Exercise and Nutrition Strategies for Combating Sarcopenia and Type 2 Diabetes Mellitus in Older Adults
Abstract
:1. Introduction
2. Methods
- “resistance training” OR “resistance exercise” OR “strength training” OR “aerobic exercise’’
- “frailty” OR “muscle loss” OR “sarcopenia”
- “muscle hypertrophy” OR “muscle strength” OR “skeletal muscle mass”
- “diabetes mellitus” OR “type 2 diabetes mellitus OR “insulin resistance”
- “diet” OR “nutrition” OR “amino acids” OR “protein” OR “antioxidant” OR “life-style modification” OR “omega-3 supplementation” OR “vitamin D supplementation”
- “older adults” OR “elderly”
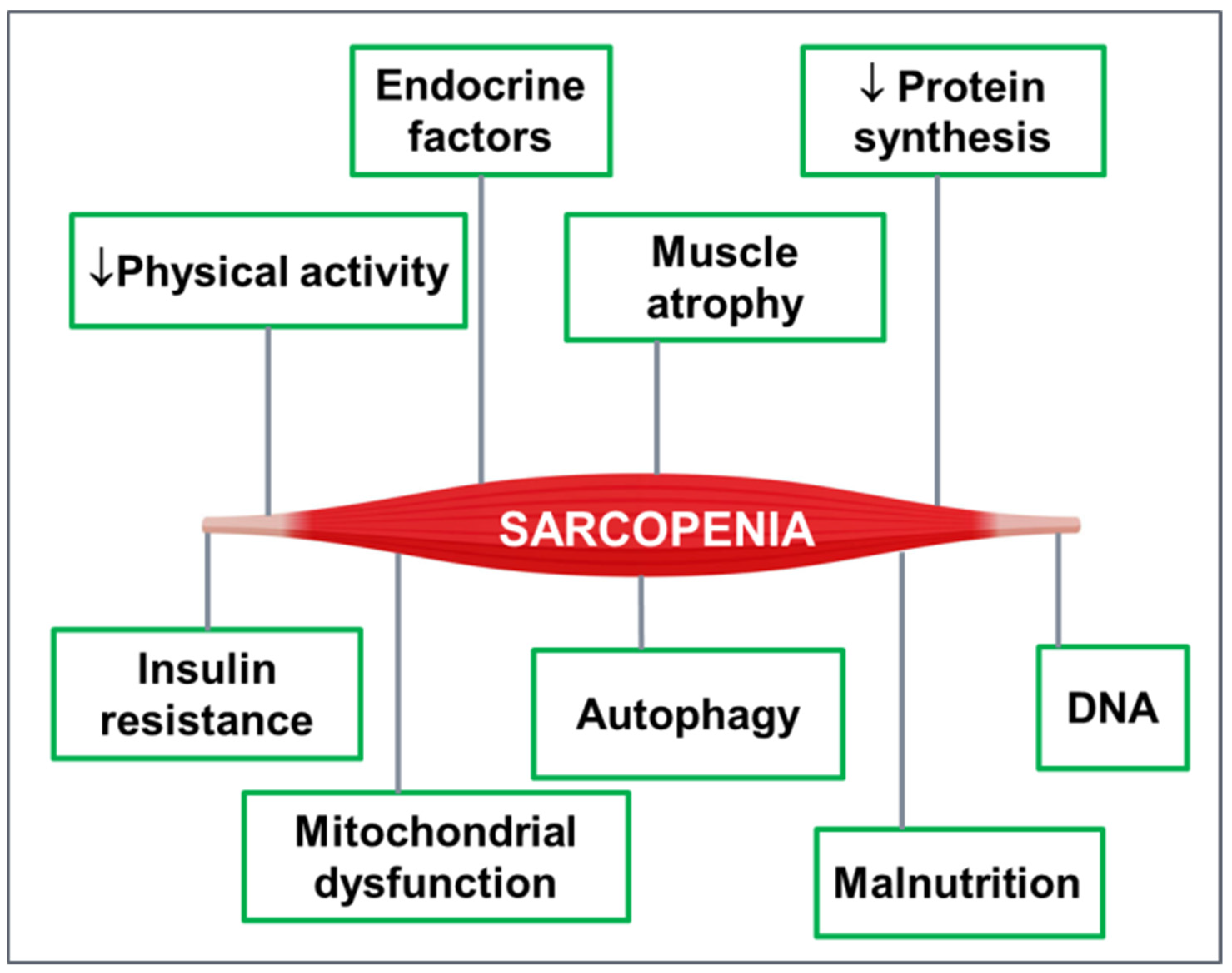
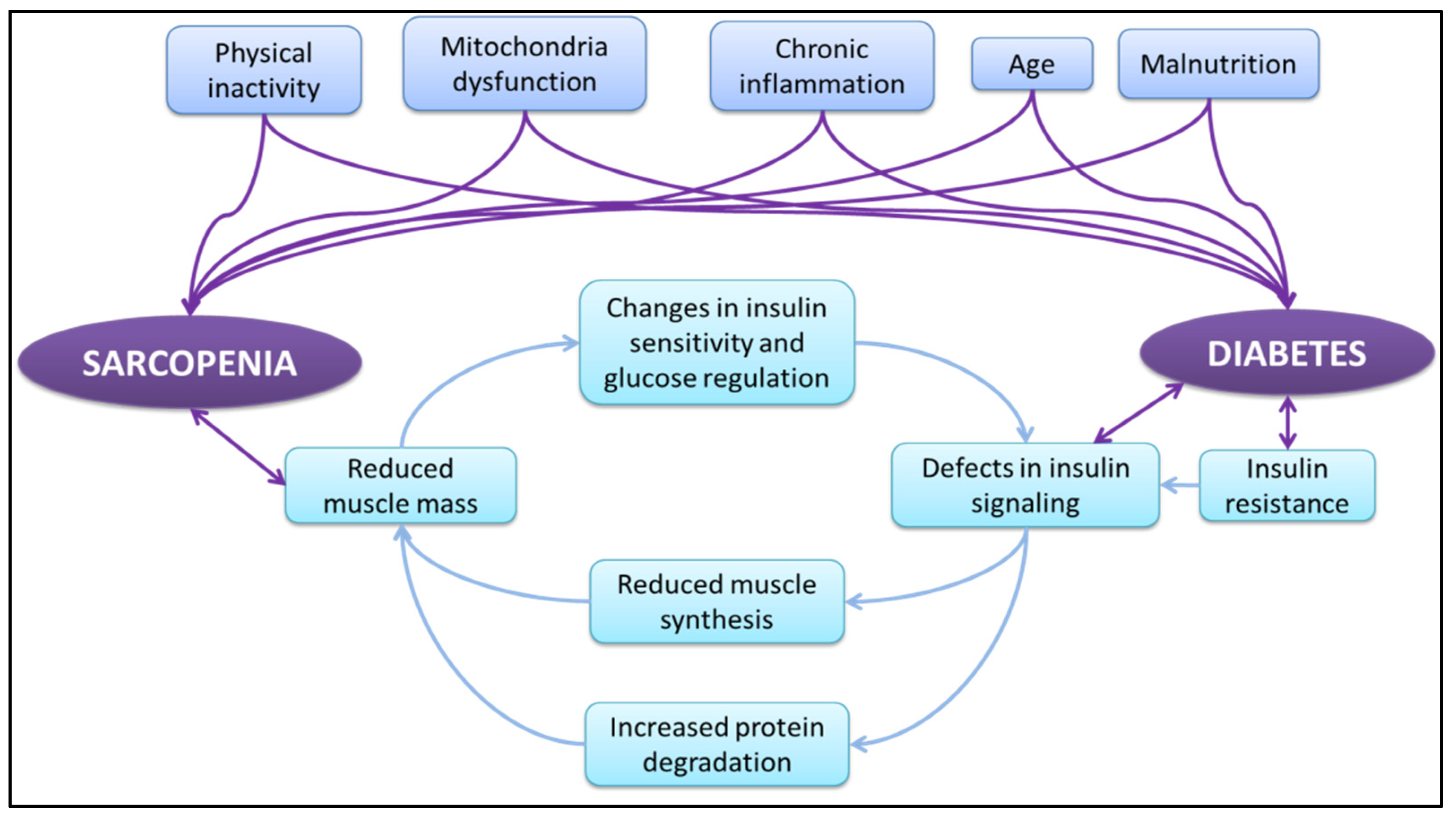
3. Sarcopenia and T2DM
4. Exercise and Diet to Control Sarcopenia and Type 2 Diabetes Mellitus in Older Adults
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADA | American Diabetes Association |
| ASM | Appendicular skeletal mass |
| AT | Aerobic training |
| AWGS | Asian Working Group for Sarcopenia |
| BMI | Body mass index |
| DEXA | Dual Energy X-ray Absorptiometry |
| EWGSOP | European Working Group on Sarcopenia in Older People |
| FNIH | Foundation of National Institute of Health |
| GNRI | Geriatric nutritional risk index |
| HbA1c | Glycosylated hemoglobin |
| HDL-C | High density lipoprotein cholesterol |
| IWGS | International Working Group on Sarcopenia |
| LMM | Low Muscle Mass |
| LMS | Low Muscle Strength |
| LSMI | Low Skeletal Mass Index |
| MWD | Minutes walking distance |
| N | Number of participants |
| RASM | Relative appendicular skeletal muscle index |
| RT | Resistance training |
| SD | Standard deviation |
| SMI | Skeletal mass Index |
| TSM | Total body skeletal mass |
| TUG | Timed Up and Go test |
| WHO | World Health Organization |
References
- Janssen, I. Influence of Sarcopenia on the Development of Physical Disability: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2006, 54, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Sreekumaran Nair, K. Aging muscle. Am. J. Clin. Nutr. 2005, 81, 953–963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wiedmer, P.; Jung, T.; Castro, J.P.; Pomatto, L.C.D.; Sun, P.Y.; Davies, K.J.A.; Grune, T. Sarcopenia—Molecular mechanisms and open questions. Ageing Res. Rev. 2021, 65, 101200. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing Group for the European Working Group on Sarcopenia in Older People 2 (EWGSOP2); and the Extended Group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 601. [Google Scholar] [CrossRef] [Green Version]
- Ali, S.; Garcia, J.M. Sarcopenia, Cachexia and Aging: Diagnosis, Mechanisms and Therapeutic Options—A Mini-Review. Gerontology 2014, 60, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Jentoft, A.J.; Baeyens, J.P.; Bauer, J.M.; Boirie, Y.; Cederholm, T.; Landi, F.; Martin, F.C.; Michel, J.P.; Rolland, Y.; Schneider, S.M.; et al. European Working Group on Sarcopenia in Older People. Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing 2010, 39, 412–423. [Google Scholar] [CrossRef] [Green Version]
- Chumlea, W.C.; Cesari, M.; Evans, W.J.; Ferrucci, L.; Fielding, R.A.; Pahor, M.; Studenski, S.; Vellas, B. International Working Group on Sarcopenia Task Force Members. Sarcopenia: Designing phase IIB trials. J. Nutr. Health Aging 2011, 15, 450–455. [Google Scholar] [CrossRef]
- Chen, L.K.; Woo, J.; Assantachai, P.; Auyeung, T.W.; Chou, M.Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S.; et al. Asian Working Group for Sarcopenia: 2019 Consensus Update on Sarcopenia Diagnosis and Treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e2. [Google Scholar] [CrossRef]
- Menetski, J.P.; Hoffmann, S.C.; Cush, S.S.; Kamphaus, T.N.; Austin, C.P.; Herrling, P.L.; Wagner, J.A. The Foundation for the National Institutes of Health Biomarkers Consortium: Past Accomplishments and New Strategic Direction. Clin. Pharmacol. Ther. 2019, 105, 829–843. [Google Scholar] [CrossRef]
- Lo, J.H.; Pong, U.K.; Yiu, T.; Ong, M.T.; Lee, W.Y. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Translat. 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Landi, F.; Schneider, S.M.; Zúñiga, C.; Arai, H.; Boirie, Y.; Chen, L.K.; Fielding, R.A.; Martin, F.C.; Michel, J.P.; et al. Prevalence of and interventions for sarcopenia in ageing adults: A systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing 2014, 43, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Spira, D.; Norman, K.; Nikolov, J.; Demuth, I.; Steinhagen-Thiessen, E.; Eckardt, R. Prevalence and definition of sarcopenia in community dwelling older people. Z. Gerontol. Geriatr. 2015, 49, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Uusi-Rasi, K.; Pasanen, M.; Kannus, P.; Karinkanta, S.; Sievänen, H. Sarcopenia and osteopenia among 70–80-year-old home-dwelling Finnish women: Prevalence and association with functional performance. Osteoporos. Int. 2012, 24, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Volpato, S.; Bianchi, L.; Cherubini, A.; Landi, F.; Maggio, M.; Savino, E.; Bandinelli, S.; Ceda, G.P.; Guralnik, J.M.; Zuliani, G.; et al. Prevalence and Clinical Correlates of Sarcopenia in Community-Dwelling Older People: Application of the EWGSOP Definition and Diagnostic Algorithm. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 69, 438–446. [Google Scholar] [CrossRef]
- Lee, W.-J.; Liu, L.-K.; Peng, L.-N.; Lin, M.-H.; Chen, L.-K. Comparisons of Sarcopenia Defined by IWGS and EWGSOP Criteria Among Older People: Results From the I-Lan Longitudinal Aging Study. J. Am. Med. Dir. Assoc. 2013, 14, 528.e1–528.e7. [Google Scholar] [CrossRef]
