J.; Cunningham, G.; Matsumoto, A.M.; Weber, T.; Berman the Testosterone Gel Study Group, N. Transdermal Testosterone Gel Improves Sexual Function, Mood, Muscle Strength, and Body Composition Parameters in Hypogonadal Men1. J. Clin. Endocrinol. Metab. 2000, 85, 2839–2853. [Google Scholar] [CrossRef] [PubMed]
- Peschansky, V.J.; Wahlestedt, C. Noncoding RNAs as Direct and Indirect Modulators of Epigenetic Regulation. Epigenetics 2014, 9, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Jacques, M.; Hiam, D.; Craig, J.; Barrès, R.; Eynon, N.; Voisin, S. Epigenetic Changes in Healthy Human Skeletal Muscle Following Exercise—A Systematic Review. Epigenetics 2019, 14, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Lacal, I.; Ventura, R. Epigenetic Inheritance: Concepts, Mechanisms and Perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar] [CrossRef]
- Pathak, R.; Singh, P.; Ananthakrishnan, S.; Adamczyk, S.; Schimmel, O.; Govind, C.K. Acetylation-Dependent Recruitment of the FACT Complex and Its Role in Regulating Pol II Occupancy Genome-Wide in Saccharomyces cerevisiae. Genetics 2018, 209, 743–756. [Google Scholar] [CrossRef]
- Bannister, A.J.; Kouzarides, T. Regulation of Chromatin by Histone Modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef]
- Gujral, P.; Mahajan, V.; Lissaman, A.C.; Ponnampalam, A.P. Histone Acetylation and the Role of Histone Deacetylases in Normal Cyclic Endometrium. Reprod. Biol. Endocrinol. 2020, 18, 84. [Google Scholar] [CrossRef]
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone Deacetylases and Mechanisms of Regulation of Gene Expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef]
- Latham, T.; Mackay, L.; Sproul, D.; Karim, M.; Culley, J.; Harrison, D.J.; Hayward, L.; Langridge-Smith, P.; Gilbert, N.; Ramsahoye, B.H. Lactate, a Product of Glycolytic Metabolism, Inhibits Histone Deacetylase Activity and Promotes Changes in Gene Expression. Nucleic Acids Res. 2012, 40, 4794–4803. [Google Scholar] [CrossRef]
- Jacobs, I.; Tesch, P.A.; Bar-Or, O.; Karlsson, J.; Dotan, R. Lactate in Human Skeletal Muscle after 10 and 30 S of Supramaximal Exercise. J. Appl. Physiol. 1983, 55, 365–367. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Walder, K.R. Exercise and the Skeletal Muscle Epigenome. Cold Spring Harb. Perspect. Med. 2017, 7, a029876. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Tang, Z.; Huang, H.; Zhou, G.; Cui, C.; Weng, Y.; Liu, W.; Kim, S.; Lee, S.; Perez-Neut, M.; et al. Metabolic Regulation of Gene Expression by Histone Lactylation. Nature 2019, 574, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Ando, K.; Ito, T.; Suda, Y.; Matsui, Y.; Oyama, A.; Kaneko, H.; Yokoyama, S.; Egawa, T.; Goto, K. Lactate Stimulates a Potential for Hypertrophy and Regeneration of Mouse Skeletal Muscle. Nutrients 2019, 11, 869. [Google Scholar] [CrossRef]
