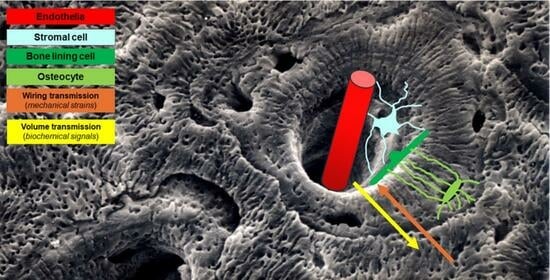
The Osteocyte: From “Prisoner” to “Orchestrator”
Abstract
1. Osteoblast-to-Osteocyte Transformation
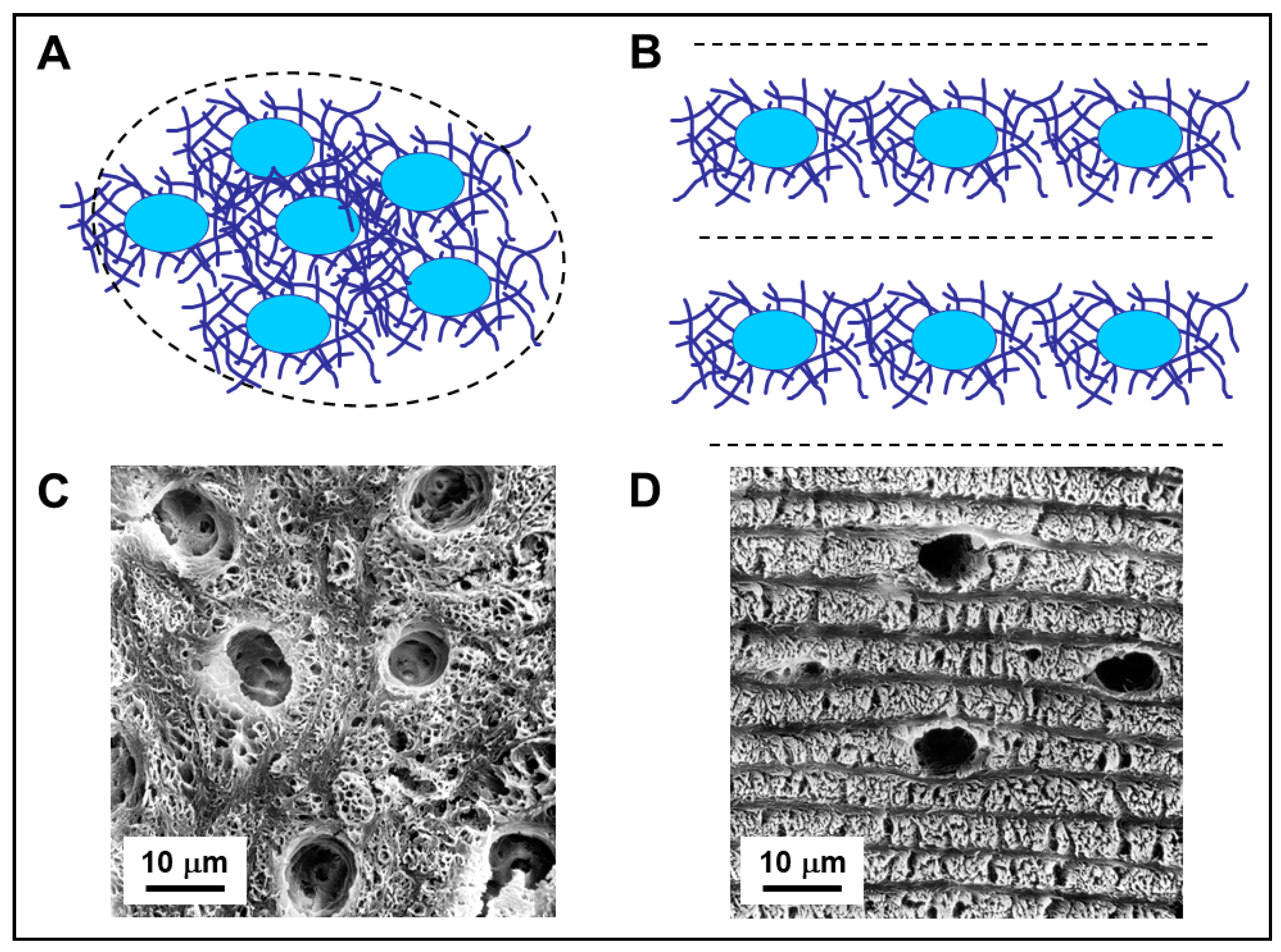
2. Osteogenesis Processes and Osteocyte Morphology
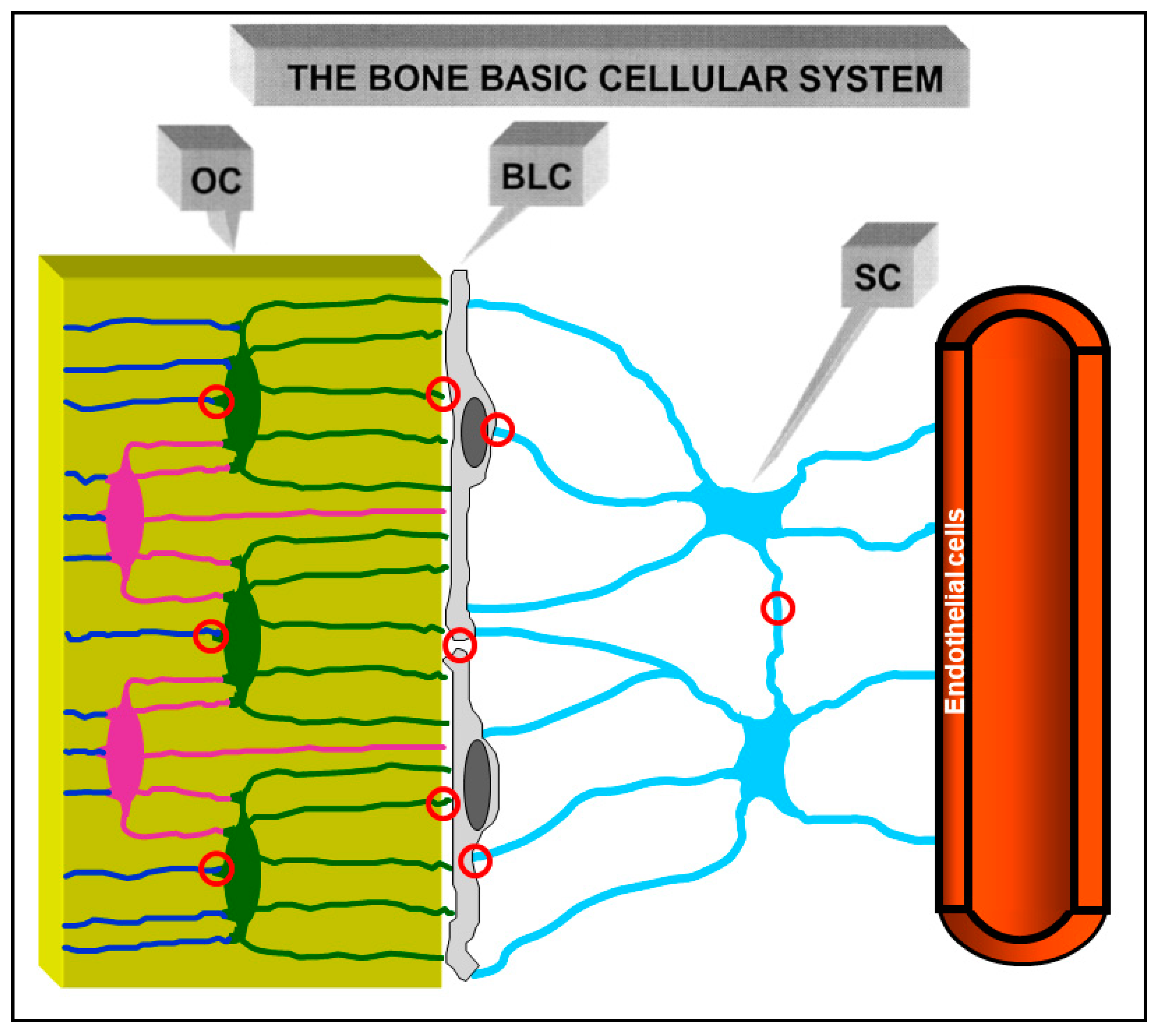
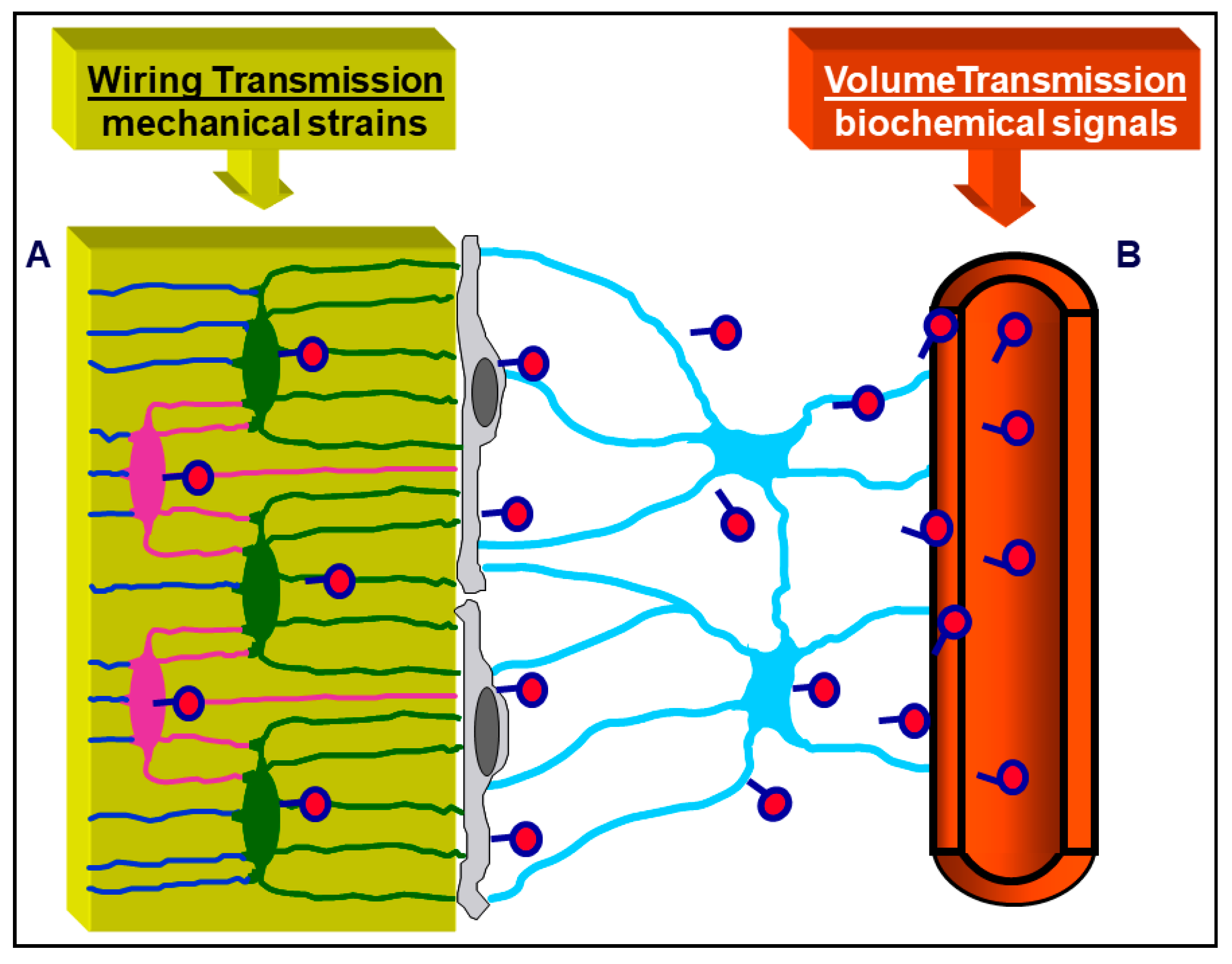
3. Osteocyte Network and Communications
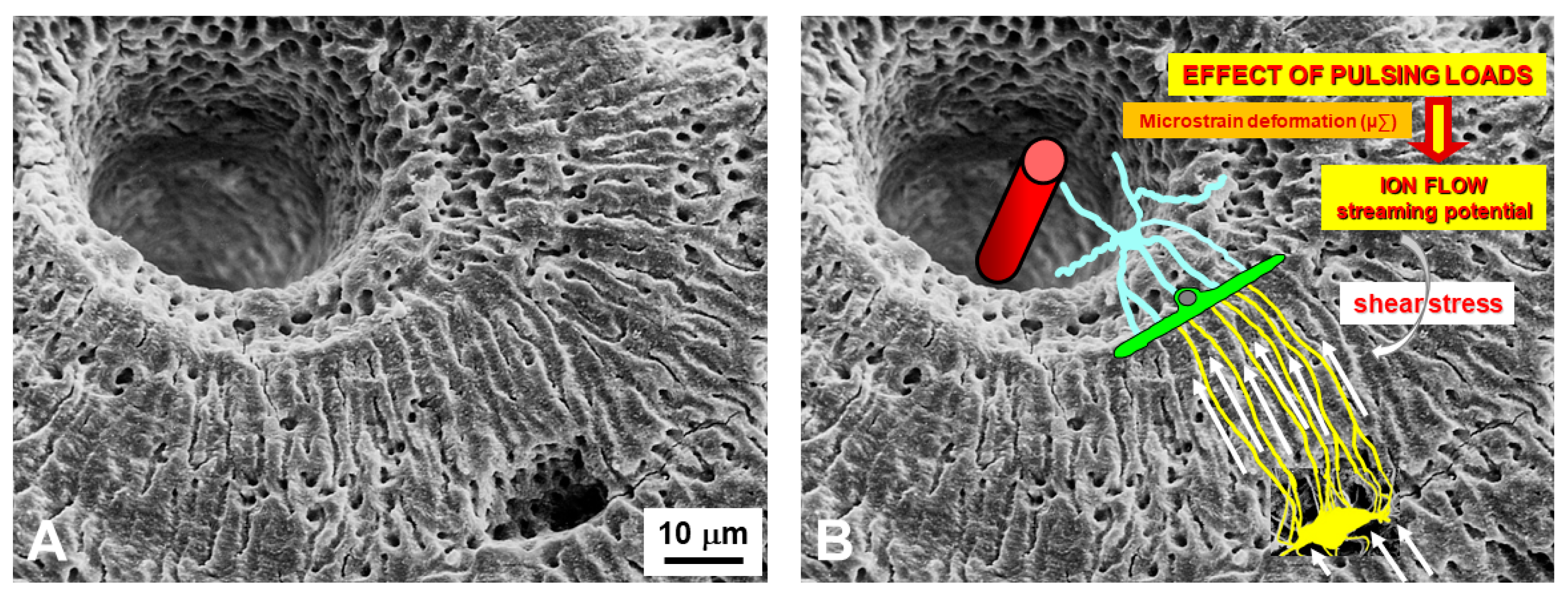
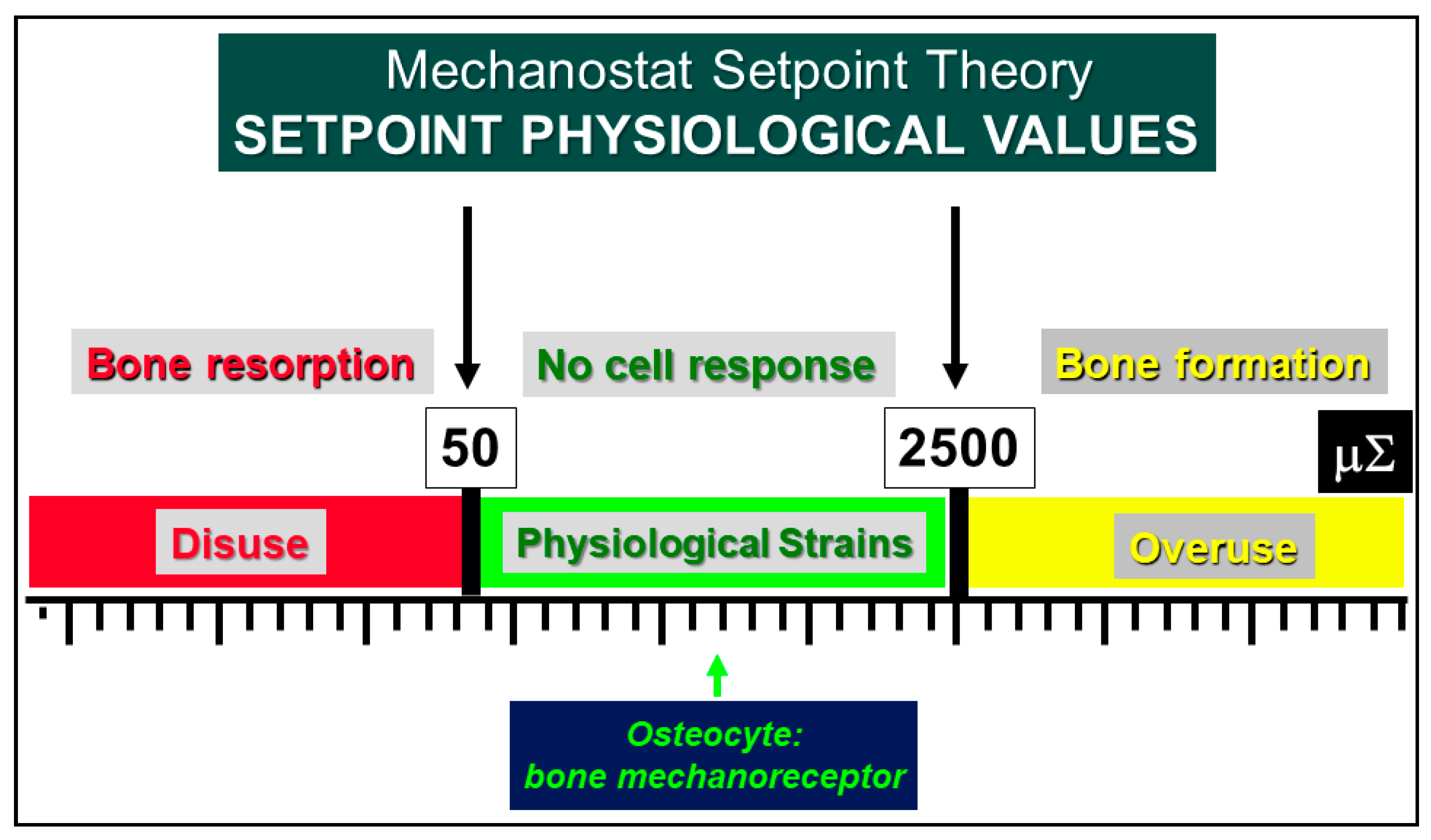
4. Osteocytes as Bone Mechanical Sensors and Transducers of Mechanical Strains into Biological Signals
5. Bone Remodeling and Sclerostin Involvement
6. Interplay between Mineral and Skeletal Homeostasis and Osteocyte Role Mediated by Sclerostin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gegenbaur, C. Über die Bildung des Knochengewebes I u II. Z. Naturwiss 1864, 1, 343–369. [Google Scholar]
- Waldeyer, W. Über den Ossifikationsprozess. Arch. Mikrosk. Anat. Entw. Mech. 1865, 1, 354–375. [Google Scholar] [CrossRef]
- Waldeyer, W. Über den Ossifikationsprozess. Zentbl. Med. Wiss 1865, 6, 113–116. [Google Scholar]
- Dudley, H.R.; Spiro, D. The fine structure of bone cells. J. Biophys. Biochem. Cytol. 1961, 11, 627–649. [Google Scholar] [CrossRef]
- Hancox, N.M.; Boothroyd, B. Electron microscopy of the early stages of osteogenesis. Clin. Orthop. 1965, 40, 153–161. [Google Scholar] [CrossRef]
- Cameron, D.A. The ultrastructure of bone. In The Biochemistry and Physiology of Bone; Bourne, G.H., Ed.; Academic Press: New York, NY, USA, 1972; Volume I, pp. 191–236. [Google Scholar]
- Rasmussen, H.; Bordier, P. The Physiological and Cellular Basis of Metabolic Bone Disease; The William & Wilkins Company: Baltimore, MD, USA, 1974. [Google Scholar]
- Njweide, P.J.; van der Plas, A.; Scherft, J.P. Biochemical and histological studies on various bone cell preparation. Calcif. Tissue Int. 1981, 33, 529–540. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C. A three-dimensional ultrastructural study of osteoid-osteocytes in the tibia of chick embryos. Cell Tissue Res. 1986, 246, 125–131. [Google Scholar] [CrossRef] [PubMed]
- Marotti, G.; Cane, V.; Palazzini, S.; Palumbo, C. Structure-function relationships in the osteocyte. Ital. J. Miner. Electrolyte Metab. 1990, 4, 93–106. [Google Scholar]
- Palumbo, C.; Palazzini, S.; Zaffe, D.; Marotti, G. Osteocyte differentiation in the tibia of newborn rabbit: An ultrastructural study of the formation of cytoplasmic processes. Acta Anat. 1990, 137, 350–358. [Google Scholar] [CrossRef]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Schaffler, M.B.; Cheung, W.Y.; Majeska, R.; Kennedy, O. Osteocytes: Master orchestrators of bone. Calcif. Tissue Int. 2014, 94, 5–24. [Google Scholar] [CrossRef]
- Palumbo, C.; Palazzini, S.; Marotti, G. Morphological study of intercellular junctions during osteocyte differentiation. Bone 1990, 11, 401–406. [Google Scholar] [CrossRef]
