Detraining and Anabolic-Androgenic Steroid Discontinuation Change Calcaneal Tendon Morphology
Abstract
:1. Introduction
2. Materials and Methods
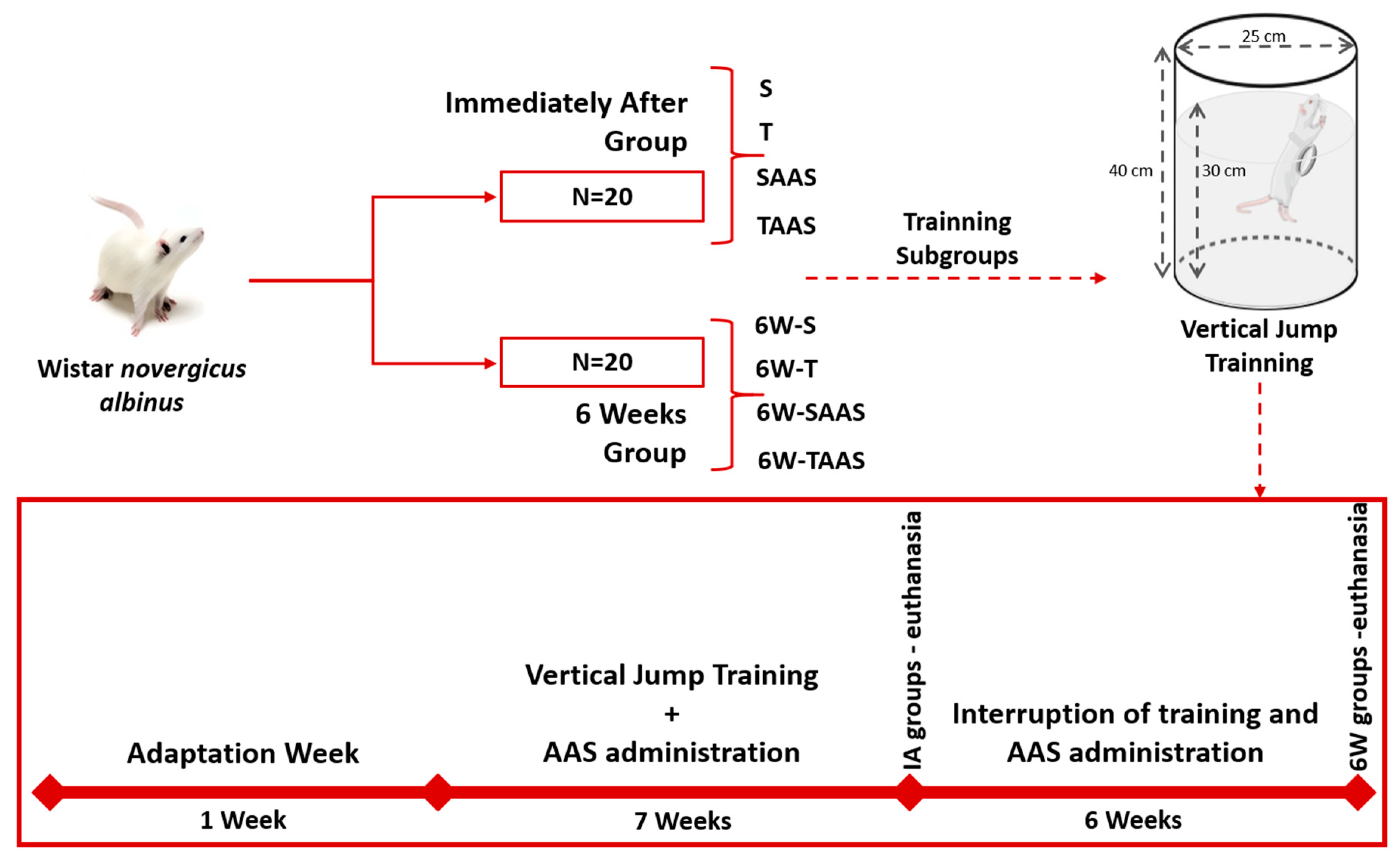