- Oishi, Y.; Tsukamoto, H.; Yokokawa, T.; Hirotsu, K.; Shimazu, M.; Uchida, K.; Tomi, H.; Higashida, K.; Iwanaka, N.; Hashimoto, T. Mixed Lactate and Caffeine Compound Increases Satellite Cell Activity and Anabolic Signals for Muscle Hypertrophy. J. Appl. Physiol. 2015, 118, 742–749. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Wang, Y.X.; Rudnicki, M.A. Building Muscle: Molecular Regulation of Myogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008342. [Google Scholar] [CrossRef]
- Friedrichs, M.; Wirsdöerfer, F.; Flohé, S.B.; Schneider, S.; Wuelling, M.; Vortkamp, A. BMP Signaling Balances Proliferation and Differentiation of Muscle Satellite Cell Descendants. BMC Cell Biol. 2011, 12, 26. [Google Scholar] [CrossRef]
- Le Grand, F.; Rudnicki, M.A. Skeletal Muscle Satellite Cells and Adult Myogenesis. Curr. Opin. Cell Biol. 2007, 19, 628–633. [Google Scholar] [CrossRef]
- Yun, K.; Wold, B. Skeletal Muscle Determination and Differentiation: Story of a Core Regulatory Network and Its Context. Curr. Opin. Cell Biol. 1996, 8, 877–889. [Google Scholar] [CrossRef]
- Seale, P.; Sabourin, L.A.; Girgis-Gabardo, A.; Mansouri, A.; Gruss, P.; Rudnicki, M.A. Pax7 Is Required for the Specification of Myogenic Satellite Cells. Cell 2000, 102, 777–786. [Google Scholar] [CrossRef]
- Olguin, H.C.; Olwin, B.B. Pax-7 Up-Regulation Inhibits Myogenesis and Cell Cycle Progression in Satellite Cells: A Potential Mechanism for Self-Renewal. Dev. Biol. 2004, 275, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Taylor, N.A.S.; Wilkinson, J.G. Exercise-Induced Skeletal Muscle Growth. Sports Med. 1986, 3, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Murach, K.A.; Englund, D.A.; Dupont-Versteegden, E.E.; McCarthy, J.J.; Peterson, C.A. Myonuclear Domain Flexibility Challenges Rigid Assumptions on Satellite Cell Contribution to Skeletal Muscle Fiber Hypertrophy. Front. Physiol. 2018, 9, 635. [Google Scholar] [CrossRef] [PubMed]
- Mauro, A. Satellite Cell Of Skeletal Muscle Fibers. J. Biophys. Biochem. Cytol. 1961, 9, 493–495. [Google Scholar] [CrossRef]
- McLoon, L.K.; Rowe, J.; Wirtschafter, J.; McCormick, K.M. Continuous Myofiber Remodeling in Uninjured Extraocular Myofibers: Myonuclear Turnover and Evidence for Apoptosis. Muscle Nerve 2004, 29, 707–715. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Shibasaki, A.; Naka, A.; Saito, H.; Iida, K. Lactate Promotes Myoblast Differentiation and Myotube Hypertrophy via a Pathway Involving MyoD in Vitro and Enhances Muscle Regeneration in Vivo. Int. J. Mol. Sci. 2018, 19, 3649. [Google Scholar] [CrossRef]
- Willkomm, L.; Schubert, S.; Jung, R.; Elsen, M.; Borde, J.; Gehlert, S.; Suhr, F.; Bloch, W. Lactate Regulates Myogenesis in C2C12 Myoblasts in Vitro. Stem Cell Res. 2014, 12, 742–753. [Google Scholar] [CrossRef]
- Willkomm, L.; Gehlert, S.; Jacko, D.; Schiffer, T.; Bloch, W. P38 MAPK Activation and H3K4 Trimethylation Is Decreased by Lactate in Vitro and High Intensity Resistance Training in Human Skeletal Muscle. PLoS ONE 2017, 12, e0176609. [Google Scholar] [CrossRef] [PubMed]