- Miller, S.C.; Bowman, B.M.; Smith, J.M.; Jee, W.S. Characterization of endosteal bone-lining cells from fatty marrow bone sites in adult beagles. Anat. Rec. 1980, 198, 163–173. [Google Scholar] [CrossRef]
- Doty, S.B. Cell-to-cell communication in bone tissue. In Davidovich V: The Biological Mechanism of Tooth Eruption and Root Resorption; Davidovitch, V., Ed.; EBSCO Media: Birmingham, AL, USA, 1988; pp. 61–69. [Google Scholar]
- Ferretti, M.; Palumbo, C.; Contri, M.; Marotti, G. Static and dynamic osteogenesis: Two different types of bone formation. Anat. Embryol. 2002, 206, 21–29. [Google Scholar] [CrossRef]
- Ferretti, M.; Palumbo, C.; Bertoni, L.; Cavani, F.; Marotti, G. Does static precede dynamic osteogenesis in endochondral ossification as occurs in intramembranous ossification? Anat. Rec. 2006, 288A, 1158–1162. [Google Scholar] [CrossRef]
- Palumbo, C.; Ferretti, M.; De Pol, A. Apoptosis during intramembranous ossification. J. Anat. 2003, 203, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Palumbo, C.; Ferretti, M.; Marotti, G. Osteocyte dendrogenesis in static and dynamic bone formation: An ultrastructural study. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2004, 278, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Palumbo, C. Static osteogenesis versus dynamic osteogenesis: A comparison between two different types of bone formation. Appl. Sci. 2021, 11, 2025. [Google Scholar] [CrossRef]
- Marotti, G. The structure of bone tissues and the cellular control of their deposition. Ital. J. Anat. Embryol. 1996, 101, 25–79. [Google Scholar] [PubMed]
- Marotti, G. The original contribution of the scanning electron microscope to the knowledge of bone structure. In Ultrastructure of Skeletal Tissues; Bonucci, E., Motta, P.M., Eds.; Kluwer Academic Publisher: Boston, MA, USA, 1990; pp. 19–39. [Google Scholar]
- Marotti, G.; Muglia, M.A.; Palumbo, C. Collagen texture and osteocyte distribution in lamellar bone. Ital. J. Anat. Embryol. 1995, 100 (Suppl. S1), 95–102. [Google Scholar]
- Rensberger, J.M.; Watabe, M. Fine structure of bone in dinosaurs, birds and mammals. Nature 2000, 406, 619–622. [Google Scholar] [CrossRef]
- D’Emic, M.D.; Benson, R.B.J. Measurement, variation, and scaling of osteocyte lacunae: A case study in birds. Bone 2013, 57, 300–310. [Google Scholar] [CrossRef]
- Dong, P.; Haupert, S.; Hesse, B.; Langer, M.; Gouttenoire, P.J.; Bousson, V.; Peyrin, F. 3D osteocyte lacunar morphometric properties and distributions in human femoral cortical bone using synchrotron radiation micro-CT images. Bone 2014, 60, 172–185. [Google Scholar] [CrossRef]
- Van Oers, R.F.M.; Wang, H.; Bacabac, R.G. Osteocyte shape and mechanical loading. Curr. Osteoporos. Rep. 2015, 13, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Marotti, G. A new theory of bone lamellation. Calcif. Tissue Int. 1993, 53 (Suppl. S1), S47–S56. [Google Scholar] [CrossRef]
