Abstract
Measurement of the adverse outcomes of repeated head trauma in athletes is often achieved using tests where the comparator is ‘accuracy’. While it is expected that ex-athletes would perform worse than controls, previous studies have shown inconsistent results. Here we have attempted to address these inconsistencies from a different perspective by quantifying not only accuracy, but also motor response times. Age-matched control subjects who have never experienced head trauma (n = 20; 41.8 ± 14.4 years) where compared to two cohorts of retired contact sport athletes with a history of head trauma/concussions; one with self-reported concerns (n = 36; 45.4 ± 12.6 years), and another with no ongoing concerns (n = 19; 43.1 ± 13.5 years). Participants performed cognitive (Cogstate) and somatosensory (Cortical Metrics) testing with accuracy and motor times recorded. Transcranial magnetic stimulation (TMS) investigated corticospinal conduction and excitability. Results showed that there was little difference between groups in accuracy scores. Conversely, motor times in all but one test revealed that ex-athletes with self-reported concerns were significantly slower compared to other groups (p ranges 0.031 to <0.001). TMS latency showed significantly increased time (p = 0.008) in the group with ongoing concerns. These findings suggest that incorporating motor times is more informative than considering accuracy scores alone.
1. Introduction
The long-term neurological sequelae of head trauma in retired contact sport athletes are of ongoing global concern and investigation. Research into the cognitive and neuropsychological health of retired athletes has by now been carried out over decades, but studies have not always provided consistent results. In particular, there is disparity with respect to self-reported symptomology or concern, and the results of commonly used objective measurements. This is a problem highlighted by Cunningham et al. [1] in a systematic review of 46 cross-sectional studies of retired athletes with a history of sports related concussion. While almost 80% of studies included ex-athletes with self-reported concerns about their cognitive health, only half to two-thirds of these studies showed any impairment in objective measurement of psychomotor function, executive function, or memory.
Assessments of cognitive function generally rely on a performance ‘outcome’, and these can be binary (such as correct detection of something or not), or they can be continuous or additive (such as the number of errors made during a given test) [2]. However, as illustrated by Cunningham et al. [1], reliance on test outcome measures may not detect subtle impairments, particularly in single testing sessions, that reflect self-reported concerns. This implies that the current objective testing regimes that rely purely on outcome measures are insufficient in measuring subtle cognitive processing abilities [3,4].
De Boek and Jeon [4] argue that while cognitive tests measure overall performance abilities, as determined by the number of correct or incorrect responses, less attention is given to quantifying process abilities, reflected by response time. While it is acceptable to determine performance outcome without knowledge of the processes involved, it is only half of the story. Providing cohorts such as retired contact sports athletes who generally are high functioning but struggle with daily activities, an explanation of not only outcomes, but also the process, allows for increased understanding as well as more informative feedback that may assist in interventional therapies [4].
Response time has long been a consideration in cognitive ability measurements, but the increasing precision in measurement by use of computerised testing has allowed for response time data collection to understand cognitive ability in healthy populations [3]. Consequently, interest in response time has revived, with a number of models being developed for use in psychology (see reviews [3,4]). However, the use of response time data as a reflection of processing ability appears to not be utilised in exploring long-term consequences of repeated head trauma in retired athletes [5]. A small number of studies have previously employed psychomotor reaction time in retired athletes [1]. However, reaction time and response time are two distinct variables with the former describing the speed of detecting the stimulus, while the latter describes a speed-accuracy trade-off for the determination of the correct response to a given stimulus, rather than simply responding to a stimulus [3,4,6].
Previous work on sensorimotor and neurophysiology of individuals with persistent post-concussion symptoms [7], and chronic long-term outcomes in retired athletes [8,9,10], has demonstrated slower sensorimotor reaction time in symptomatic individuals when compared with controls. Following these studies, the aim of this study was to present data on cognitive testing response time in two groups of retired contact sport athletes’: one with ongoing concerns about their cognitive health and the other with no ongoing concerns. Comparisons were made between these two groups to age-matched controls using two different computerised testing applications. The data present both performance outcome and response time data, as well as single pulse transcranial magnetic stimulation (TMS) for quantification of corticospinal conduction time and excitability. It was hypothesised that the cohort with ongoing concerns would perform worse than the both the retired athlete group with no ongoing concerns and controls in both outcome accuracy scores and motor response times. A secondary hypothesis was that increased self-reported fatigue would correlate with corticospinal conduction latency time.
2. Methods
As part of a larger research project, studies reported here were conducted on a convenience sample of 75 male participants (retired contact sport athletes n = 55; age-matched male controls n = 20; Table 1). Participants were pre-screened for TMS suitability [11] and provided written informed consent to participate in the study as approved by the La Trobe University Ethics Committee (HEC18005, 25 February 2018).