- Hiroki, E.; Abe, S.; Iwanuma, O.; Sakiyama, K.; Yanagisawa, N.; Shiozaki, K.; Ide, Y. A Comparative Study of Myostatin, Follistatin and Decorin Expression in Muscle of Different Origin. Anat. Sci. Int. 2011, 86, 151–159. [Google Scholar] [CrossRef]
- Baar, K.; Blough, E.; Dineen, B.; Esser, K. 11 Transcriptional Regulation in Response to Exercise. Exerc. Sport Sci. Rev. 1999, 27, 333–380. [Google Scholar] [CrossRef]
- Gordon, B.S.; Kelleher, A.R.; Kimball, S.R. Regulation of Muscle Protein Synthesis and the Effects of Catabolic States. Int. J. Biochem. Cell Biol. 2013, 45, 2147–2157. [Google Scholar] [CrossRef]
- Tang, J.E.; Phillips, S.M. Maximizing Muscle Protein Anabolism: The Role of Protein Quality. Curr. Opin. Clin. Nutr. Metab. Care 2009, 12, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Cerda-Kohler, H.; Henríquez-Olguín, C.; Casas, M.; Jensen, T.E.; Llanos, P.; Jaimovich, E. Lactate Administration Activates the ERK1/2, MTORC1, and AMPK Pathways Differentially according to Skeletal Muscle Type in Mouse. Physiol. Rep. 2018, 6, e13800. [Google Scholar] [CrossRef] [PubMed]
- Kyun, S.; Yoo, C.; Park, H.-Y.; Kim, J.; Lim, K. The Effects of Exogenous Lactate Administration on the IGF1/Akt/MTOR Pathway in Rat Skeletal Muscle. Int. J. Environ. Res. Public Health 2020, 17, 7805. [Google Scholar] [CrossRef]
- Goldspink, D.F.; Garlick, P.J.; McNurlan, M.A. Protein Turnover Measured in Vivo and in Vitro in Muscles Undergoing Compensatory Growth and Subsequent Denervation Atrophy. Biochem. J. 1983, 210, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Johnson, S.E. ERK2 Is Required for Efficient Terminal Differentiation of Skeletal Myoblasts. Biochem. Biophys. Res. Commun. 2006, 345, 1425–1433. [Google Scholar] [CrossRef]
- Li, G.; Wang, H.; Wang, L.; Chen, R.; Liu, J. Distinct Pathways of ERK1/2 Activation by Hydroxy-Carboxylic Acid Receptor-1. PLoS ONE 2014, 9, e93041. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sakuma, K.; Yamaguchi, A. The Functional Role of Calcineurin in Hypertrophy, Regeneration, and Disorders of Skeletal Muscle. J. Biomed. Biotechnol. 2010, 2010, 721219. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.K.; Levin, J.B.; Hamilton, A.M.; Borodinsky, L.N. Calcium Signaling in Skeletal Muscle Development, Maintenance and Regeneration. Cell Calcium 2016, 59, 91–97. [Google Scholar] [CrossRef]
- Kadi, F.; Thornell, L.-E. Concomitant Increases in Myonuclear and Satellite Cell Content in Female Trapezius Muscle following Strength Training. Histochem. Cell Biol. 2000, 113, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Petrella, J.K.; Kim, J.; Cross, J.M.; Kosek, D.J.; Bamman, M.M. Efficacy of Myonuclear Addition May Explain Differential Myofiber Growth among Resistance-Trained Young and Older Men and Women. Am. J. Physiol.-Endocrinol. Metab. 2006, 291, E937–E946. [Google Scholar] [CrossRef] [PubMed]