- Landi, F.; Liperoti, R.; Fusco, D.; Mastropaolo, S.; Quattrociocchi, D.; Proia, A.; Russo, A.; Bernabei, R.; Onder, G. Prevalence and Risk Factors of Sarcopenia Among Nursing Home Older Residents. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 67A, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Tanimoto, Y.; Watanabe, M.; Sun, W.; Sugiura, Y.; Tsuda, Y.; Kimura, M.; Hayashida, I.; Kusabiraki, T.; Kono, K. Association between sarcopenia and higher-level functional capacity in daily living in community-dwelling elderly subjects in Japan. Arch. Gerontol. Geriatr. 2012, 55, e9–e13. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Syddall, H.; Martin, H.; Patel, H.; Baylis, D.; Cooper, C. The developmental origins of sarcopenia. J. Nutr. Health Aging 2008, 12, 427–432. [Google Scholar] [CrossRef] [Green Version]
- Murphy, R.A.; Ip, E.H.; Zhang, Q.; Boudreau, R.M.; Cawthon, P.M.; Newman, A.B.; Tylavsky, F.A.; Visser, M.; Goodpaster, B.H.; Harris, T.B. Transition to Sarcopenia and Determinants of Transitions in Older Adults: A Population-Based Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2013, 69, 751–758. [Google Scholar] [CrossRef]
- DeFronzo, R.A.; Tripathy, D. Skeletal Muscle Insulin Resistance Is the Primary Defect in Type 2 Diabetes. Diabetes Care 2009, 32, S157–S163. [Google Scholar] [CrossRef] [Green Version]
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2014, 37 (Suppl. 1), S81–S90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Kaabi, J.; Mustafa, H.; Govender, R.D.; King, J.K.; Hashim, M.J.; Khan, M.A.B. Epidemiology of Type 2 Diabetes—Global Burden of Disease and Forecasted Trends. J. Epidemiol. Glob. Health 2019, 10, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.N.; Park, M.S.; Yang, S.J.; Yoo, H.J.; Kang, H.J.; Song, W.; Seo, J.A.; Kim, S.G.; Kim, N.H.; Baik, S.H.; et al. Prevalence and Determinant Factors of Sarcopenia in Patients with Type 2 Diabetes. Diabetes Care 2010, 33, 1497–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, A.B.F.; Nascimento, D.A.C.; Rodrigues, I.J.M.; Charone, C.C.O.; Lopes, G.L.; Lima, R.S.; Sá, A.A.; Carneiro, T.X.; Moraes, N.S. Association between sarcopenia and diabetes in community dwelling elderly in the Amazon region—Viver Mais Project. Arch. Gerontol. Geriatr. 2019, 83, 121–125. [Google Scholar] [CrossRef]
- Nomura, T.; Kawae, T.; Kataoka, H.; Ikeda, Y. Assessment of lower extremity muscle mass, muscle strength, and exercise therapy in elderly patients with diabetes mellitus. Environ. Health Prev. Med. 2018, 23, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, S.; Van Laethem, C.; Van Acker, K.; Calders, P. Influence of combined exercise training on indices of obesity, diabetes and cardiovascular risk in type 2 diabetes patients. Clin. Rehabil. 2008, 22, 483–492. [Google Scholar] [CrossRef]
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength.;mass.;and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef]
- Chan, L.-C.; Yang, Y.-C.; Lin, H.-C.; Wahlqvist, M.L.; Hung, Y.-J.; Lee, M.-S. Nutrition counseling is associated with less sarcopenia in diabetes: A cross-sectional and retrospective cohort study. Nutrition 2021, 91–92, 111269. [Google Scholar] [CrossRef]
- Robinson, S.M.; Reginster, J.Y.; Rizzoli, R.; Shaw, S.C.; Kanis, J.A.; Bautmans, I.; Bischoff-Ferrari, H.; Bruyère, O.; Cesari, M.; Dawson-Hughes, B.; et al. Does nutrition play a role in the prevention and management of sarcopenia? Clin. Nutr. 2018, 37, 1121–1132. [Google Scholar] [CrossRef] [Green Version]
- Cooper, C.; Fielding, R.; Visser, M.; van Loon, L.J.; Rolland, Y.; Orwoll, E.; Reid, K.; Boonen, S.; Dere, W.; Epstein, S.; et al. Tools in the assessment of sarcopenia. Calcif. Tissue Int. 2013, 93, 201–210. [Google Scholar] [CrossRef]
- American Diabetes Association Professional Practice Committee. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2022. Diabetes Care 2022, 45 (Suppl. 1), S17–S38. [Google Scholar] [CrossRef] [PubMed]
- Teppala, S.; Shankar, A. Association between serum IGF-1 and diabetes among U.S. adults. Diabetes Care 2010, 33, 2257–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulou, S. Sarcopenia: A Contemporary Health Problem among Older Adult Populations. Nutrients 2020, 12, 1293. [Google Scholar] [CrossRef]
- Korhonen, M.T.; Cristea, A.; Alén, M.; Häkkinen, K.; Sipilä, S.; Mero, A.; Viitasalo, J.T.; Larsson, L.; Suominen, H. Aging, muscle fiber type, and contractile function in sprint-trained athletes. J. Appl. Physiol. 2006, 101, 906–917. [Google Scholar] [CrossRef] [PubMed]
- Chawla, R.; Chawla, A.; Jaggi, S. Microvascular and macrovascular complications in diabetes mellitus: Distinct or continuum? Indian J. Endocrinol. Metab. 2016, 20, 546. [Google Scholar] [CrossRef]
- Park, S.W.; Goodpaster, B.H.; Strotmeyer, E.S.; de Rekeneire, N.; Harris, T.B.; Schwartz, A.V.; Tylavsky, F.A.; Newman, A.B. Decreased Muscle Strength and Quality in Older Adults with Type 2 Diabetes. Diabetes 2006, 55, 1813–1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palicka, V. Pathophysiology of Diabetes Mellitus. EJIFCC 2002, 13, 140–144. [Google Scholar]
- Park, S.W.; Goodpaster, B.H.; Lee, J.S.; Kuller, L.H.; Boudreau, R.; de Rekeneire, N.; Harris, T.B.; Kritchevsky, S.; Tylavsky, F.A.; Nevitt, M.; et al. Excessive Loss of Skeletal Muscle Mass in Older Adults with Type 2 Diabetes. Diabetes Care 2009, 32, 1993–1997. [Google Scholar] [CrossRef] [Green Version]
- Fielding, R.A.; Vellas, B.; Evans, W.J.; Bhasin, S.; Morley, J.E.; Newman, A.B.; Abellan van Kan, G.; Andrieu, S.; Bauer, J.; Breuille, D.; et al. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. J. Am. Med. Dir. Assoc. 2011, 12, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Wolfe, R.R. Effects of insulin on muscle tissue. Curr. Opin. Clin. Nutr. Metab. Care. 2000, 3, 67–71. [Google Scholar] [CrossRef]