Table 1.
Participant demographics (mean ± SD).
The retired playing group were divided into sub-groups based on their self-reported fatigue and related symptoms score [9]: participants with ongoing self-reported concerns regarding their mental and cognitive health relating to their history of head trauma experienced in sport (‘self-concern’: n = 36), and those who acknowledged they had a history of head trauma from sport but did not express any enduring concerns (‘no concern’: n = 19). Athlete participants were excluded if they reported any head/brain trauma outside of their sport (e.g., car accidents, fights, etc.). For the retired athlete groups, the definition of concussion was based on their recollection of having been involved in a collision that results in signs and symptoms of concussion [12] and to further assist with recollection, participants needed to recall that they had been medically restricted from play for one week due to their concussion [13]. Both groups were compared to age-match controls (n = 20) who had no neurological impairment/disease, and no history of head trauma, either by playing contact sports, or trauma from accidents. All data were completed during one visit to the laboratory and cognitive and TMS testing was randomised to reduce any potential serial order effects.
2.1. Symptom Self-Report
All participants completed a questionnaire regarding their concussion injury history [9], and a self-assessment regarding fatigue and related concerns affecting their daily activities [14]. The self-assessment required participants to respond to 15 questions covering a range of concerns including fatigue (general and mental), perception of thinking speed and mental recovery, emotional, irritability and sensitivity changes, and sleep variability, using a Likert rating scale from 0 to 3, in 0.5 increments. Higher scores reflect greater severity for each symptom-related question. The questionnaire has been previously validated by Johansson and colleagues [14,15,16].
2.2. Cognitive Assessment
Participants completed a computerised brief battery (Cogstate, Melbourne, Victoria, Australia) that comprised of a subset of tasks from the full Cogstate battery taking about 8–10 min in total [17]. Prior to data collection, participants were given a five-minute interactive demonstration and familiarisation. Once participants had demonstrated they were aware of the assessment protocol, data collection began.
Participants completed two separate reaction time tests; a simple reaction time ‘detection test’ where the individual was instructed to respond as quickly as possible by pressing a keyboard key as soon as the card was revealed (‘turned up’), and a choice reaction time ‘identification test’ where the participant pressed one of two keys; one representing the ‘yes’ button if the card was revealed red in colour, or another key representing the ‘no’ button if the card was black in colour. For both the detection and identification assessments, if a key was pressed before the card was revealed, this would be recorded as an error, contributing to the accuracy metric. The test was completed when 25 correct responses were recorded or the maximum time (three min) had elapsed [18].
Tests for response times included the One-Back and Visual Learning tasks. The one-back task required the participant to respond to the question “is this card the same as the previous card?” Participants were instructed to press a particular key for a ‘yes’ or an alternative key for a ‘no’ response as soon as possible. Cards (n = 42) were shown, and the correct response was 50% each of the trials presented. The test was completed when all 42 trials were completed or the maximum allowed time of three minutes had passed [18]. The visual learning tasked required the participant to view the card presented in the middle of the screen and respond to the question “have you seen this card before?” Similar to the one-back task, participants were instructed to press a particular key for a ‘yes’ or an alternative key for a ‘no’ response. Participants were required to learn a series of six cards repeated throughout the task, intermixed with eight non-repeating ‘distracter’ cards in series of 14 cards. Three 14-card series were presented, and this task continued until the participant had made 42 complete responses or the maximum time allowed (3 min) had elapsed. The primary outcome measure for this task was the number of correct responses (i.e., true-positive and true-negative) expressed as a proportion of the total trials [18].
2.3. Somatosensory Assessment
As described in previously published studies [19,20,21], somatosensory assessment was undertaken by utilising a portable vibrotactile stimulation device (Brain Gauge, Cortical Metrics, NC, USA). Physically similar to a standard computer mouse, the device contains two cylindrical probes (5 mm diameter) positioned at the top and front of the device. These probes, driven by the computer via a USB cable, provided a light vibration stimulus, at frequencies between 25 and 50 Hz that is sensed by the participant’s index and middle digits of their non-dominant hand [21].
Participants completed the battery involving four discrete tasks, one reaction time and three discriminative tasks (amplitude, duration and temporal order judgement), whereby the participant used their non-dominant hand to detect the stimulus, and their dominant hand to respond via a computer mouse. Testing time took approximately 15 min. For the discrimination tasks in the battery, a simple tracking procedure that utilised a two-alternative forced choice paradigm was used to determine an individual’s difference distinguished threshold for stimulus [20].