- Gehlert, S.; Suhr, F.; Gutsche, K.; Willkomm, L.; Kern, J.; Jacko, D.; Knicker, A.; Schiffer, T.; Wackerhage, H.; Bloch, W. High Force Development Augments Skeletal Muscle Signalling in Resistance Exercise Modes Equalized for Time under Tension. Pflügers Arch.-Eur. J. Physiol. 2014, 467, 1343–1356. [Google Scholar] [CrossRef]
- Duncan, G.E.; Howley, E.T.; Johnson, B.N. Applicability of VO2max Criteria: Discontinuous versus Continuous Protocols. Med. Sci. Sports Exerc. 1997, 29, 273–278. [Google Scholar] [CrossRef]
- Menzies, P.; Menzies, C.; McIntyre, L.; Paterson, P.; Wilson, J.; Kemi, O.J. Blood Lactate Clearance during Active Recovery after an Intense Running Bout Depends on the Intensity of the Active Recovery. J. Sports Sci. 2010, 28, 975–982. [Google Scholar] [CrossRef]
- Shirai, T.; Kitaoka, Y.; Uemichi, K.; Tokinoya, K.; Takeda, K.; Takemasa, T. Effects of Lactate Administration on Hypertrophy and MTOR Signaling Activation in Mouse Skeletal Muscle. Physiol. Rep. 2022, 10, 1–14. [Google Scholar] [CrossRef]
- Khodabukus, A.; Madden, L.; Prabhu, N.K.; Koves, T.R.; Jackman, C.P.; Muoio, D.M.; Bursac, N. Electrical Stimulation Increases Hypertrophy and Metabolic Flux in Tissue-Engineered Human Skeletal Muscle. Biomaterials 2019, 198, 259–269. [Google Scholar] [CrossRef]
- Roberts, M.D.; Mobley, C.B.; Vann, C.G.; Haun, C.T.; Schoenfeld, B.J.; Young, K.C.; Kavazis, A.N. Synergist Ablation-Induced Hypertrophy Occurs More Rapidly in the Plantaris than Soleus Muscle in Rats due to Different Molecular Mechanisms. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2020, 318, R360–R368. [Google Scholar] [CrossRef]
- Jones, N.C.; Tyner, K.J.; Nibarger, L.; Stanley, H.M.; Cornelison, D.D.W.; Fedorov, Y.V.; Olwin, B.B. The P38α/β MAPK Functions as a Molecular Switch to Activate the Quiescent Satellite Cell. J. Cell Biol. 2005, 169, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Ohno, Y.; Oyama, A.; Kaneko, H.; Egawa, T.; Yokoyama, S.; Sugiura, T.; Ohira, Y.; Yoshioka, T.; Goto, K. Lactate Increases Myotube Diameter via Activation of MEK/ERK Pathway in C2C12 Cells. Acta Physiol. 2018, 223, e13042. [Google Scholar] [CrossRef]
- Shirai, T.; Uemichi, K.; Hidaka, Y.; Kitaoka, Y.; Takemasa, T. Effect of Lactate Administration on Mouse Skeletal Muscle under Calorie Restriction. Curr. Res. Physiol. 2021, 4, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Boroujerdi, S.; Rahimi, R. Acute GH and IGF-I Responses to Short vs. Long Rest Period between Sets during Forced Repetitions Resistance Training System. S. Afr. J. Res. Sport Phys. Educ. Recreat. 2008, 30, 31–38. [Google Scholar] [CrossRef][Green Version]
- Weakley, J.J.S.; Till, K.; Read, D.B.; Roe, G.A.B.; Darrall-Jones, J.; Phibbs, P.J.; Jones, B. The Effects of Traditional, Superset, and Tri-Set Resistance Training Structures on Perceived Intensity and Physiological Responses. Eur. J. Appl. Physiol. 2017, 117, 1877–1889. [Google Scholar] [CrossRef]
- Couto, B.; Silva, H.; Filho, A.; da Silveira Neves, S.; Ramos, M.; Szmuchrowski, L.; Barbosa, M. Acute Effects of Resistance Training with Local Vibration. Int. J. Sports Med. 2013, 34, 814–819. [Google Scholar] [CrossRef]