- Morley, J.E.; Argiles, J.M.; Evans, W.J.; Bhasin, S.; Cella, D.; Deutz, N.E.P.; Doehner, W.; Fearon, K.C.H.; Ferrucci, L.; Hellerstein, M.K.; et al. Nutritional Recommendations for the Management of Sarcopenia. J. Am. Med. Dir. Assoc. 2010, 11, 391–396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, M.; Gang, X.; Wang, G.; Xiao, X.; Li, Z.; Jiang, Z.; Wang, G. A cross-sectional study: Associations between sarcopenia and clinical characteristics of patients with type 2 diabetes. Medicine 2020, 99, e18708. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.G.; Halter, J.B. The Pathophysiology of Hyperglycemia in Older Adults: Clinical Considerations. Diabetes Care 2017, 40, 444–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinclair, A.; Morley, J.E.; Rodriguez-Mañas, L.; Paolisso, G.; Bayer, T.; Zeyfang, A.; Bourdel-Marchasson, I.; Vischer, U.; Woo, J.; Chapman, I.; et al. Diabetes Mellitus in Older People: Position Statement on behalf of the International Association of Gerontology and Geriatrics (IAGG), the European Diabetes Working Party for Older People (EDWPOP), and the International Task Force of Experts in Diabetes. J. Am. Med. Dir. Assoc. 2012, 13, 497–502. [Google Scholar] [CrossRef]
- Landi, F.; Onder, G.; Bernabei, R. Sarcopenia and Diabetes: Two Sides of the Same Coin. J. Am. Med. Dir. Assoc. 2013, 14, 540–541. [Google Scholar] [CrossRef]
- Umegaki, H. Sarcopenia and diabetes: Hyperglycemia is a risk factor for age-associated muscle mass and functional reduction. J. Diabetes Investig. 2015, 6, 623–624. [Google Scholar] [CrossRef]
- Anagnostis, P.; Gkekas, N.K.; Achilla, C.; Pananastasiou, G.; Taouxidou, P.; Mitsiou, M.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. Type 2 Diabetes Mellitus is Associated with Increased Risk of Sarcopenia: A Systematic Review and Meta-analysis. Calcif. Tissue Int. 2020, 107, 453–463. [Google Scholar] [CrossRef]
- Veronese, N.; Pizzol, D.; Demurtas, J.; Soysal, P.; Smith, L.; Sieber, C.; Strandberg, T.; Bourdel-Marchasson, I.; Sinclair, A.; Petrovic, M.; et al. Association between sarcopenia and diabetes: A systematic review and meta-analysis of observational studies. Eur. Geriatr. Med. 2019, 10, 685–696. [Google Scholar] [CrossRef]
- Wang, T.; Feng, X.; Zhou, J.; Gong, H.; Xia, S.; Wei, Q.; Hu, X.; Tao, R.; Li, L.; Qian, F.; et al. Type 2 diabetes mellitus is associated with increased risks of sarcopenia and pre-sarcopenia in Chinese elderly. Sci. Rep. 2016, 6, 38937. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.-S.; Park, K.-S.; Kim, M.-J.; Kim, S.-K.; Cho, Y.-W.; Park, S.W. Type 2 diabetes is associated with low muscle mass in older adults. Geriatr. Gerontol. Int. 2014, 14, 115–121. [Google Scholar] [CrossRef]
- Ai, Y.; Xu, R.; Liu, L. The prevalence and risk factors of sarcopenia in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Diabetol. Metab. Syndr. 2021, 13, 93. [Google Scholar] [CrossRef] [PubMed]
- Kalyani, R.R.; Metter, E.J.; Egan, J.; Golden, S.H.; Ferrucci, L. Hyperglycemia predicts persistently lower muscle strength with aging. Diabetes Care 2015, 38, 82–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenders, M.; Verdijk, L.B.; van der Hoeven, L.; Adam, J.J.; van Kranenburg, J.; Nilwik, R.; van Loon, L.J. Patients with type 2 diabetes show a greater decline in muscle mass, muscle strength, and functional capacity with aging. J. Am. Med. Dir. Assoc. 2013, 14, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.M.; Moon, J.S.; Chang, M.C. Prevalence of Sarcopenia and Its Association With Diabetes: A Meta-Analysis of Community-Dwelling Asian Population. Front. Med. 2021, 8, 681232. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.P.; Sacks, J.; Nieuwoudt, S. The essential role of exercise in the management of type 2 diabetes. Clevel. Clin. J. Med. 2017, 84, S15–S21. [Google Scholar] [CrossRef]
- Mitranun, W.; Deerochanawong, C.; Tanaka, H.; Suksom, D. Continuous vs interval training on glycemic control and macro- and microvascular reactivity in type 2 diabetic patients. Scand. J. Med. Sci. Sports 2014, 24, e69–e76. [Google Scholar] [CrossRef] [PubMed]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-Based Recommendations for Optimal Dietary Protein Intake in Older People: A Position Paper From the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef]
- Terada, T.; Friesen, A.; Chahal, B.S.; Bell, G.J.; McCargar, L.J.; Boulé, N.G. Feasibility and preliminary efficacy of high intensity interval training in type 2 diabetes. Diabetes Res. Clin. Pract. 2013, 99, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-T.; Chung, Y.-C.; Chen, Y.-J.; Ho, S.-Y.; Wu, H.-J. Effects of Different Types of Exercise on Body Composition, Muscle Strength, and IGF-1 in the Elderly with Sarcopenic Obesity. J. Am. Geriatr. Soc. 2017, 65, 827–832. [Google Scholar] [CrossRef]
- Vlietstra, L.; Hendrickx, W.; Waters, D.L. Exercise interventions in healthy older adults with sarcopenia: A systematic review and meta-analysis. Australas. J. Ageing 2018, 37, 169–183. [Google Scholar] [CrossRef]
- Fleg, J.L. Aerobic exercise in the elderly: A key to successful aging. Discov. Med. 2012, 13, 223–228. [Google Scholar] [PubMed]
- Yoshimura, Y.; Wakabayashi, H.; Yamada, M.; Kim, H.; Harada, A.; Arai, H. Interventions for Treating Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Studies. J. Am. Med. Dir. Assoc. 2017, 18, 553.e1–553.e16. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.Y.; Huang, K.S.; Chen, K.M.; Chou, C.P.; Tu, Y.K. Exercise, Nutrition, and Combined Exercise and Nutrition in Older Adults with Sarcopenia: A Systematic Review and Network Meta-analysis. Maturitas 2021, 145, 38–48. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, L.P.; Silva, J.P.; Coelho, F.M.; Pereira, D.S.; Parentoni, A.N.; Pereira, L.S. Impact of resistance exercise program on functional capacity and muscular strength of knee extensor in pre-frail community-dwelling older women: A randomized crossover trial. Rev. Bras. Fisioter. 2011, 15, 318–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seynnes, O.; Fiatarone Singh, M.A.; Hue, O.; Pras, P.; Legros, P.; Bernard, P.L. Physiological and functional responses to low-moderate versus high-intensity progressive resistance training in frail elders. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 503–509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.-H.; Cheng, F.-C.; Tsai, L.-C.; Lee, N.-Y.; Lu, Y.-F. Appropriate physical activity and dietary intake achieve optimal metabolic control in older type 2 diabetes patients. J. Diabetes Investig. 2014, 5, 418–427. [Google Scholar] [CrossRef] [Green Version]