Familiarisation was performed before each test for participant orientation, requiring correct responses on three consecutive trials before progressing the test where data would be acquired. Participants were verbally instructed to respond as quickly as possible, and during testing no feedback or knowledge of the results were provided.
2.4. Corticospinal Excitability
Employing previously published methods in similar cohorts [8,9,10], corticospinal excitability was quantified via single-pulse TMS, delivered over the contralateral primary motor cortex. Surface electromyography (sEMG) measured motor evoked potentials (MEPs) recording 500 ms sweeps (100 ms pre-trigger, 400 ms post-trigger; PowerLab 4/35, ADInstruments, Sydney, Australia). Electromyography, adhering to the Non-Invasive Assessment of Muscles (SENIAM) guidelines for sEMG [22], was recorded using bipolar Ag/AgCl electrodes, with an intra-electrode distance of 2 cm positioned over the first dorsal interosseous (FDI) muscle of the participant’s dominant hand, and the ground electrode placed over metacarpophalangeal joint of the third digit.
Single pulse TMS was delivered using a MagStim 2002 stimulator (Magstim, Whitland, Dyfed, UK) and a figure-of-eight coil (Magstim, Whitland, Dyfed, UK). Reliability of coil placement was maintained by participants wearing a snugly fitted cap (EasyCap, Wörthsee, Bavaria, Germany), positioned with reference to the nasion-inion and interaural lines. The cap was marked with sites at 1 × 1 cm spacing in a latitude-longitude matrix to provide reliable coil position throughout the testing protocol [23].
Following identification of the ‘optimal site’, defined as the site with the largest observed MEP [23], active motor threshold (aMT) was determined via a low-level voluntary static contraction of the FDI muscle at 10% of Maximal Voluntary Contraction (MVC). The aMT was identified by delivering TMS stimuli (5% of stimulator output steps, and in 1% steps closer to threshold) at intensities from a level below the participant’s threshold until an observable MEP of at 200 µV and associated cSP could be measured in at least five of ten stimuli [24,25]. Once aMT was established, 20 stimuli (four sets of five pulses per set) were delivered in random intervals (between 6 and 10 s) at intensities to evoke a MEP of 1 mV. A break of 30 s was provided between sets to reduce any possibility of muscular fatigue [26].
2.5. Data and Statistical Analyses
Self-report symptom scores were totaled from the responses of the 15 questions, giving a maximum of 44 points [14]. Outcome measures from Cogstate included percentage of correct responses and mean reaction time for the detection test, and mean response time for the identification test, One-Back and Visual Learning tasks [18]. For the somatosensory testing, apart from the mean reaction time for the detection of the sensory stimulus, the discrimination assessments measured response time and calculated score for following presentation of the stimulus [6,27]. Single pulse MEP latency was calculated as the time between stimulation of the motor cortex to the onset of the MEP [28]. MEP amplitudes were measured from the peak-to-trough difference of the waveform. Duration of the cSP was calculated from the onset (deflection) of the MEP waveform to the return of uninterrupted EMG [29]. With the most influencing confounding factor on cSP duration being the preceding MEP [30], this study employed MEP:cSP ratio to compare between groups and reduce between-participant variability [31]. Previously published MEP:cSP ratios have been published in cohorts with persistent post-concussion symptoms [7] and retired contact sport athletes [8].
All statistical analyses were conducted using Jamovi software (Jamovi, Sydney, NSW, Australia, Version 1.0.8). Data were tested for normality using Shapiro-Wilks (S-W) tests showing data to be skewed (all variables p < 0.05). Data were analysed using Kruskal-Wallis tests with Dwass, Steel, Critchlow-Fligner post hoc comparisons, except for comparison for competitive career, the number of concussions, and time since last concussion between ‘self-concern’ and ‘no-concern’ groups which was analysed using a Mann-Whitney test. Effect sizes are presented as rank-biserial correlation (rrb) for 2-group or partial eta squared (n2p) for 3-group comparisons. The number of previous concussions and the fatigue and related symptom scores to cortical metrics and TMS variables were correlated using Kendall’s Tau B. Data in Tables and Figures are presented as mean (± SD) and statistical significance as set as alpha < 0.05.
3. Results
There were no differences in participant age (H(2) = 0.61, p = 0.74, n2p = 0.01), and education (H(2) = 1.89, p = 0.11, n2p = 0.23) between all groups. Between retired athlete groups there was no difference in career length (U = 242, p = 0.33), the number of concussions (U = 263, p = 0.33), or time since last reported concussion (U = 233, p = 0.34; Table 1).