2.1. Animals
2.2. Experimental Groups
2.3. Anabolic Androgenic Steroid Administration
2.4. Training Protocol
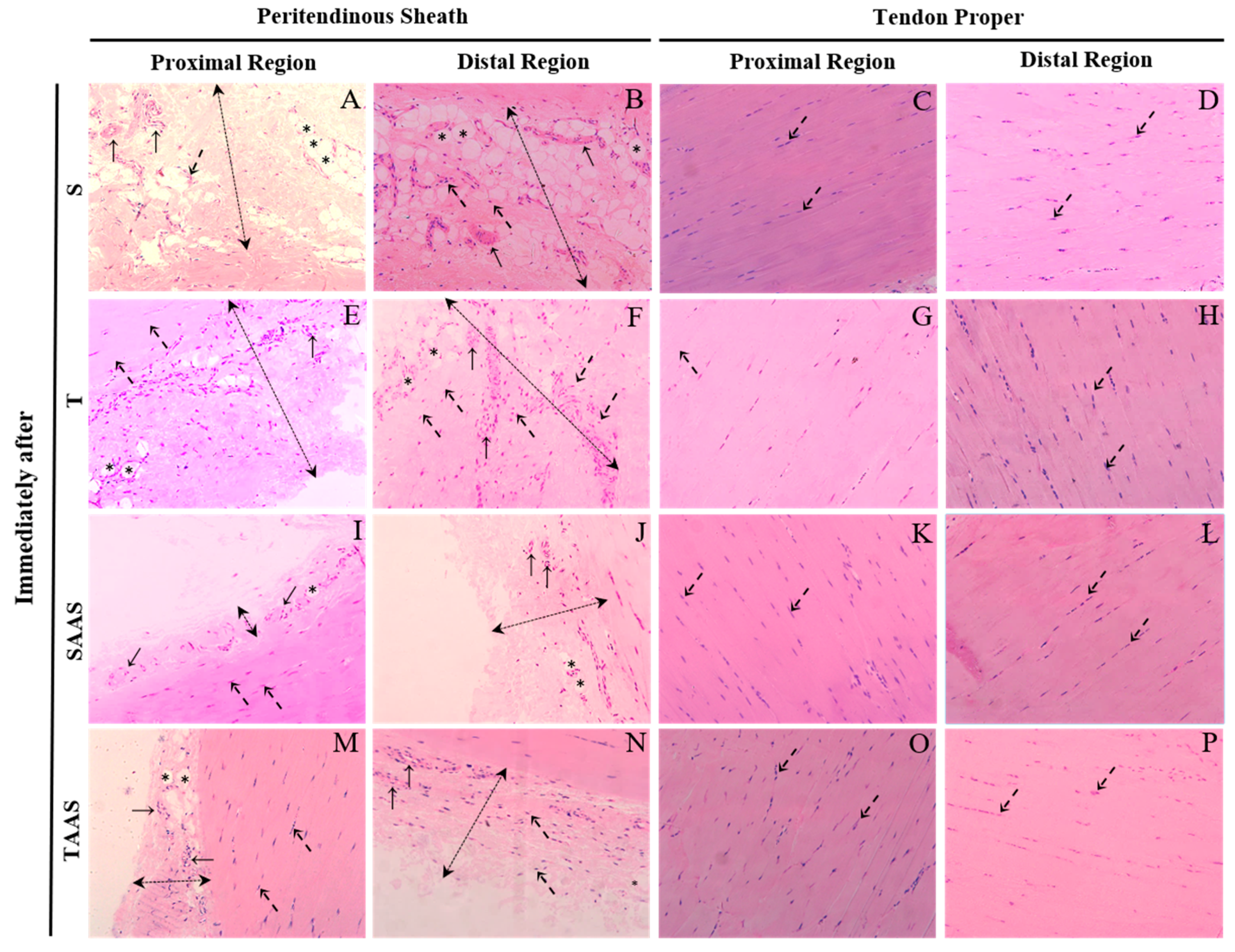
2.5. Histological Preparation