- Wang, Y.; McNamara, L.M.; Schaffler, M.B.; Weinbaum, S. Strain amplification and integrin based signaling in osteocytes. J. Musculoskelet. Neuronal Interact. 2008, 8, 332–334. [Google Scholar] [PubMed]
- Marotti, G. The osteocyte as a wiring transmission system. J. Musculoskelet. Neuronal Interact. 2000, 1, 133–136. [Google Scholar] [PubMed]
- Marotti, G.; Palumbo, C. The mechanism of transduction of mechanical strains into biological signals at the bone cellular level. Eur. J. Histochem. 2007, 51 (Suppl. S1), 15–19. [Google Scholar] [PubMed]
- Palazzini, S.; Palumbo, C.; Ferretti, M.; Marotti, G. Stromal cell structure and relationships in perimedullary spaces of chick embryo shaft bones. Anat. Embryol. 1998, 197, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Parfitt, A.M. The physiologic and clinical significance of bone histomorphometric data. In Bone Histomorphometry; Recker, R.R., Ed.; CRC Press: Boca Raton, FL, USA; Ann Arbor, MI, USA; London, UK; Tokyo, Japan, 1983; pp. 143–223. [Google Scholar]
- Marotti, G.; Palazzini, S.; Palumbo, C. Evidence of a twofold regulation of osteoblast activity: “Volume transmission” and “Wiring transmission”. Calcif. Tissue Int. 1993, 53, 440. [Google Scholar]
- Marotti, G.; Palazzini, S.; Palumbo, C.; Ferretti, M. Ultrastructural evidence of the existence of a dendritic network throughout the cells of the osteogenic lineage: The novel concept of wiring- and volume-transmission in bone. Bone 1996, 19, 151S. [Google Scholar] [CrossRef]
- Zaman, G.; Pitsillides, A.A.; Rawlinson, S.C.; Suswillo, R.F.; Mosley, J.R.; Cheng, M.Z.; Platts, L.A.; Hukkanen, M.; Polak, J.M.; Lanyon, L.E. Mechanical strain stimulates nitric oxide production by rapid activation of endothelial nitric oxide synthase in osteocytes. J. Bone Miner. Res. 1999, 14, 1123–1131. [Google Scholar] [CrossRef] [PubMed]
- Bakker, A.D.; Soejima, K.; Klein-Nulend, J.; Burger, E.H. The production of nitric oxide and prostaglandin E(2) by primary bone cells is shear stress dependent. J. Biomech. 2001, 34, 671–677. [Google Scholar] [CrossRef]
- Watanuki, M.; Sakai, A.; Sakata, T.; Tsurukami, H.; Miwa, M.; Uchida, Y.; Watanabe, K.; Ikeda, K.; Nakamura, T. Role of inducible nitric oxide synthase in skeletal adaptation to acute increases in mechanical loading. J. Bone Miner. Res. 2002, 17, 1015–1025. [Google Scholar] [CrossRef]
- Basso, N.; Heersche, J.N. Effects of hind limb unloading and reloading on nitric oxide synthase expression and apoptosis of osteocytes and chondrocytes. Bone 2006, 39, 807–814. [Google Scholar] [CrossRef]
- Klein-Nulend, J.; van der Plas, A.; Semeins, C.M.; Ajubi, N.E.; Frangos, J.A.; Nijweide, P.J.; Burger, E.H. Sensitivity of osteocytes to biomechanical stress in vitro. FASEB J. 1995, 9, 441–445. [Google Scholar] [CrossRef]
- Rubinacci, A.; Villa, I.; Dondi Benelli, F.; Borgo, E.; Ferretti, M.; Palumbo, C.; Marotti, G. Osteocyte-bone lining cell system at the origin of steady ionic current in amphibian bone. Calcif. Tissue Int. 1998, 63, 331–339. [Google Scholar] [CrossRef]