Table 2 presents all items of the fatigue and related symptom questionnaire. There were significant differences observed between groups for total score (H(2) = 63.27, p < 0.001, n2p = 0.85). Post hoc comparisons showed the group reporting ongoing concerns with their mental or cognitive health (‘self-concern’) had significantly higher total scores than both control participants (W = 8.72, p < 0.001), and those ex-players with no ongoing concerns (‘no concern’; (W = 8.57, p < 0.001). This pattern was seen in almost every item within the survey except for sensitivity to light and noise, where post hoc differences were observed between ongoing concerns and no concerns groups, and ongoing concerns and control groups (p < 0.001). For decreased sleep, differences were found only between the ongoing concern and control groups (p < 0.001). While the no concern group rated higher on the decreased sleep compared to controls, this was not statistically significant (p = 0.103). There was no difference in increased sleep between groups (H(2) = 4.51, p = 0.105). The total fatigue and related symptom score was not correlated to age (Kendall’s Tau B = 0.11, p = 0.26), nor to the number of concussions (Kendall’s Tau B = 0.005, p = 0.96), nor the time since last concussion (Kendall’s Tau B = 0.12, p = 0.28).

Table 2.
Fatigue and related symptoms scores (mean ± SD).
Cognitive assessment revealed no differences in accuracy between groups in each of the four the Cogstate tests performed (Figure 1A). However, the time taken to respond to the questions in three of four of these tests was significantly longer in the group with ongoing self concern (Figure 1B). Reaction times for the visual detection and attention, tasks showed significant differences (H(2) = 10.61, p = 0.005, n2p = 0.14) with post hocs revealing a significantly greater time in the group with self concern than both no concern (W = 3.88, p = 0.017) and control groups (W = 3.88, p = 0.017), despite near identical accuracy scores. Response times in the visual learning task was significantly longer between groups (H(2) = 11.32, p = 0.003, n2p = 0.15) and post hoc comparison showing a significant difference in response times with ongoing concerns relative to ex-players with no ongoing concern (W = 3.60, p = 0.029) and controls (W = 3.94, p = 0.015; Figure 1B). No differences were detected between groups in the response time of the working memory task (H(2) = 4.61, p = 0.1, n2p = 0.06). The time taken to respond in all cognitive tasks was positively correlated to fatigue score; however, the task result (i.e., the accuracy of response) was not (Table 3).
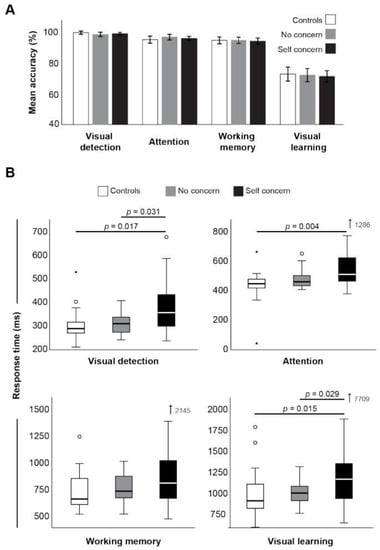
Figure 1.
CogState accuracy (A) and median response times (B) between groups. * indicates statistical significance (p < 0.05).

Table 3.
Correlation a between self-reported fatigue score and objective measures.
Like cognitive testing, somatosensory testing using Cortical Metrics showed no difference in the mean score between groups (Figure 2A), but again, response times were consistently longer in the self concern group (Figure 2B). Specifically, there were significantly delayed reaction times in sensory detection, and response times for sequential amplitude, simultaneous amplitude, and duration discrimination, relative to the control group. Again, response times, not overall scores, were significantly positively correlated with fatigue scores (Table 3).
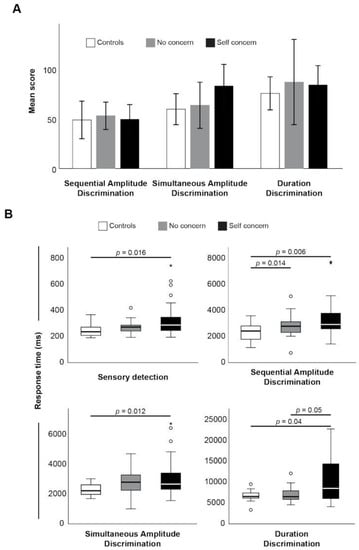
Figure 2.
Mean score (A) and response times (B) between groups. * indicates statistical significance (p < 0.05).
Differences among groups were also found during transcranial magnetic stimulation (TMS). While the median MEP:cSP ratio (Figure 3A) was increased in ex-players both with self concern and no concern, compared to control (37.8 and 48.0 vs. 23.6, respectively), this did not reach statistical significance. However, MEP latency was significantly prolonged in the players with self concern relative to control (H(2) = 9.73, p = 0.008, n2p = 0.13; Figure 3B), suggesting the presence of damage to motor pathways in this group that cannot be discerned from MEP amplitudes alone. TMS latency was, like the response times of cognitive and somatosensory tests, significantly positively correlated with fatigue score (Kendall’s Tau B = 0.182, p = 0.024).