- Gentil, P.; Oliveira, E.; Bottaro, M. Time under Tension and Blood Lactate Response during Four Different Resistance Training Methods. J. Physiol. Anthropol. 2006, 25, 339–344. [Google Scholar] [CrossRef]
- Lacerda, L.T.; Martins-Costa, H.C.; Diniz, R.C.R.; Lima, F.V.; Andrade, A.G.P.; Tourino, F.D.; Bemben, M.G.; Chagas, M.H. Variations in Repetition Duration and Repetition Numbers Influence Muscular Activation and Blood Lactate Response in Protocols Equalized by Time under Tension. J. Strength Cond. Res. 2016, 30, 251–258. [Google Scholar] [CrossRef]
- Vargas-Molina, S.; Martín-Rivera, F.; Bonilla, D.A.; Petro, J.L.; Carbone, L.; Romance, R.; deDiego, M.; Schoenfeld, B.J.; Benítez-Porres, J. Comparison of Blood Lactate and Perceived Exertion Responses in Two Matched Time-Under-Tension Protocols. PLoS ONE 2020, 15, e0227640. [Google Scholar] [CrossRef]
- Haddock, B.L.; Wilkin, L.D. Resistance Training Volume and Post Exercise Energy Expenditure. Int. J. Sports Med. 2006, 27, 143–148. [Google Scholar] [CrossRef]
- Ojasto, T.; Häkkinen, K. Effects of Different Accentuated Eccentric Loads on Acute Neuromuscular, Growth Hormone, and Blood Lactate Responses during a Hypertrophic Protocol. J. Strength Cond. Res. 2009, 23, 946–953. [Google Scholar] [CrossRef]
- Kelleher, A.R.; Hackney, K.J.; Fairchild, T.J.; Keslacy, S.; Ploutz-Snyder, L.L. The Metabolic Costs of Reciprocal Supersets vs. Traditional Resistance Exercise in Young Recreationally Active Adults. J. Strength Cond. Res. 2010, 24, 1043–1051. [Google Scholar] [CrossRef]
- Sánchez-Medina, L.; González-Badillo, J.J. Velocity Loss as an Indicator of Neuromuscular Fatigue during Resistance Training. Med. Sci. Sports Exerc. 2011, 43, 1725–1734. [Google Scholar] [CrossRef]
- Paoli, A.; Moro, T.; Marcolin, G.; Neri, M.; Bianco, A.; Palma, A.; Grimaldi, K. High-Intensity Interval Resistance Training (HIRT) influences resting energy expenditure and respiratory ratio in non-dieting individuals. J. Transl. Med. 2012, 10, 237. [Google Scholar] [CrossRef] [PubMed]
- Suginohara, T.; Wakabayashi, K.; Ato, S.; Ogasawara, R. Effect of 2-Deoxyglucose-Mediated Inhibition of Glycolysis on the Regulation of MTOR Signaling and Protein Synthesis before and after High-Intensity Muscle Contraction. Metabolism 2021, 114, 154419. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. Postexercise Hypertrophic Adaptations. J. Strength Cond. Res. 2013, 27, 1720–1730. [Google Scholar] [CrossRef] [PubMed]
- Schoenfeld, B.J. Potential Mechanisms for a Role of Metabolic Stress in Hypertrophic Adaptations to Resistance Training. Sports Med. 2013, 43, 179–194. [Google Scholar] [CrossRef] [PubMed]
- van Gemert, L.A.; de Galan, B.E.; Wevers, R.A.; ter Heine, R.; Willemsen, M.A. Lactate Infusion as Therapeutical Intervention: A Scoping Review. Eur. J. Pediatrics 2022, 181, 2227–2235. [Google Scholar] [CrossRef]