- Irvine, C.; Taylor, N.F. Progressive resistance exercise improves glycaemic control in people with type 2 diabetes mellitus: A systematic review. Aust. J. Physiother. 2009, 55, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Kadoglou, N.P.; Fotiadis, G.; Kapelouzou, A.; Kostakis, A.; Liapis, C.D.; Vrabas, I.S. The differential anti-inflammatory effects of exercise modalities and their association with early carotid atherosclerosis progression in patients with type 2 diabetes. Diabet. Med. 2013, 30, e41–e50. [Google Scholar] [CrossRef]
- Hovanec, N.; Sawant, A.; Overend, T.J.; Petrella, R.J.; Vandervoort, A.A. Resistance Training and Older Adults with Type 2 Diabetes Mellitus: Strength of the Evidence. J. Aging Res. 2012, 2012, 284635. [Google Scholar] [CrossRef]
- Castaneda, C.; Layne, J.E.; Munoz-Orians, L.; Gordon, P.L.; Walsmith, J.; Foldvari, M.; Roubenoff, R.; Tucker, K.L.; Nelson, M.E. A randomized controlled trial of resistance exercise training to improve glycemic control in older adults with type 2 diabetes. Diabetes Care 2002, 25, 2335–2341. [Google Scholar] [CrossRef] [Green Version]
- Moses, A.C. Insulin resistance and type 2 diabetes mellitus: Is there a therapeutic role for IGF-1? Endocr. Dev. 2005, 9, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Latham, N.K.; Anderson, C.S.; Lee, A.; Bennett, D.A.; Moseley, A.; Cameron, I.D.; Fitness Collaborative Group. A randomized, controlled trial of quadriceps resistance exercise and vitamin D in frail older people: The Frailty Interventions Trial in Elderly Subjects (FITNESS). J. Am. Geriatr. Soc. 2003, 51, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Li, W.; Wang, J. Effects of six months of combined aerobic and resistance training for elderly patients with a long history of type 2 diabetes. J. Sports Sci. Med. 2012, 11, 495–501. [Google Scholar] [PubMed]
- Børsheim, E.; Bui, Q.U.; Tissier, S.; Kobayashi, H.; Ferrando, A.A.; Wolfe, R.R. Effect of amino acid supplementation on muscle mass, strength and physical function in elderly. Clin. Nutr. 2008, 27, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Tieland, M.; van de Rest, O.; Dirks, M.L.; van der Zwaluw, N.; Mensink, M.; van Loon, L.J.C.; de Groot, L.C.P.G.M. Protein Supplementation Improves Physical Performance in Frail Elderly People: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Am. Med. Dir. Assoc. 2012, 13, 720–726. [Google Scholar] [CrossRef]
- Van Loon, L.J.; Kruijshoop, M.; Menheere, P.P.; Wagenmakers, A.J.; Saris, W.H.; Keizer, H.A. Amino acid ingestion strongly enhances insulin secretion in patients with long-term type 2 diabetes. Diabetes Care 2003, 26, 625–630. [Google Scholar] [CrossRef] [Green Version]
- Moslehi, N.; Shab-Bidar, S.; Mirmiran, P.; Sadeghi, M.; Azizi, F. Associations between dairy products consumption and risk of type 2 diabetes: Tehran lipid and glucose study. Int. J. Food Sci. Nutr. 2015, 66, 692–699. [Google Scholar] [CrossRef]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kondo, Y.; Fukuda, T.; Kitagawa, N.; Okada, H.; et al. Vitamin Intake and Loss of Muscle Mass in Older People with Type 2 Diabetes: A Prospective Study of the KAMOGAWA-DM Cohort. Nutrients 2021, 13, 2335. [Google Scholar] [CrossRef]
- Bischoff, H.A.; Stahelin, H.B.; Urscheler, N.; Ehrsam, R.; Vonthein, R.; Perrig-Chiello, P.; Tyndall, A.; Theiler, R. Muscle strength in the elderly: Its relation to vitamin D metabolites. Arch. Phys. Med. Rehabil. 1999, 80, 54–58. [Google Scholar] [CrossRef]
- Lucato, P.; Solmi, M.; Maggi, S.; Bertocco, A.; Bano, G.; Trevisan, C.; Manzato, E.; Sergi, G.; Schofield, P.; Kouidrat, Y.; et al. Low vitamin D levels increase the risk of type 2 diabetes in older adults: A systematic review and meta-analysis. Maturitas 2017, 100, 8–15. [Google Scholar] [CrossRef]
- Okamura, T.; Hashimoto, Y.; Miki, A.; Kaji, A.; Sakai, R.; Iwai, K.; Osaka, T.; Ushigome, E.; Hamaguchi, M.; Yamazaki, M.; et al. Reduced dietary omega-3 fatty acids intake is associated with sarcopenia in elderly patients with type 2 diabetes: A cross-sectional study of KAMOGAWA-DM cohort study. J. Clin. Biochem. Nutr. 2020, 66, 233–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Semba, R.D.; Blaum, C.; Guralnik, J.M.; Moncrief, D.T.; Ricks, M.O.; Fried, L.P. Carotenoid and vitamin E status are associated with indicators of sarcopenia among older women living in the community. Aging Clin. Exp. Res. 2003, 15, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.-D.; Chen, H.-C.; Huang, S.-W.; Liou, T.-H. The Role of Muscle Mass Gain Following Protein Supplementation Plus Exercise Therapy in Older Adults with Sarcopenia and Frailty Risks: A Systematic Review and Meta-Regression Analysis of Randomized Trials. Nutrients 2019, 11, 1713. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egger, A.; Niederseer, D.; Diem, G.; Finkenzeller, T.; Ledl-Kurkowski, E.; Forstner, R.; Pirich, C.; Patsch, W.; Weitgasser, R.; Niebauer, J. Different types of resistance training in type 2 diabetes mellitus: Effects on glycaemic control, muscle mass and strength. Eur. J. Prev. Cardiol. 2012, 20, 1051–1060. [Google Scholar] [CrossRef]
- Amamou, T.; Normandin, E.; Pouliot, J.; Dionne, I.J.; Brochu, M.; Riesco, E. Effect of a High-Protein Energy-Restricted Diet Combined with Resistance Training on Metabolic Profile in Older Individuals with Metabolic Impairments. J. Nutr. Health Aging 2017, 21, 67–74. [Google Scholar] [CrossRef]