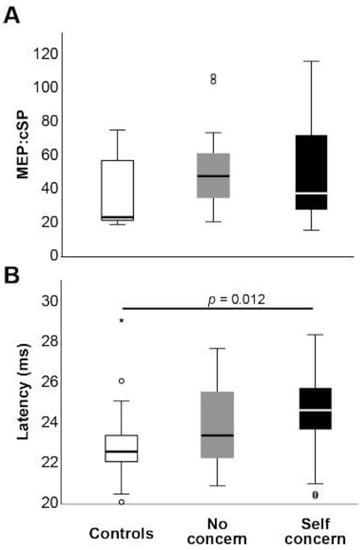
Figure 3.
Mean TMS MEP:cSP ratio (A) and MEP latency (B) between groups. * indicates statistical significance (p < 0.05).
4. Discussion
The present study has found that retired contact sport athletes with self-concerns were significantly slower in both reaction time and response time, compared to retired athletes with no concerns, and age-matched controls. Moreover, reaction and response times correlated with self-reported fatigue and related symptom scale total score, and corticospinal latency. While groups did not differ in outcome performance (i.e., accuracy), the difference in response times suggest a lack of efficiency at processing ability [6] which appeared unrelated to sleep concerns (i.e., the two retired athlete groups did not differ in either decreased in increased sleep concerns). The slowing of responses in lieu of accuracy scores is an important finding as the majority of studies investigating cognitive health outcomes in these cohorts report performance outcomes, with only a minority presenting abilities via psychomotor reaction times [1]. Moreover, the novel finding of impaired response times suggests that cognitive impairment of retired athletes with a history of head trauma should include response times in future studies.
Concussion-focussed studies have previously employed the fatigue and related symptom survey to characterise and quantify these specific cohorts, particularly those who express ongoing self-reported symptoms compared to those who report no ongoing symptoms [7,8]. These investigations have reported significant differences between the groups studied, with the self-concern group having the highest scores. However, it was also reported that players with no concern scored on average above the 10.5 clinical cut-off score for “normal” as suggested by Johansson and Rönnbäck [15]. This may imply that there is an underlying clinical issue for the individual, although a serious problem whereby activities of daily living are significantly affected, is not always the case. For this study, the current sample was derived from those who volunteered for testing who explicitly expressed they had no ongoing concerns, and the total score from the survey was employed to characterise between groups.
Slowed response times in the acute period (one to two weeks) following a concussion injury has been previously reported [6], but to the best of the authors’ knowledge this is the first study to report slowed response times in a long-term cohort with a history of repeated head trauma. While the visual learning task was not statistically significant, the response times reflected the same pattern as the other reaction time and response time tasks: the control group showed the fastest while the self-concern group showed the slowest. Coupled with the TMS data demonstrating altered corticospinal latency, the data suggest that those with a history of repeated neurological insults have some effects on processing ability, with those reporting greater severity of symptoms reflected in worse response times and significantly reduced corticospinal excitability ratio.
Previous studies primarily focussed on neurophysiological alterations in both persistent post concussion symptoms [7,19] and chronic outcomes of repeated head trauma [8,9,10]. In contrast, this study aimed to quantify response times, while TMS was used to provide a potential physiological mechanism to explain differences between groups [4]. While it is acknowledged that TMS is an indirect measure of corticospinal excitability and latency is a raw measure of conduction speed, the correlations between significantly prolonged corticospinal latency and cognitive response times in the ‘self-concern’ group was surprising. However, this is not the first time that slowed TMS latency has been reported. Livingston et al. reported a slowing of TMS latency in the acute phase following a concussion [32,33], while others have recently reported increased TMS latencies in young athletes (18–22 years) who had reported a history of concussions (>1 year) [34]. These findings, in a group of older retired athletes, may reflect alterations in white matter in the pyramidal pathways [34], where MEP latencies have been shown to increase with demyelination associated with neurogenerative disease [35,36]; it has also been postulated that slowed conduction time may be due to neurochemical changes associated with a history of physical brain trauma [37]. While further research, particularly studies where co-registration of TMS and neuroimaging can be performed, is required, employing response times in computerised cognitive-motor and sensorimotor testing, along with low-cost physiological techniques such as TMS may provide a more accurate picture of long-term cognitive health concerns in those with a history of repeated head trauma.