| Study | Variable | Results | In Vitro or In Vivo |
|---|---|---|---|
| Wilkomm et al. (2014) [122] | Casp-3 | ↑ @ 20 mmol @ Days 1 & 3 | in vitro |
| Ki67 | ↓ @ 10 & 20 mmol | ||
| Pax7 | ↓ on Day 1, → Day 3, ↑ Day 5 @ 10 & 20 mmol | ||
| Myf5 | ↓ on Day 1, → Day 3, ↑ Day 5 @ 10 & 20 mmol | ||
| Myogenin | ↑ quickest in control, 10 mm reached same value by Day 5, 20 mmol reached same value at Day 10 | ||
| Oishi et al. (2015) [110] | mTOR | ↑ @ 10 mmol | in vitro |
| p70S6K | ↑ @ 10 mmol | ||
| Fst | ↑ @ 10 mmol | ||
| Mstn | ↓ @ 10 mmol | ||
| Myofibrillar protein content | → | in vivo | |
| Akt | → | ||
| Myogenin | ↑ (LC; TA/GA) | ||
| Wilkomm et al. (2017) [123] | p38 | ↓ @ 20 mmol | in vitro |
| H3k4me3 | ↓ @ 20 mmol | ||
| Myf5 | ↓ @ 20 mmol | ||
| Myogenin | ↓ @ 20 mmol | ||
| MHC 1 | ↓ @ 20 mmol | ||
| pp38 MAPK | ↓ @ 30 min, ↑ @ 1, 4, 24-h. post | in vivo | |
| H3k4me3 | → or ↓ @ 4 h. | ||
| Tsukomoto et al., 2018 [121] | Myf5 | → @ 10 mmol | in vitro |
| Myogenin | → @ 10 mmol | ||
| MyoD | ↑ @ Days 3 & 5 | ||
| Myf4 | ↑ @ Days 3 & 5 | ||
| p70S6K | → | ||
| Myh1 | ↑ @ ≥ 8 mmol | ||
| Myh4 | ↑ @ ≥ 8 mmol | In vivo | |
| MyoD | → (trend towards increase) | ||
| Myogenin | → | ||
| Ohno et al. (2018) [144] | GPR81 | ↑ expression @ mRNA & protein levels in myotubes and myoblasts with the highest levels in myotubes. | in vitro |
| Myotube diameter | ↑ @ 20 mmol | ||
| MEK1/2 | ↑ @ 20 mmol | ||
| p-ERK1/2 | ↑ @ 20 mmol | ||
| p-p90RSK | ↑ @ 20 mmol | ||
| p-Akt | → @ 20 mmol | ||
| p-mTOR | → @ 20 mmol | ||
| p-p70S6K | → @ 20 mmol | ||
| p-FoxO3a | → @ 20 mmol | ||
| p-ULKI | → @ 20 mmol | ||
| LC3B-II | → @ 20 mmol | ||
| Cerda-Kohler (2018) [128] | Lactate | ↑ in lactate vs. control | in vivo |
| Blood glucose | → in lactate vs. control | ||
| Insulin | → in lactate vs. control | ||
| ERK1/2 | ↑ @ 40 min in lactate for quadricep, → EDL or Soleus | ||
| IGF-1 | → | ||
| Akt | ↑ @ 40 min in lactate for quadricep & EDL | ||
| p70S6K | ↑ in quadricep only | ||
| S6 | ↑ for quadricep & EDL, → Soleus | ||
| AMPK | → in quadriceps or EDL, ↑ in Soleus | ||
| ACC phosphorylation | ↑ in Soleus | ||
| TBC1D1 | → for all muscle groups | ||
| TBC1D4 | ↑ for Soleus, → quadriceps & EDL | ||
| PDH-E1α | ↓ in Soleus, → or ↓ in EDL, → or ↑ in EDL | ||
| Ohno et al. (2019) [109] | Bodyweight | → (oral administration) | in vivo |
| TA muscle weight | ↑ (oral administration) | ||
| Fiber CSA | ↑ (oral administration) | ||
| Pax7-positive nuclei | ↑ 200% and 138% at Weeks 1 & 2 (oral administration) | ||
| Bodyweight (CTX) | → CTX-injected groups | ||
| TA muscle weight (CTX) | ↑ in LX (24% absolute & 29% relative) | ||
| Fiber CSA (CTX) | ↑ 44% at 2 weeks in LX vs. 1 week | ||
| Pax7-positive nuclei (CTX) | ↑ (0.08/myofiber) vs. control (0.02/myofiber) | ||
| ↑ in LX vs. CX | |||
| Myotube | ↑ in diameter, length, and myonuclei @ 20 mmol for 5 days | in vitro | |
| Kyun et al. 2020 [129] | Blood lactate | ↑ | in vivo |