- Fragala, M.S.; Cadore, E.L.; Dorgo, S.; Izquierdo, M.; Kraemer, W.J.; Peterson, M.D.; Ryan, E.D. Resistance Training for Older Adults: Position Statement from the National Strength and Conditioning Association. J. Strength Cond. Res. 2019, 33, 2019–2052. [Google Scholar] [CrossRef]
- Lynch, C.J.; Adams, S.H. Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 2014, 10, 723–736. [Google Scholar] [CrossRef] [Green Version]
- Algoblan, A.; Alalfi, M.; Khan, M. Mechanism linking diabetes mellitus and obesity. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 587–591. [Google Scholar] [CrossRef] [Green Version]
- Advika, T.S.; Idiculla, J.; Kumari, S.J. Exercise in patients with Type 2 diabetes: Facilitators and barriers—A qualitative study. J. Family Med. Prim. Care 2017, 6, 288–292. [Google Scholar] [CrossRef]
- Phu, S.; Boersma, D.; Duque, G. Exercise and Sarcopenia. J. Clin. Densitom. 2015, 18, 488–492. [Google Scholar] [CrossRef]
- Yoo, S.-Z.; No, M.-H.; Heo, J.-W.; Park, D.-H.; Kang, J.-H.; Kim, S.H.; Kwak, H.-B. Role of exercise in age-related sarcopenia. J. Exerc. Rehabil. 2018, 14, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Colberg, S.R.; Sigal, R.J.; Yardley, J.E.; Riddell, M.C.; Dunstan, D.W.; Dempsey, P.C.; Horton, E.S.; Castorino, K.; Tate, D.F. Physical Activity/Exercise and Diabetes: A Position Statement of the American Diabetes Association. Diabetes Care 2016, 39, 2065–2079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neville, S.E.; Boye, K.S.; Montgomery, W.S.; Iwamoto, K.; Okamura, M.; Hayes, R.P. Diabetes in Japan: A review of disease burden and approaches to treatment. Diabetes/Metab. Res. Rev. 2009, 25, 705–716. [Google Scholar] [CrossRef]
- Mumu, S.J.; Saleh, F.; Ara, F.; Afnan, F.; Ali, L. Non-adherence to life-style modification and its factors among type 2 diabetic patients. Indian J. Public Health 2014, 58, 40–44. [Google Scholar] [CrossRef]
- Greco, M.; Chiefari, E.; Montalcini, T.; Accattato, F.; Costanzo, F.S.; Pujia, A.; Foti, D.; Brunetti, A.; Gulletta, E. Early Effects of a Hypocaloric, Mediterranean Diet on Laboratory Parameters in Obese Individuals. Mediat. Inflamm. 2014, 2014, 750860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sardeli, A.; Komatsu, T.; Mori, M.; Gáspari, A.; Chacon-Mikahil, M. Resistance Training Prevents Muscle Loss Induced by Caloric Restriction in Obese Elderly Individuals: A Systematic Review and Meta-Analysis. Nutrients 2018, 10, 423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinheimer, E.M.; Sands, L.P.; Campbell, W.W. A systematic review of the separate and combined effects of energy restriction and exercise on fat-free mass in middle-aged and older adults: Implications for sarcopenic obesity. Nutr. Rev. 2010, 68, 375–388. [Google Scholar] [CrossRef]
- Bilsborough, S.; Mann, N. A review of issues of dietary protein intake in humans. Int. J. Sport Nutr. Exerc. Metab. 2006, 16, 129–152. [Google Scholar] [CrossRef]
- Borack, M.S.; Volpi, E. Efficacy and Safety of Leucine Supplementation in the Elderly. J. Nutr. 2016, 146, 2625S–2629S. [Google Scholar] [CrossRef] [Green Version]
- Robinson, S.; Cooper, C.; Aihie Sayer, A. Nutrition and Sarcopenia: A Review of the Evidence and Implications for Preventive Strategies. J. Aging Res. 2012, 2012, 5108016. [Google Scholar] [CrossRef]
- Wilkinson, D.J.; Hossain, T.; Hill, D.S.; Phillips, B.E.; Crossland, H.; Williams, J.; Loughna, P.; Churchward-Venne, T.A.; Breen, L.; Phillips, S.M.; et al. Effects of leucine and its metabolite β-hydroxy-β-methylbutyrate on human skeletal muscle protein metabolism. J. Physiol. 2013, 591, 2911–2923. [Google Scholar] [CrossRef]
- Kuriyan, R.; Lokesh, D.P.; Selvam, S.; Jayakumar, J.; Philip, M.G.; Shreeram, S.; Kurpad, A.V. The relationship of endogenous plasma concentrations of β-Hydroxy β-Methyl Butyrate (HMB) to age and total appendicular lean mass in humans. Exp. Gerontol. 2016, 81, 13–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesinovic, J.; Zengin, A.; De Courten, B.; Ebeling, P.R.; Scott, D. Sarcopenia and type 2 diabetes mellitus: A bidirectional relationship. Diabetes, Metabolic Syndrome and Obesity: Targets Ther. 2019, 12, 1057–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stockton, K.A.; Mengersen, K.; Paratz, J.D.; Kandiah, D.; Bennell, K.L. Effect of vitamin D supplementation on muscle strength: A systematic review and meta-analysis. Osteoporos. Int. 2010, 22, 859–871. [Google Scholar] [CrossRef]
- Beaudart, C.; Buckinx, F.; Rabenda, V.; Gillain, S.; Cavalier, E.; Slomian, J.; Petermans, J.; Reginster, J.-Y.; Bruyère, O. The Effects of Vitamin D on Skeletal Muscle Strength, Muscle Mass, and Muscle Power: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Endocrinol. Metab. 2014, 99, 4336–4345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facchini, F.S.; Humphreys, M.H.; DoNascimento, C.A.; Abbasi, F.; Reaven, G.M. Relation between insulin resistance and plasma concentrations of lipid hydroperoxides, carotenoids, and tocopherols. Am. J. Clin. Nutr. 2000, 72, 776–779. [Google Scholar] [CrossRef] [Green Version]
- Cesari, M.; Pahor, M.; Bartali, B.; Cherubini, A.; Penninx, B.W.; Williams, G.R.; Atkinson, H.; Martin, A.; Guralnik, J.M.; Ferrucci, L. Antioxidants and physical performance in elderly persons: The Invecchiare in Chianti (InCHIANTI) study. Am. J. Clin. Nutr. 2004, 79, 289–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozawa, A.; Jacobs, D.R.; Steffes, M.W.; Gross, M.D.; Steffen, L.M.; Lee, D.-H. Associations of Serum Carotenoid Concentrations with the Development of Diabetes and with Insulin Concentration: Interaction with Smoking. Am. J. Epidemiol. 2006, 163, 929–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franz, M.; Boucher, J.L.; Evert, A.B. Evidence-based diabetes nutrition therapy recommendations are effective: The key is individualization. Diabetes Metab. Syndr. Obes. Targets Ther. 2014, 7, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Fukushima, Y.; Kurose, S.; Shinno, H.; Cao Thu, H.; Takao, N.; Tsutsumi, H.; Kimura, Y. Importance of Lean Muscle Maintenance to Improve Insulin Resistance by Body Weight Reduction in Female Patients with Obesity. Diabetes Metab. J. 2016, 40, 147–153. [Google Scholar] [CrossRef]