It is outside of the scope of the study to speculate on why some of the retired playing cohort were more affected than others in their self-report. However, the aim of the study was to address the concerns regarding potentially biased sampling that has previously been suggested [38]. Similar to more recent studies [8], the aim was specifically to recruit retired athletes with a history of head trauma both with and without ongoing self-reported concerns. In line with previous work, this study found that the group with no reported symptoms fared significantly better than the group with self-reported concerns but did show small-to-moderate effects compared to the age-matched control group. Collectively, these data show that repeated head trauma may affect cortical processing; however, there may be a ‘threshold’ before this becomes a clinical concern. Further research is required to ascertain what this threshold may be from a physiological perspective.
There are a number of limitations to consider in this study. Firstly was the reliance on self-report for participants’ concussion history. To assist with recollection, this study used the criteria of missing playing the following week [13], which has been previously used in quantifying concussions [9]. However, it is acknowledged that this may still underestimate the number of concussions players experienced. Moreover, while similar career lengths between the two retired playing groups were observed, this does not take into consideration the exposure of repetitive sub-concussions, that may contribute to the neural degradation suggested by increased TMS latency data. Secondly, similar to previous work [8,9,10,39], this study used a retrospective cross-sectional design. While it is impossible to obtain data of the players’ pre-morbid functioning, the aim to address this was by having a three-group design incorporating an ‘active-control’ group of retire players with a similar history of reported concussions, but no ongoing concerns. Future studies would benefit from prospective designs with players being tested prior to starting their careers, but in light of current cohorts, future studies should consider repeated measures to quantify time-related progressive changes between groups of currently retired athletes.
In conclusion, this study is the first to present slowed response times in a cohort of older, retired contact sport athletes with ongoing concerns regarding their head trauma history. While outcome results did not differ between groups, the finding of poorer response time performance suggests less cognitive processing efficiency and neural conduction integrity, and may underpin the concerns, expressed by some retired players, with regard to struggling with activities of daily living. With computerised testing that collects response time data, the data suggest that analyses of cognitive health will be more informative with the inclusion of cognitive-motor and/or sensorimotor response times.
Author Contributions
Conceptualization A.J.P.; Methodology A.J.P. and M.T.; Data analysis A.J.P. and C.M.S.; Writing—Original draft and preparation A.J.P.; Writing—Review and editing A.J.P., D.K., D.J.K., A.K.F. and C.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding, nor any support for the work.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of La Trobe University (HEC18005).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are available upon reasonable request from an authorised institution.
Acknowledgments
A.J.P. currently receives partial research salary funding from Erasmus+ strategic partnerships program (2019-1-IE01-KA202-051555). A.J.P. has previously received partial research funding from the Sports Health Check Charity (Australia), Australian Football League, Impact Technologies Inc., and Samsung Corporation, and is remunerated for expert advice to medico-legal practices.
Conflicts of Interest
The development and manufacture of the Cortical Metrics device used in this study has received partial funding from the Office of Naval Research (USA). MT is a director of Cortical Metrics LLC who has a license from the University of North Carolina to distribute the Brain Gauge device used in this study. No other author has any declaration of conflict of interest.
References
- Cunningham, J.; Broglio, S.P.; O’Grady, M.; Wilson, F. History of Sport-Related Concussion and Long-Term Clinical Cognitive Health Outcomes in Retired Athletes: A Systematic Review. J. Athl. Train. 2020, 55, 132–158. [Google Scholar] [CrossRef] [PubMed]