| Insulin | ↓ | ||
| IGF1 | ↓ | ||
| Akt mRNA | ↑ | ||
| mTOR mRNA | ↑ | ||
| IGF receptor mRNA | ↑ | ||
| phosphorylation of Akt | ↑ | ||
| phosphorylation of mTOR | ↑ | ||
| Atrogin-1 | → | ||
| MuRF1 | → | ||
| Shirai et al., 2021 [145] | Myofiber CSA | ↓ vs. control, ↑ with CR + lactate vs. CR alone | in vivo |
| Bodyweight | ↓ vs. control, → with CR + lactate vs. a ↓ CR alone | ||
| Food intake | ↓ vs. control, same between both CR groups. | ||
| Akt | ↓ vs. control | ||
| mTOR | ↓ vs. control | ||
| p70S6K | ↓ vs. control, ↑ vs. CR. | ||
| 4EBP1 | ↓ vs. control | ||
| S6 | ↓ vs. control, ↑ vs. CR. | ||
| MAFbx | → between groups | ||
| MuRF1 | → between groups | ||
| LC3-II/LC3-I | ↑ in CR, but not in CR + Lactate | ||
| p62 | ↓ in CR, but not in CR + Lactate | ||
| Ubiquitinated protein level | ↓ in CR, but not in CR + Lactate | ||
| AMPK | → between groups | ||
| PGC-1α | ↑ in CR + Lactate & CR | ||
| UQCRC2 | ↑ in CR + Lactate & CR | ||
| MTCO1 | ↑ in CR + Lactate & CR | ||
| ATP5A | ↑ in CR + Lactate & CR | ||
| NDUB | ↑ in CR + Lactate & CR | ||
| SDHB | ↑ in CR + Lactate & CR | ||
| CS activity | ↑ in CR + Lactate vs. CR | ||
| Enzyme activity | ↑ in CR + Lactate vs. CR | ||
| Shirai et al., 2022 [140] | Plantaris weight | → between Lactate-OL and PBS-OL | in vivo |
| Plantaris CSA | → between Lactate-OL and PBS-OL | ||
| p-p70S6K (Thr389) | → between Lactate-OL and PBS-OL | ||
| total-p70S6K | → between Lactate-OL and PBS-OL | ||
| p-S6 | → between Lactate-OL and PBS-OL | ||
| total-S6 | → between Lactate-OL and PBS-OL | ||
| p-4EBP1 (Thr37/46) | → between Lactate-OL and PBS-OL | ||
| total-4EBP1 | → between Lactate-OL and PBS-OL | ||
| p-p70S6K (Thr389) | → between Lactate-ES and PBS-ES | ||
| total-p70S6K | → between Lactate-ES and PBS-ES | ||
| p-S6 | → between Lactate-ES and PBS-ES | ||
| total-S6 | → between Lactate-ES and PBS-ES | ||
| p-4EBP1 (Thr37/46) | → between Lactate-ES and PBS-ES | ||
| total-4EBP1 | → between Lactate-ES and PBS-ES | ||
| p-ERK1/2 (Thr202/Try204) | → between Lactate-ES and PBS-ES | ||
| total-ERK1/2 | → between Lactate-ES and PBS-ES | ||
| p-p38 (Thr180/Try182) | → between Lactate-ES and PBS-ES | ||
| total-p38 | → between Lactate-ES and PBS-ES | ||
| Puromycin | → between Lactate-ES and PBS-ES |
| Study | Training Protocol | Bla- (mmol/L) | Measurement Site | Exercise(s) |
|---|---|---|---|---|
| Haddock & Wilkin (2006) [152] | 3 sets of nine exercises @ 8 RM to volitional fatigue | 10.2 | Fingertip | Bench press, lateral pull down, leg curl, overhead press, knee extension, biceps curl, triceps pull down, and abdominal crunch. |
| Boroujerdi & Rahimi et al. (2008) [146] | 4 sets × 10RM + 15% additional load with 1-min rest period * 3–4 forced reps were completed for both protocols once subjects could not complete a rep on their own. | 14.5 | Antecubital vein | Bench Press and Half Squat |