- Ross, R.; Janssen, I.; Dawson, J.; Kungl, A.M.; Kuk, J.L.; Wong, S.L.; Nguyen-Duy, T.B.; Lee, S.; Kilpatrick, K.; Hudson, R. Exercise-induced reduction in obesity and insulin resistance in women: A randomized controlled trial. Obes. Res. 2004, 12, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Weinstock, R.S.; Dai, H.; Wadden, T.A. Diet and exercise in the treatment of obesity: Effects of 3 interventions on insulin resistance. Arch. Intern. Med. 1998, 158, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.; Jeong, I.-K.; Kim, M.Y.; Kim, Y.S.; Shin, S.; Kim, S.S.; Kim, J.H. Effects of Resistance Training and Aerobic Exercise on Insulin Sensitivity in Overweight Korean Adolescents: A Controlled Randomized Trial. Diabetes Metab. J. 2011, 35, 418–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takamura, Y.; Nomura, M.; Uchiyama, A.; Fujita, S. Effects of Aerobic Exercise Combined with Panaxatriol Derived from Ginseng on Insulin Resistance and Skeletal Muscle Mass in Type 2 Diabetic Mice. J. Nutr. Sci. Vitaminol. (Tokyo) 2017, 63, 339–348. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, F.; Hashimoto, Y.; Kaji, A.; Sakai, R.; Kawate, Y.; Okamura, T.; Kitagawa, N.; Okada, H.; Nakanishi, N.; Majima, S.; et al. Association between Geriatric Nutrition Risk Index and The Presence of Sarcopenia in People with Type 2 Diabetes Mellitus: A Cross-Sectional Study. Nutrients 2021, 13, 3729. [Google Scholar] [CrossRef]
| Study | Muscle Mass Criteria | Study Design | Result |
|---|---|---|---|
| Kim et al., 2010 [23] | SMI < 2 SDs | Cross-sectional study 2 groups: -T2DM: n = 414 -control group: n = 396 | SMI values were significantly decreased in patients with diabetes compared with control group Patients with diabetes had three times higher risk of having a low SMI than control group |
| Wang et al., 2016 [49] | AWGS criteria | Cross-sectional study Community-dwelling Chinese citizens (≥60 years) 2 groups: -T2DM Patients: n = 116 men + 120 women -control group: n = 404 men + 450 women | The prevalence of sarcopenia and pre-sarcopenia was significantly higher in diabetic patients than in healthy controls Diabetic patients was associated with a 1.56-fold increased risk of sarcopenia using AWGS criteria |
| Kim et al., 2014 [50] | Three different formulas for skeletal mass: (a) ASM/height2, (b) ASM/weight, (c) TSM/weight | Cross-sectional study Adults (≥65 years) 2 groups: -T2DM: n = 59 men + 85 women -control: n = 130 men + 140 women | Older men with T2DM showed significantly lower ASM than those without diabetes The risk of low muscle mass (in all formulas) was significantly higher in older men with T2DM |
| Souza et al., 2019 [24] | EWGSOP criteria | Cross-sectional study Older adults (>60 years) n = 1078 | Diabetes mellitus was present in 36.87% of the patients with sarcopenia using EWGSOP criteria |
| Leenders et al., 2013 [53] | DEXA, sit-to-stand test, handgrip test | Cross-sectional study (3 months) Community dwelling and still living Independently older men 2 groups: -T2DM: n = 60 men -control group: n = 32 men | Leg lean mass and ASM were significantly lower in older men with T2DM compared with normoglycemic controls Leg extension strength was significantly lower in the group with T2DM Significant longer sit-to-stand time for group with T2DM compared with normoglycemic group Significant lower handgrip strength for group with T2DM when compared with normoglycemic controls. |
| Kalyani et al., 2015 [52] | Knee extensor strength divided by DEXA-derived leg lean body mass | Longitudinal study (7.5 years) Adults (25–96 years) n = 984 | Muscle strength (knee extensor strength) and muscle quality (knee extensor strength/leg lean mass) were all significantly decreased from lower to higher HbA1c. Hyperglycemia is associated with persistently lower muscle strength with aging |
| Park et al., 2009 [38] | DEXA | Longitudinal study (6 years) well-functioning community-dwelling Adults (70–79 years) n = 2675 | Older adults with either diagnosed or undiagnosed Type 2 diabetes showed excessive loss of appendicular lean mass and trunk fat mass compared with nondiabetic subjects Thigh muscle cross-sectional area declined two times faster in older women with diabetes than their nondiabetic counterparts |
| Anagnostis et al., 2020 [47] | Multiple criteria depending on studies (EWGSOP, AWGS, FNIH) | Systematic review Patients with T2DM or sarcopenia n = 1832 + 1159 | Patients with T2DM demonstrated a higher risk of sarcopenia (using EWGSOP or AWGS or FNIH criteria) compared with euglycemic subjects Patients with T2DM have an increased risk of sarcopenia (using EWGSOP or AWGS or FNIH criteria) compared with euglycemic subjects |
| Ai et al., 2021 [51] | Multiple criteria depending on studies (EWGSOP, AWGS, FNIH, LMM, LMS, LSMI) | Systematic review Patients with T2DM n = 16634 | The pooled prevalence of sarcopenia in patients with T2DM was 18% Elder age, male gender and chronic hyperglycemia, Osteoporosis were significant risk factors for Sarcopenia |
| Veronese et al., 2019 [48] | AWGS, EWGSOP criteria | Systematic review Adults with mean age = 65.4 years n = 54676 | Diabetic participants had an increased prevalence of sarcopenia (using EWGSOP or AWGS criteria) compared to controls Sarcopenia (using EWGSOP or AWGS criteria) was associated with an increased odds of having diabetes |
| Chung et al., 2019 [54] | AWGS criteria | Systematic review Asian aged ≥60 years 2 groups: -diabetics: n = 1537 -non- diabetics: n = 5485 | Diabetics showed a significantly higher risk of sarcopenia (using AWGS criteria) than non-diabetics |