- Harvey, P.D. Domains of cognition and their assessment. Dialogues Clin. Neurosci. 2019, 21, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Kyllonen, P.C.; Zu, J. Use of response time for measuring cognitive ability. J. Intell. 2016, 4, 14. [Google Scholar] [CrossRef]
- De Boeck, P.; Jeon, M. An Overview of Models for Response Times and Processes in Cognitive Tests. Front. Psychol. 2019, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, D.; Crewther, S.G.; MacCalman, K.; Brown, A.; Crewther, D.P. Cognitive Processing Speed across the Lifespan: Beyond the Influence of Motor Speed. Front. Aging Neurosci. 2017, 9, 62. [Google Scholar] [CrossRef]
- Tommerdahl, A.; Francisco, E.M.; Lensch, R.; Holden, J.K.; Favorov, O.V.; Tommerdahl, M. Response Time in Somatosensory Discrimination Tasks is Sensitive to Neurological Insult. Neurol. Neurobiol. 2019, 2, 1–7. [Google Scholar]
- Pearce, A.J.; Kidgell, D.J.; Frazer, A.K.; King, D.; Buckland, M.E.; Tommerdahl, M. Corticomotor correlates of somatosensory reaction time and variability in individuals with post concussion symptoms. Somatosens. Mot. Res. 2020, 37, 14–21. [Google Scholar] [CrossRef]
- Pearce, A.J.; Kidgell, D.J.; Tommerdahl, M.; Frazer, A.K.; Rist, B.; Mobbs, R.; Batchelor, J.; Buckland, M.E. Chronic Neurophysiological Effects of Repeated Head Trauma in Retired Australian Male Sport Athletes. Front. Neurol. 2021, 12, 633320. [Google Scholar] [CrossRef]
- Pearce, A.J.; Hoy, K.; Rogers, M.A.; Corp, D.T.; Maller, J.J.; Drury, H.G.; Fitzgerald, P.B. The long-term effects of sports concussion on retired Australian football players: A study using transcranial magnetic stimulation. J. Neurotraum. 2014, 31, 1139–1145. [Google Scholar] [CrossRef]
- Pearce, A.J.; Rist, B.; Fraser, C.L.; Cohen, A.; Maller, J.J. Neurophysiological and cognitive impairment following repeated sports concussion injuries in retired professional rugby league players. Brain Inj. 2018, 32, 498–505. [Google Scholar] [CrossRef]
- Rossi, S.; Hallett, M.; Rossini, P.M.; Pascual-Leone, A. Screening questionnaire before TMS: An update. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2011, 122, 1686. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.A.; Daneshvar, D.H.; Picano, J.D.; Gavett, B.E.; Baugh, C.M.; Riley, D.O.; Nowinski, C.J.; McKee, A.C.; Cantu, R.C.; Stern, R.A. Self-reported concussion history: Impact of providing a definition of concussion. Open Access J. Sport. Med. 2014, 5, 99–103. [Google Scholar] [CrossRef]
- AFL Medical Officers Association. The Management of Concussion in Australian Football; Australian Football League: Melbourne, Australia, 2011. [Google Scholar]
- Johansson, B.; Berglund, P.; Rönnbäck, L. Mental fatigue and impaired information processing after mild and moderate traumatic brain injury. Brain Inj. 2009, 23, 1027–1040. [Google Scholar] [CrossRef]
- Johansson, B.; Rönnbäck, L. Long-lasting mental fatigue after traumatic brain injury—A major problem most often neglected diagnostic criteria, assessment, relation to emotional and cognitive problems, cellular background, and aspects on treatment. In Traumatic Brain Injury Croatia; Sadaka, F., Ed.; Intech Open: London, UK, 2014. [Google Scholar]
- Johansson, B.; Starmark, A.; Berglund, P.; Rödholm, M.; Rönnbäck, L. A self-assessment questionnaire for mental fatigue and related symptoms after neurological disorders and injuries. Brain Inj. 2010, 24, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Maruff, P.; Lim, Y.Y.; Darby, D.; Ellis, K.A.; Pietrzak, R.H.; Snyder, P.J.; Bush, A.I.; Szoeke, C.; Schembri, A.; Ames, D.; et al. Clinical utility of the cogstate brief battery in identifying cognitive impairment in mild cognitive impairment and Alzheimer’s disease. BMC Psychol. 2013, 1, 30. [Google Scholar] [CrossRef]
- Maruff, P.; Thomas, E.; Cysique, L.; Brew, B.; Collie, A.; Snyder, P.; Pietrzak, R.H. Validity of the CogState brief battery: Relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch. Clin. Neuropsychol. 2009, 24, 165–178. [Google Scholar] [CrossRef] [PubMed]
- Pearce, A.J.; Tommerdahl, M.; King, D. Neurophysiological abnormalities in individuals with persistent post-concussion symptoms. Neuroscience 2019, 408, 272–281. [Google Scholar] [CrossRef]