- Rubinacci, A.; Covini, M.; Bisogni, C.; Villa, I.; Galli, M.; Palumbo, C.; Ferretti, M.; Muglia, M.A.; Marotti, G. Bone as an ion exchange system: Evidence for a link between mechanotrasduction and metabolic needs. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E851–E864. [Google Scholar] [CrossRef][Green Version]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Pead, M.J.; Suswillo, R.; Skerry, T.M.; Vedi, S.; Lanyon, L.E. Increased 3H uridine levels in osteocytes following a single period of dynamic bone loading in vivo. Calcif. Tissue Int. 1988, 43, 92–96. [Google Scholar] [CrossRef]
- Skerry, T.M.; Bitensky, L.; Chayen, J.; Lanyon, L.E. Early strain-related changes in enzyme activity in osteocytes following bone loading in vivo. J. Bone Miner. Res. 1989, 4, 783–788. [Google Scholar] [CrossRef]
- El-Haj, A.J.; Minter, S.L.; Rawlinson, S.C.; Suswillo, R.; Lanyon, L.E. Cellular responses to mechanical loading in vitro. J. Bone Miner. Res. 1990, 5, 923–932. [Google Scholar] [CrossRef]
- Turner, C.H. Homeostatic control of bone structure: An application of feedback theory. Bone 1991, 12, 203–217. [Google Scholar] [CrossRef]
- Turner, C.H. Functional determinants of bone structure: Beyond Wolff’s law of bone transformation. Bone 1992, 13, 403–409. [Google Scholar] [CrossRef]
- Lozupone, E.; Favia, A.; Grimaldi, A. Effect of intermittent mechanical force of bone tissue in vitro: Preliminary results. J. Bone Miner. Res. 1992, 7 (Suppl. S2), S407–S409. [Google Scholar] [CrossRef]
- Ypey, D.L.; Weidema, A.F.; Höld, K.M.; Van der Laarse, A.; Ravesloot, J.H.; Van Der Plas, A.; Nijweide, P.J. Voltage, calcium and stretch activated ionic channels and intracellular calcium in bone cells. J. Bone Miner. Res. 1992, 7 (Suppl. S2), S377–S387. [Google Scholar] [CrossRef] [PubMed]
- Burger, E.H.; Veldhuijzen, J.P. Influence of mechanical factors on bone formation, resorption and growth in vitro. In Bone Growth-B.; Hall, B.K., Ed.; CRC Press: Boca Raton, FL, USA; Ann Arbor, MI, USA; London, UK; Tokyo, Japan, 1993; pp. 37–56. [Google Scholar]
- Dallas, S.L.; Zaman, G.; Pead, M.J.; Lanyon, L.E. Early strain-related changes in cultured embryonic chick tibiotarsi parallel those associated with adaptive modeling in vivo. J. Bone Miner. Res. 1993, 8, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Dodds, R.A.; Ali, N.; Pead, M.J.; Lanyon, L.E. Early loading-related changes in the activity of glucose-6-phosphate dehydrogenase and alkaline phosphatase in osteocytes and periosteal osteoblasts in rat fibulae in vivo. J. Bone Miner. Res. 1993, 8, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.L.; Turner, C.H. Mechanotransduction and functional response of bone to mechanical strain. Calcif. Tissue Int. 1995, 57, 344–358. [Google Scholar] [CrossRef]