| Study | Study Design | Duration | Exercise Intervention | Nutritional Intervention | Sarcopenic Markers | T2DM Markers |
|---|---|---|---|---|---|---|
| Mitranun et al., 2014 [56] | Parallel-group randomized trial Diabetic patients (50–70 years) n = 16 men + 29 women 2 groups: continuous AT, interval AT | 12 weeks | 3 sessions/week AT: 30–40 min walking | Significant increase in leg muscle strength for both AT No significant change in upper body strength | Significant decrease in fasting glucose concentration and insulin resistance in both exercise groups Significant decrease in HbA1c in interval AT | |
| Tan et al., 2015 [73] | Randomized control trial Diabetic patients (>60 years) n = 13 men + 12 women 2 groups: CT, CON | 6-month | 3 sessions/week CT: 30 min moderate AT; 10 min RT | Significant increase in the leg muscle strength for CT group Significant increase in 6-MWD for the CT group | Significant decrease for CT group in concentrations of fasting and 2-h post-glucose challenge plasma glucose, serum insulin, HbA1c | |
| Egger et al., 2013 [84] | Parallel-group randomized trial Diabetic patients (64.8 ± 7.8 years) n = 13 men and 19 women 2 groups: CON, Hypertrophy CT, Endurance CT | 8 weeks | 7 sessions/week Hypertrophy CT: RT:10–12 repetitions,70% 1-RM; AT 1 h/day Endurance CT: RT: 25–30 repetitions, 40% 1-RM; AT 1 h/day | Significant increase in muscle strength and muscle mass for both groups Significant increase in strength in hypertrophy CT versus endurance CT | HbA1c did not change significanlty Significant reductions in fasting glucose, fructosamine | |
| Chen, et al., 2017 [59] | Randomized control trial Sarcopenic patients with obesity (>65 years) n = 60 4 groups: AT, RT, CT, CON | 12 weeks | RT: 60–70% 1-RM, 3 sets 8–10 repetitions, 2 sessions/week AT: 1 h, 2 sessions/week CT: 1 session/week RT, 1 session/week AT | Significant increase in skeletal muscle mass and strength for AT, RT, CT groups vs. CON Significant increase in grip strength at weeks 8 and 12 in the RT group vs. AT, CT, CON groups | Significant increase in IGF-1 concentration at week 8 for the RT, CT groups vs. AT, CON groups. At Week 12, no significant differences were observed among the four groups. | |
| Lustosa et al. 2011 [64] | Randomized, crossover trial Pre-frail community-dwelling women (>65) n = 322 groups: RT, CON | 10 weeks | 3 sessions/week RT 75% 1-RM, 8 repetitions | Significant improvement in TUG, 10-MWT and knee extensor’s muscle strength | Not measured | |
| Latham et al., 2013 [72] | Randomized control trial Frail patients (>65 years) n = 243 4 groups: RT, CON, Vitamin D, placebo | 10 weeks | RT 60–80% 1-RM | Single dose of vitamin D | No significant results in TUG and MWD tests Exercise non-significantly increased the risk of musculoskeletal injury | Not measured |
| Seynnes et al., 2004 [65] | Randomized control trial Frail patients (>70 years) n = 22 3 groups: high intensity RT, low intensity RT, CON | 10 weeks | 3 sessions/week Highly intensive RT: 80% 1-RM, 3 sets of 8 repetitions Low intensive RT: 40% 1-RM, 3 sets of 8 repetitions | RT groups significantly improved knee extensor strength, endurance, stair-climbing power, and chair-rising time 6-MWD significantly improved only in the high intensity RT | Not measured | |
| Semba et al., 2003 [82] | Cross-sectional study Non-disabled to severely disabled women (>65 years) n = 669 | 8 years | Data analysis | Higher plasma concentrations of α-carotene, β-carotene, β-cryptoxanthin, and lutein/zeaxanthin were associated with reduced grip, hip, and knee strength with 95% confidence interval | Not measured | |
| Bischoff, et al., 1999 [79] | Cross-sectional study Frail patients (>65 years) n = 216 men + 103 women | 2 years | Data analysis | Vitamin D related significantly to both sexes with the strength degradation | Not measured | |
| Okamura et al., 2020 [81] | Cohort study Diabetic people (>65) n = 180 men + 162 women | 2 years | Data analysis | Low omega-3 fatty acids levels intake was significantly associated with the presence of sarcopenia based on Japan Society of Hepatology | Not measured | |
| Takahashi et al., 2021 [78] | Cohort study Diabetic people (>65) n = 112 men + 85 women | 2 years | Data analysis | Low levels of vitamin D, intake was significantly related to the loss of muscle mass Low levels of vitamin B1 and vitamin B12 intake was non-significantly related to the loss of muscle mass Vitamin A, vitamin B1, vitamin B2, vitamin B6, vitamin C and vitamin E were not found to be significantly related to the loss of muscle mass | Not measured | |
| Tieland et al., 2012 [75] | Randomized, Double-Blind, Placebo-Controlled Trial Frail patients (>65 years) n = 65 | 24 weeks | 15 g protein 2 times/day | Skeletal muscle mass did not change significantly in the protein or placebo group Leg extension strength significantly increased in protein group Physical performance improved significantly in protein group TUG, MWD and handgrip strength were not significantly associated with protein or placebo group | Not measured | |
| Terada et al., 2013 [58] | Parallel-group randomized Diabetic patients (55–75 years) n = 15 = 8 males + 7 women 2 groups: interval AT, continuous AT | 12 weeks | 5 sessions/week AT high intensity interval exercise: cycling and treadmill walking; 1-min intervals at 100% VO2R followed by 3-min recovery intervals at 20% VO2R; AT moderate intensity continuous exercise: stationary cycling and treadmill walking; continuous exercise at 40% VO2R; | Not measured | HbA1c, fasting blood glucose did not significantly change from baseline | |
| Amamou et al., 2017 [85] | Parallel-group randomized trial Overweight adults (60 -75 years) with at least 2 factors of metabolic syndrome n = 17 men + 14 women 2 groups: high protein, high protein + RT | 6-weeks | 3 sessions/week RT: 65–80% 1-RM, 2 sets of 8–15 repetitions | Caloric intake was reduced by 500 kcal/d in all participants and protein intake ~1.4 g/kg/d | Not measured | Significant decrease in fasting glucose and triglycerides in both groups |