2.6. Histomorphometric Analysis
2.7. Statistical Analysis
3. Results
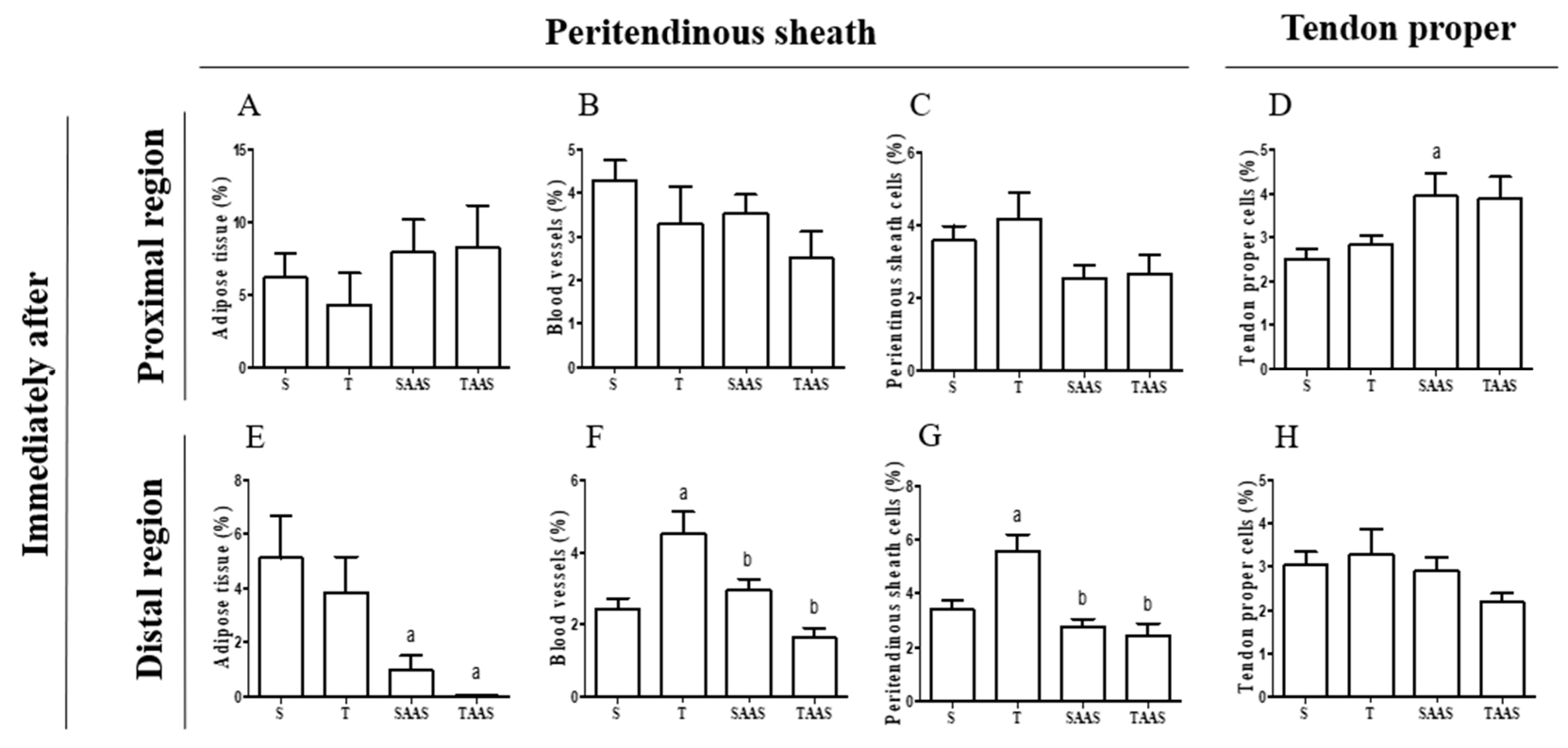
3.1. Histomorphometry Immediately After Seven Weeks of Training (IA)