- Marotti, G. Morphological evidence of “wiring transmission” throughout the cell of the osteogenic lineage. Calcif. Tissue Int. 1995, 56, 437. [Google Scholar]
- Vatsa, A.; Breuls, R.G.; Semeins, C.M.; Salmon, P.L.; Smit, T.H.; Klein-Nulend, J. Osteocyte morphology in fibula and calvaria—Is there a role for mechanosensing? Bone 2008, 43, 452–458. [Google Scholar] [CrossRef]
- Bertacchini, J.; Benincasa, M.; Checchi, M.; Cavani, F.; Smargiassi, A.; Ferrett, M.; Palumbo, C. Expression and functional proteomic analyses of osteocytes from Xenopus laevis tested under mechanical stress conditions: Preliminary observations on an appropriate new animal model. J. Anat. 2017, 231, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. The Utah paradigm of skeletal physiology: An overview of its insights for bone, cartilage and collagenous tissue organs. J. Bone Miner. Metab. 2000, 18, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Frost, H.M. The Utah paradigm on animal models of skeletal disorders: Quo vadis? J. Musculoskelet. Neuronal Interact. 2001, 1, 185–191. [Google Scholar]
- Jee, W.S.S. Principles in bone physiology. J. Musculoskelet. Neuronal Interact. 2000, 1, 11–13. [Google Scholar]
- Lozupone, E.; Palumbo, C.; Favia, A.; Ferretti, M.; Palazzini, S.; Cantatore, F.P. Intermittent compressive load stimulates osteogenesis and improves osteocyte viability in bones cultured “in vitro”. Clin. Rheumatol. 1996, 15, 563–572. [Google Scholar] [CrossRef] [PubMed]
- Doty, S.B. Morphological evidence of gap junctions between bone cells. Calcif. Tissue Int. 1981, 33, 509–512. [Google Scholar] [CrossRef]
- Piekarski, K.; Munro, M. Transport mechanism operating between blood supply and osteocytes in long bones. Nature 1977, 269, 80–82. [Google Scholar] [CrossRef]
- Palumbo, C.; Ferretti, M.; Ardizzoni, A.; Zaffe, D.; Marotti, G. Osteocyte-osteoclast morphological relationships and the putative role of osteocytes in bone remodeling. J. Musculoskeletal. Neuronal Interact. 2001, 1, 327–332. [Google Scholar]
- Toscani, D.; Bolzoni, M.; Ferretti, M.; Palumbo, C.; Giuliani, N. Role of Osteocytes in Myeloma Bone Disease: Anti-sclerostin Antibody as New Therapeutic Strategy. Front. Immunol. 2018, 9, 2467. [Google Scholar] [CrossRef]
- Everts, V.; Delaissé, J.M.; Korper, W.; Jansen, D.C.; Tigchelaar-Gutter, W.; Saftig, P.; Beertsen, W. The bone lining cell: Its role in cleaning Howship’s lacunae and initiating bone formation. J. Bone Miner. Res. 2002, 17, 77–90. [Google Scholar] [CrossRef]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Delaisse, J.M. The reversal phase of the bone-remodeling cycle: Cellular prerequisites for coupling resorption and formation. Bonekey Rep. 2014, 3, 561. [Google Scholar] [CrossRef]
- Matsuo, K.; Otaki, N. Bone cell interactions through Eph/ephrin: Bone modeling, remodeling and associated diseases. Cell Adh. Migr. 2012, 6, 148–156. [Google Scholar] [CrossRef]