| Kadoglou et al., 2013 [68] | Randomized control trial Diabetic people (>55) n = 100 4 groups: AT, RT, CT, CON | 16 weeks | AT: 60–75% of maximum heart rate RT: 60–80% 1-RM CT: above AT + RT | Not measured | All exercise groups significantly ameliorated glycemic profile, since the reduction in fasting plasma glucose, HbA1c, fasting insulin levels, insulin resistance and triglycerides levels was significant compared with the control group | |
| Castaneda et al., 2002 [70] | Randomized control trial Diabetic people (>55) n = 62 2 groups: RT, CON | 3 sessions/week RT: 60–70% 1-RM | Not measured | Significant decrease in HbA1c, fasting plasma glucose concentrations for RT group Non-significant decrease in serum triglyceride concentrations in RT group | ||
| Van Loon et al., 2003 [76] | Cross-sectional study T2DM + healthy people (>55) n = 20 10 were T2DM patients and 10 were CON | 1 week | 2 sessions Session 1: both groups received carbohydrates Session 2: both groups received carbohydrates with amino acid/protein mixture | Not measured | Significant increase in insulin responses for mixture intervention group Non-significant differences were observed in plasma glucose, glucagon, growth hormone, IGF-I, within 2-h time frame for the intervention groups. | |
| Huang et al., 2014 [66] | Cross-sectional study Diabetic people (>65) n = 210 4 groups: low protein (<0.6 gr/d/kg), moderate protein (>0.6, <0.8 gr/d/kg), high protein (>0.8, <1 gr/d/kg), very high protein (>1 gr/d/kg) | unclear | Data analysis | Not measured | Significant reduction in HbA1c and triglycerides in high and very high protein groups There were observed non-significant reductions in fasting plasma glucose | |
| Moslehi et al., 2015 [77] | Nested case–control study Healthy people that developed T2DM n = 698 2 groups: T2DM, CON | 7 years | Data analysis | Not measured | Milk intake decreased non-significantly the T2DM risk in men but not in women. There was no significant association between diabetes and total dairy intake No significant association was observed between diabetes and carbohydrate, protein, fermented dairy, grain, fruit, vegetable, meat | |
| Vlietstra et al., 2018 [60] | Systematic review Adults with sarcopenia (>60 years) | 3–6 months | Multiple exercise interventions | Knee-extension strength, TUG, appendicular muscle mass and leg muscle mass significantly improved in response to exercise interventions MWD did not significantly improved | Not measured | |
| Yoshimura et al., 2017 [62] | Systematic Review Adults with sarcopenia(>60 years) | 3–6 months | Multiple exercise interventions | Multiple nutritional interventions | Exercise interventions did not significantly change muscle mass, muscle strength, and walking speed Nutritional interventions did not significantly change muscle strength A combined intervention of exercise and nutrition did not significantly change the walking speed. | Not measured |
| Wu et al., 2021 [63] | Systematic Review older adults with sarcopenia (>65 years) | RT | Multiple nutritional interventions | RT alone and the combination of RT and nutrition significantly increased handgrip strength and improved dynamic balance | Not measured | |
| Liao et al., 2019 [83] | Systematic Review Older adults with a high risk of sarcopenia or frailty and physical limitations (>60 years) | RT or a multicomponent exercise regime that consisted of MSE, aerobic exercise, balance training, and physical activity training | Protein supplementation | The protein supplement + exercise group exhibited significant improvements in the whole-body lean mass, appendicular lean mass, leg strength, and walking capability | Not measured | |
| Lucato et al., 2017 [80] | Systematic review Adults evolved T2DM (>60 years) n = 28258 | Vitamin D analysis | Not measured | Hypovitaminosis D is significantly associated with an elevated risk of future diabetes in older people | ||
| Hovanec et al., 2012 [69] | Systematic review Adults with T2DM (>65 years) | RT | RT had increased significantly lower body muscle strength, upper body muscle strength and whole body muscle strength | RT did not cause any significant decrease in HbA1c and fasting glucose | ||
| Irvine et al., 2009 [67] | Systematic review Adults with T2DM (mean age = 58.4) n = 372 | Progressive RT | Progressive RT resulted in significant improvements in strength when compared to AT or no exercise | Compared to control, progressive RT led to small and statistically significant absolute reductions in HbA1c of 0.3% Compared to AT there were no significant differences in HbA1c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Argyropoulou, D.; Geladas, N.D.; Nomikos, T.; Paschalis, V. Exercise and Nutrition Strategies for Combating Sarcopenia and Type 2 Diabetes Mellitus in Older Adults. J. Funct. Morphol. Kinesiol. 2022, 7, 48. https://doi.org/10.3390/jfmk7020048
Argyropoulou D, Geladas ND, Nomikos T, Paschalis V. Exercise and Nutrition Strategies for Combating Sarcopenia and Type 2 Diabetes Mellitus in Older Adults. Journal of Functional Morphology and Kinesiology. 2022; 7(2):48. https://doi.org/10.3390/jfmk7020048
Chicago/Turabian StyleArgyropoulou, Dionysia, Nikolaos D. Geladas, Tzortzis Nomikos, and Vassilis Paschalis. 2022. "Exercise and Nutrition Strategies for Combating Sarcopenia and Type 2 Diabetes Mellitus in Older Adults" Journal of Functional Morphology and Kinesiology 7, no. 2: 48. https://doi.org/10.3390/jfmk7020048
APA StyleArgyropoulou, D., Geladas, N. D., Nomikos, T., & Paschalis, V. (2022). Exercise and Nutrition Strategies for Combating Sarcopenia and Type 2 Diabetes Mellitus in Older Adults. Journal of Functional Morphology and Kinesiology, 7(2), 48. https://doi.org/10.3390/jfmk7020048