| Ojasto & Häkkinen (2009) [153] | 4 sets × 10 repetitions @ 70% of 1RM for the eccentric phase and 70% of 1RM for concentric phase with 2-min rest period | 10.23 | NA | Bench Press |
| 4 sets × 10 repetitions @ 80% of 1RM for the eccentric phase and 70% of 1RM for concentric phase with 2-min rest period | 10.99 | NA | Bench Press | |
| 4 sets × 10 repetitions @ 90% of 1RM for the eccentric phase and 70% of 1RM for concentric phase with 2-min rest period | 12.12 | NA | Bench Press | |
| 4 sets × 10 repetitions @ 100% of 1RM for the eccentric phase and 70% of 1RM for concentric phase with 2-min rest period | 11.77 | NA | Bench Press | |
| Kelleher et al. (2010) [154] | 4 supersets × 10 reps @ 70% to volitional failure with 60-s rest periods. * agonist-antagonist supersets | 10.79 | Fingertip | Bench Press, bent-over row, biceps curl, lying triceps extension, leg extension, and leg curl |
| Sánchez-Medina & González-Badillo (2011) [155] | 3 sets × 10 reps @ RIR 2 with 5-min rest periods | 10.6 | Fingertip | Back Squat |
| 3 sets × 12 reps @ RIR 0 with 5-min rest periods | 12.4 | Fingertip | Back Squat | |
| 3 sets × 10 reps @ RIR 0 with 5-min rest periods | 11.7 | Fingertip | Back Squat | |
| 3 sets × 8 reps @ RIR 0 with 5-min rest periods | 10.4 | Fingertip | Back Squat | |
| 3 sets × 6 reps @ RIR 0 with 5-min rest periods | 10 | Fingertip | Back Squat | |
| Paoli et al. (2012) [156] | 2–3 sets @ 6RM w/2–3 additional reps with 20-s rest period between exercises and 2:30 min between rounds. | 10.5 | Earlobe | Leg press, bench press, dorsal machine |
| Couto et al. (2013) [148] | 4 sets to failure @ 55% of 1RM with 2-min rest periods | 14.76 | Ulnar vein | Lat Pulldown |
| 4 sets to failure @ 55% of 1RM with 2-min rest periods * Local vibration applied to cable | 16.92 | Ulnar vein | Lat Pulldown | |
| Gorostiaga et al. (2014) [48] | 5 sets × 10 reps @ 10RM with 2-min rest periods | 10.3 | Earlobe | Leg Press |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lawson, D.; Vann, C.; Schoenfeld, B.J.; Haun, C. Beyond Mechanical Tension: A Review of Resistance Exercise-Induced Lactate Responses & Muscle Hypertrophy. J. Funct. Morphol. Kinesiol. 2022, 7, 81. https://doi.org/10.3390/jfmk7040081
Lawson D, Vann C, Schoenfeld BJ, Haun C. Beyond Mechanical Tension: A Review of Resistance Exercise-Induced Lactate Responses & Muscle Hypertrophy. Journal of Functional Morphology and Kinesiology. 2022; 7(4):81. https://doi.org/10.3390/jfmk7040081
Chicago/Turabian StyleLawson, Daniel, Christopher Vann, Brad J. Schoenfeld, and Cody Haun. 2022. "Beyond Mechanical Tension: A Review of Resistance Exercise-Induced Lactate Responses & Muscle Hypertrophy" Journal of Functional Morphology and Kinesiology 7, no. 4: 81. https://doi.org/10.3390/jfmk7040081
APA StyleLawson, D., Vann, C., Schoenfeld, B. J., & Haun, C. (2022). Beyond Mechanical Tension: A Review of Resistance Exercise-Induced Lactate Responses & Muscle Hypertrophy. Journal of Functional Morphology and Kinesiology, 7(4), 81. https://doi.org/10.3390/jfmk7040081