- Sims, N.A.; Martin, T.J. Coupling signals between the osteoclast and osteoblast: How are messages transmitted between these temporary visitors to the bone surface? Front. Endocrinol. 2015, 6, 41. [Google Scholar] [CrossRef]
- Delgado-Calle, J.; Sañudo, C.; Bolado, A.; Fernández, A.F.; Arozamena, J.; Pascual-Carra, M.A.; Rodriguez-Rey, J.C.; Fraga, M.F.; Bonewald, L.; Riancho, J.A. DNA methylation contributes to the regulation of sclerostin expression in human osteocytes. J. Bone Miner. Res. 2012, 27, 926–937. [Google Scholar] [CrossRef]
- Sapir-Koren, R.; Livshits, G. Osteocyte control of bone remodeling: Is sclerostin a key molecular coordinator of the balanced bone resorption–formation cycles? Osteoporos. Int. 2014, 25, 2685–2700. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Kang, H.; Liu, W.; Liu, P.; Zhang, J.; Harris, S.E.; Wu, D. Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J. Biol. Chem. 2005, 280, 19883–19887. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Bryant, H.U.; Macdougald, O.A. Regulation of bone mass by Wnt signaling. J. Clin. Investig. 2006, 116, 1202–1209. [Google Scholar] [CrossRef] [PubMed]
- Baron, R.; Kneissel, M. WNT signaling in bone homeostasis and disease: From human mutations to treatments. Nat. Med. 2013, 19, 179–192. [Google Scholar] [CrossRef] [PubMed]
- You, L.; Temiyasathit, S.; Lee, P.; Kim, C.H.; Tummala, P.; Yao, W.; Kingery, W.; Malone, A.M.; Kwon, R.Y.; Jacobs, C.R. Osteocytes as mechanosensors in the inhibition of bone resorption due to mechanical loading. Bone 2008, 42, 172–179. [Google Scholar] [CrossRef]
- Wijenayaka, A.R.; Kogawa, M.; Lim, H.P.; Bonewald, L.F.; Findlay, D.M.; Atkins, G.J. Sclerostin stimulates osteocyte support of osteoclast activity by a RANKL-dependent pathway. PLoS ONE 2011, 6, e25900. [Google Scholar] [CrossRef]
- Honma, M.; Ikebuchi, Y.; Kariya, Y.; Hayashi, M.; Hayashi, N.; Aoki, S.; Suzuki, H. RANKL subcellular trafficking and regulatory mechanisms in osteocytes. J. Bone Miner. Res. 2013, 28, 1936–1949. [Google Scholar] [CrossRef]
- Li, X.; Niu, Q.T.; Warmington, K.S.; Asuncion, F.J.; Dwyer, D.; Grisanti, M.; Han, C.Y.; Stolina, M.; Eschenberg, M.J.; Kostenuik, P.J.; et al. Progressive increases in bone mass and bone strength in an ovariectomized rat model of osteoporosis after 26 weeks of treatment with a sclerostin antibody. Endocrinology 2014, 155, 4785–4797. [Google Scholar] [CrossRef][Green Version]
- Nioi, P.; Taylor, S.; Hu, R.; Pacheco, E.; He, Y.D.; Hamadeh, H.; Paszty, C.; Pyrah, I.; Ominsky, M.S.; Boyce, R.W. Transcriptional profiling of laser capture microdissected subpopulations of the osteoblast lineage provides insight into the early response to Sclerostin antibody in rats. J. Bone Miner. Res. 2015, 30, 1457–1467. [Google Scholar] [CrossRef]
- Toscani, D.; Dalla Palma, B.; Palumbo, C.; Ferretti, M.; Bolzoni, M.; Guasco, D.; Mancini, C.; Martella, E.; Lazzaretti, M.; Pedrazzoni, M.; et al. Proteasome Inhibitors Block Myeloma-Induced Osteocyte Death in Vitro and in Vivo in Multiple Myeloma Patients. BLOOD 2012, 120, 3978. [Google Scholar] [CrossRef]