- Tommerdahl, M.; Dennis, R.G.; Francisco, E.M.; Holden, J.K.; Nguyen, R.; Favorov, O.V. Neurosensory assessments of concussion. Mil. Med. 2016, 181, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Francisco, E.; Holden, J.; Dennis, R.; Tommerdahl, M. Somatosensory Information Processing in the Aging Population. Front. Aging Neurosci. 2011, 3, 18. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Merletti, R.; Stegeman, D.; Blok, J.; Rau, G.; Disselhorst-Klug, C.; Hägg, G. European Recommendations for Surface Electromyography; Roessingh Research and Development The Netherlands: Enschede, The Netherlands, 1999. [Google Scholar]
- Pearce, A.J.; Thickbroom, G.W.; Byrnes, M.L.; Mastaglia, F.L. The corticomotor representation of elite racquet sport athletes. Exp. Brain Res. 2000, 130, 238–243. [Google Scholar] [CrossRef]
- Wilson, S.A.; Thickbroom, G.W.; Mastaglia, F.L. Comparison of the magnetically mapped corticomotor representation of a muscle at rest and during low-level voluntary contraction. Electroencephalogr. Clin. Neurophysiol. Electromyogr. Mot. Control. 1995, 97, 246–250. [Google Scholar]
- Pearce, A.J.; Clark, R.A.; Kidgell, D.J. A Comparison of Two Methods in Acquiring Stimulus–Response Curves with Transcranial Magnetic Stimulation. Brain Stimul. 2013, 6, 306–309. [Google Scholar] [CrossRef] [PubMed]
- Kidgell, D.J.; Pearce, A.J. Corticospinal properties following short-term strength training of an intrinsic hand muscle. Hum. Mov. Sci. 2010, 29, 631–641. [Google Scholar] [CrossRef] [PubMed]
- King, D.A.; Hume, P.; Tommerdahl, M. Use of the Brain-Gauge somatosensory assessment for monitoring recovery from concussion: A case study. J. Physiother. Res. 2018, 2, 13. [Google Scholar]
- Brasil-Neto, J.P.; Cohen, L.G.; Panizza, M.; Nilsson, J.; Roth, B.J.; Hallett, M. Optimal focal transcranial magnetic activation of the human motor cortex: Effects of coil orientation, shape of the induced current pulse, and stimulus intensity. J. Clin. Neurophysiol. Off. Publ. Am. Electroencephalogr. Soc. 1992, 9, 132–136. [Google Scholar] [CrossRef]
- Wilson, S.A.; Lockwood, R.J.; Thickbroom, G.W.; Mastaglia, F.L. The muscle silent period following transcranial magnetic cortical stimulation. J. Neurol. Sci. 1993, 114, 216–222. [Google Scholar] [CrossRef]
- Škarabot, J.; Mesquita, R.N.; Brownstein, C.G.; Ansdell, P. Myths and Methodologies: How loud is the story told by the transcranial magnetic stimulation-evoked silent period? Exp. Physiol. 2019, 104, 635–642. [Google Scholar] [CrossRef]
- Orth, M.; Rothwell, J.C. The cortical silent period: Intrinsic variability and relation to the waveform of the transcranial magnetic stimulation pulse. Clin. Neurophysiol. 2004, 115, 1076–1082. [Google Scholar] [CrossRef]
- Livingston, S.C.; Goodkin, H.P.; Hertel, J.N.; Saliba, E.N.; Barth, J.T.; Ingersoll, C.D. Differential rates of recovery after acute sport-related concussion: Electrophysiologic, symptomatic, and neurocognitive indices. J. Clin. Neurophysiol. 2012, 29, 23–32. [Google Scholar] [CrossRef]
- Livingston, S.C.; Saliba, E.N.; Goodkin, H.P.; Barth, J.T.; Hertel, J.N.; Ingersoll, C.D. A preliminary investigation of motor evoked potential abnormalities following sport-related concussion. Brain Inj. 2010, 24, 904–913. [Google Scholar] [CrossRef]
- Stokes, W.; Choynowki, J.; St Pierre, M.; Anaya, M.A.; Statton, M.A.; Celnik, P.A.; Cantarero, G. Altered corticomotor latencies but normal motor neuroplasticity in concussed athletes. J. Neurophysiol. 2020, 123, 1600–1605. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, K.; Irlbacher, K.; Grosse, P.; Röricht, S.; Meyer, B.-U. Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology 2002, 59, 1218–1224. [Google Scholar] [CrossRef] [PubMed]
- Britton, T.; Meyer, B.-U.; Benecke, R. Variability of cortically evoked motor responses in multiple sclerosis. Electroencephalogr. Clin. Neurophysiol. Evoked Potentials Sect. 1991, 81, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.P.; Ramadan, S.; Stern, R.A.; Box, H.C.; Nowinski, C.J.; Ross, B.D.; Mountford, C.E. Changes in the neurochemistry of athletes with repetitive brain trauma: Preliminary results using localized correlated spectroscopy. Alzheimer’s Res. Ther. 2015, 7, 13. [Google Scholar] [CrossRef]
- Carman, A.J.; Ferguson, R.; Cantu, R.; Comstock, R.D.; Dacks, P.A.; DeKosky, S.T.; Gandy, S.; Gilbert, J.; Gilliland, C.; Gioia, G.; et al. Mind the gaps—Advancing research into short-term and long-term neuropsychological outcomes of youth sports-related concussions. Nat. Rev. Neurol. 2015, 11, 230–244. [Google Scholar] [CrossRef]
- De Beaumont, L.; Théoret, H.; Mongeon, D.; Messier, J.; Leclerc, S.; Tremblay, S.; Ellemberg, D.; Lassonde, M. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain 2009, 132, 695–708. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).