- McClung, M.R.; Brown, J.P.; Diez-Perez, A.; Resch, H.; Caminis, J.; Meisner, P.; Bolognese, M.A.; Goemaere, S.; Bone, H.G.; Zanchetta, J.R.; et al. Effects of 24 months of treatment with romosozumab followed by 12 months of denosumab or placebo in postmenopausal women with low bone mineral density: A randomized, double-blind, phase 2, parallel group study. J. Bone Miner. Res. 2018, 33, 1397–1406. [Google Scholar] [CrossRef]
- Lewiecki, E.M.; Blicharski, T.; Goemaere, S.; Lippuner, K.; Meisner, P.D.; Miller, P.D.; Miyauchi, A.; Maddox, J.; Chen, L.; Horlait, S. A phase III randomized placebo-controlled trial to evaluate efficacy and safety of romosozumab in men with osteoporosis. J. Clin. Endocrinol. Metab. 2018, 103, 3183–3193. [Google Scholar] [CrossRef]
- Kendler, D.L.; Bone, H.G.; Massari, F.; Gielen, E.; Palacios, S.; Maddox, J.; Yan, C.; Yue, S.; Dinavahi, R.V.; Libanati, C.; et al. Bone mineral density gains with a second 12-month course of romosozumab therapy following placebo or denosumab. Osteoporos. Int. 2019, 30, 2437–2448. [Google Scholar] [CrossRef]
- Lewiecki, E.M. Romosozumab, clinical trials, and real-world care of patients with osteoporosis. Ann. Transl. Med. 2020, 8, 974. [Google Scholar] [CrossRef]
- Sakata, T.; Sakai, A.; Tsurukami, H.; Okimoto, N.; Okazaki, Y.; Ikeda, S.; Norimura, T.; Nakamura, T. Trabecular bone turnover and bone marrow cell development in tail-suspended mice. J. Bone Miner. Res. 1999, 14, 1596–1604. [Google Scholar] [CrossRef]
- Bikle, D.D.; Sakata, T.; Halloran, B.P. The impact of skeletal unloading on bone formation. Gravit. Space Biol. Bull. 2003, 16, 45–54. [Google Scholar]
- Kerstetter, J.E.; O’Brien, K.O.; Insogna, K.L. Dietary protein, calcium metabolism, and skeletal homeostasis revisited. Am. J. Clin. Nutr. 2003, 78 (Suppl. S3), S584–S592. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, M.; Cavani, F.; Smargiassi, A.; Roli, L.; Palumbo, C. Mineral and skeletal homeostasis influence the manner of bone loss in metabolic osteoporosis due to calcium-deprived diet in different sites of rat vertebra and femur. BioMed Res. Int. 2015, 304178. [Google Scholar] [CrossRef]
- Ferretti, M.; Cavani, F.; Roli, L.; Checchi, M.; Magarò, M.S.; Bertacchini, J.; Palumbo, C. Interaction among calcium diet content, PTH(1-34) treatment and balance of bone homeostasis in rat model: The trabecular bone as keystone. Int. J. Mol. Sci. 2019, 20, 753. [Google Scholar] [CrossRef] [PubMed]
- Skripitz, R.; Andreassen, T.T.; Aspenberg, P. Strong effect of PTH (1-34) on regenerating bone: A time sequence study in rats. Acta Orthop. Scand. 2000, 71, 619–624. [Google Scholar] [CrossRef] [PubMed]
- Orwoll, E.S.; Scheele, W.H.; Paul, S.; Adami, S.; Syversen, U.; Diez-Perez, A.; Kaufman, J.M.; Clancy, A.D.; Gaich, G.A. The effect of teriparatide [human parathyroid hormone (1–34)] therapy on bone density in men with osteoporosis. J. Bone Miner. Res. 2003, 18, 9–17. [Google Scholar] [CrossRef]
- Lindsay, R.; Zhou, H.; Cosman, F.; Nieves, J.; Dempster, D.W.; Hodsman, A.B. Effects of a one-month treatment with PTH (1-34) on bone formation on cancellous, endocortical, and periosteal surfaces of the human ilium. J. Bone Miner. Res. 2007, 22, 495–502. [Google Scholar] [CrossRef]
- Tanaka, S.; Kuroda, T.; Sugimoto, T.; Nakamura, T.; Shiraki, M. Changes in bone mineral density, bone turnover markers, and vertebral fracture risk reduction with once weekly teriparatide. Curr. Med. Res. Opin. 2014, 30, 931–936. [Google Scholar] [CrossRef]
- Hasegawa, T.; Amizuka, N. Bone remodeling and modeling/mini-modeling. Clin. Calcium 2017, 27, 1713–1722. [Google Scholar]
- Kumabe, Y.; Lee, S.Y.; Waki, T.; Iwakura, T.; Takahara, S.; Arakura, M.; Kuroiwa, Y.; Fukui, T.; Matsumoto, T.; Matsushita, T.; et al. 3Triweekly administration of parathyroid hormone (1–34) accelerates bone healing in a rat refractory fracture model. BMC Musculoskelet. Disord. 2017, 18, 545. [Google Scholar] [CrossRef]
- Canalis, E. Management of endocrine disease: Novel anabolic treatments for osteoporosis. Eur. J. Endocrinol. 2018, 178, R33–R44. [Google Scholar] [CrossRef] [PubMed]
- Dehority, W.; Halloran, B.P.; Bikle, D.D.; Curren, T.; Kostenuik, P.J.; Wronski, T.J.; Shen, Y.; Rabkin, B.; Bouraoui, A.; Morey-Holton, E. Bone and hormonal changes induced by skeletal unloading in the mature male rat. Am. J. Physiol. 1999, 276P, E62–E69. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, J.; Yeh, J.K.; Aloia, J.F. Differential effect of treadmill exercise on three cancellous bone sites in the young growing rat. Bone 1999, 24, 163–169. [Google Scholar] [CrossRef]
- Lecoq, B.; Potrel-Burgot, C.; Granier, P.; Sabatier, J.P.; Marcelli, C. Comparison of bone loss induced in female rats by hindlimb unloading, ovariectomy, or both. Jt. Bone Spine 2006, 73, 189–195. [Google Scholar] [CrossRef]
- Burgers, T.A.; Williams, B.O. Regulation of Wnt/β-catenin signaling within and from osteocytes. Bone 2013, 54, 244–249. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palumbo, C.; Ferretti, M. The Osteocyte: From “Prisoner” to “Orchestrator”. J. Funct. Morphol. Kinesiol. 2021, 6, 28. https://doi.org/10.3390/jfmk6010028
Palumbo C, Ferretti M. The Osteocyte: From “Prisoner” to “Orchestrator”. Journal of Functional Morphology and Kinesiology. 2021; 6(1):28. https://doi.org/10.3390/jfmk6010028
Chicago/Turabian StylePalumbo, Carla, and Marzia Ferretti. 2021. "The Osteocyte: From “Prisoner” to “Orchestrator”" Journal of Functional Morphology and Kinesiology 6, no. 1: 28. https://doi.org/10.3390/jfmk6010028
APA StylePalumbo, C., & Ferretti, M. (2021). The Osteocyte: From “Prisoner” to “Orchestrator”. Journal of Functional Morphology and Kinesiology, 6(1), 28. https://doi.org/10.3390/jfmk6010028