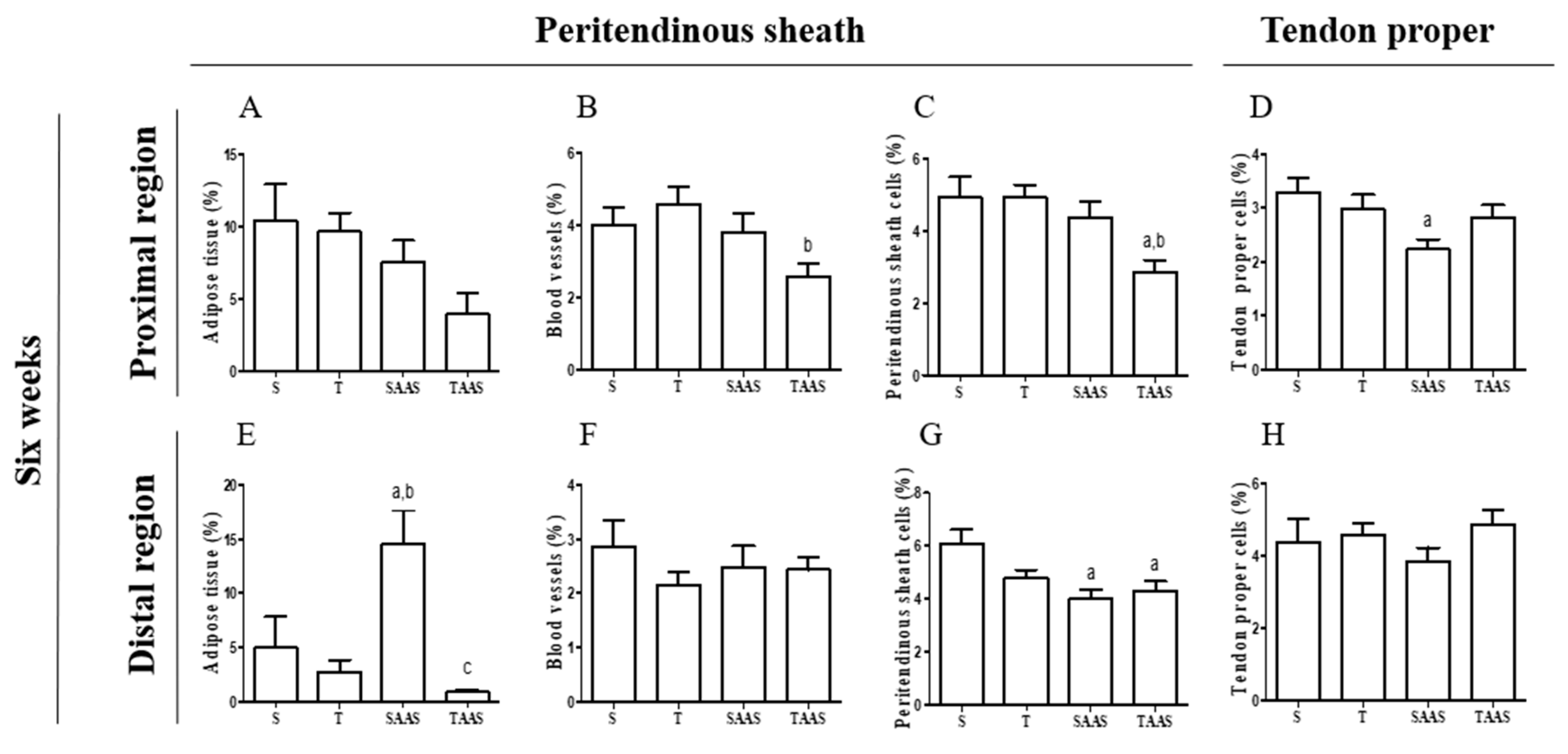
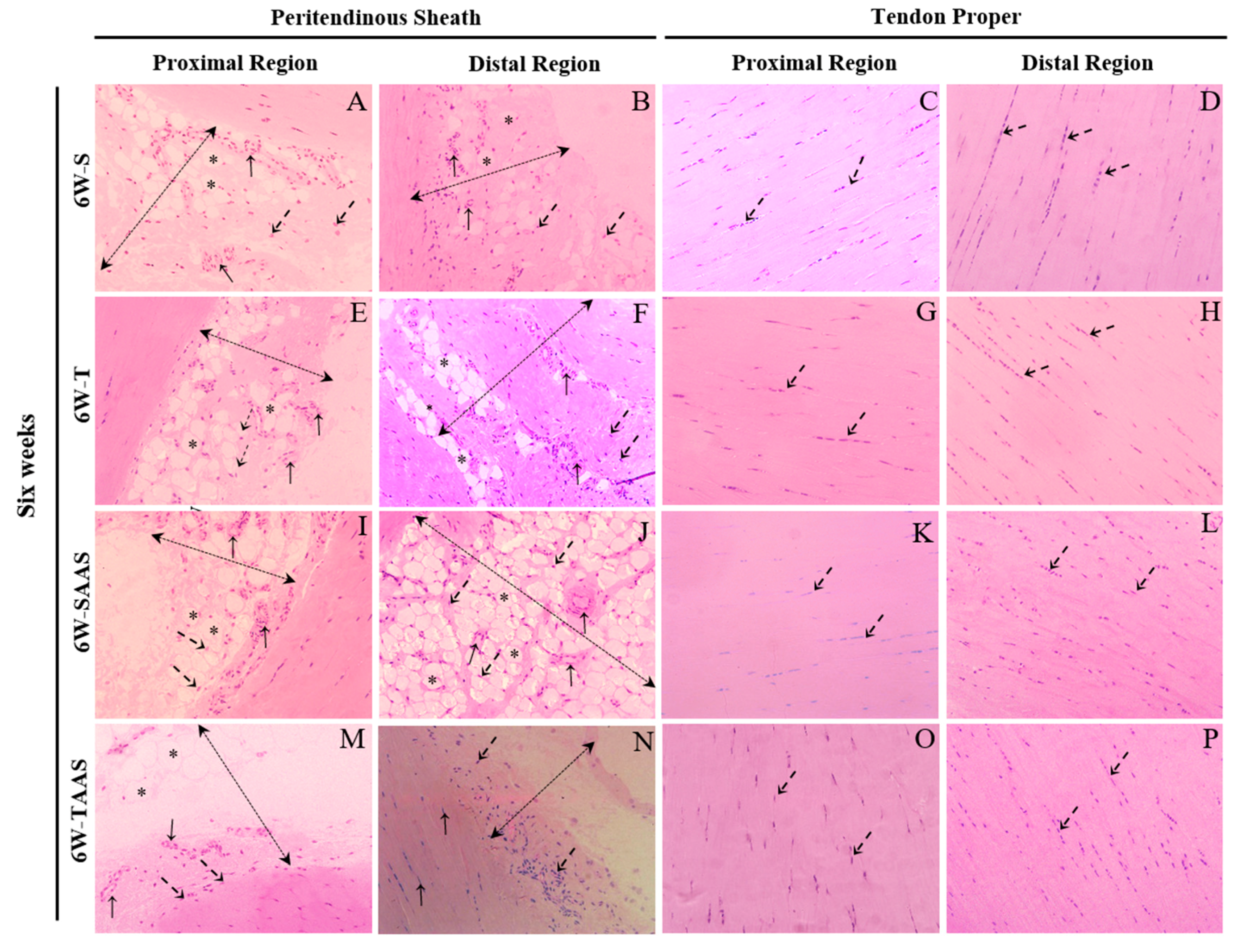
3.2. Histomorphometry after 6 Weeks of Detraining (6W)
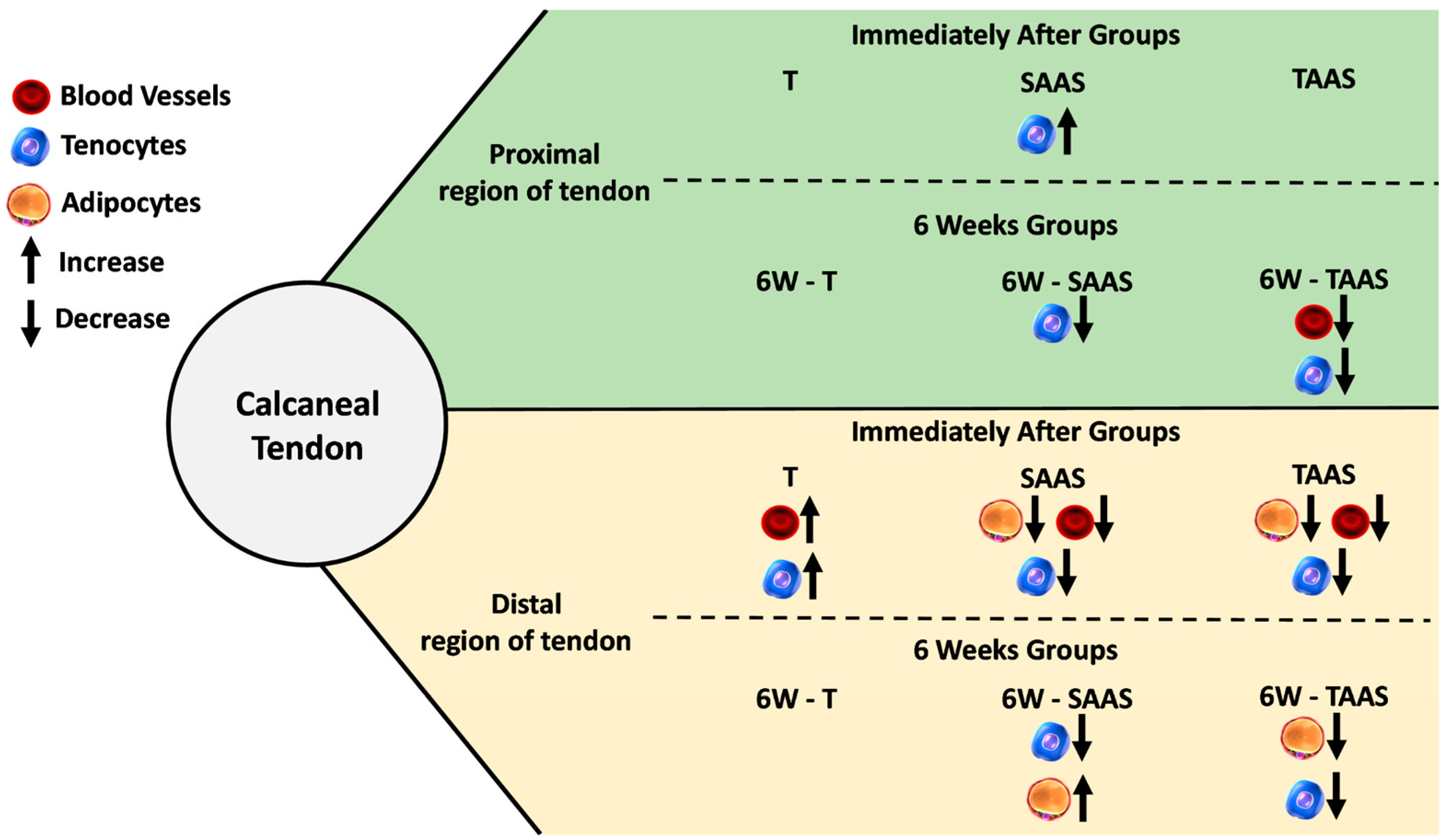
3.3. Temporal Comparison between IA and 6W Groups
4. Discussion
4.1. Training, AAS, and AAS Associated with Training Effects
4.2. AAS Discontinuation Effects
4.3. Effects of AAS Discontinuation and Detraining
4.4. Temporal Comparison between IA and 6W Groups
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ballal, M.S.; Walker, C.R.; Molloy, A.P. The anatomical footprint of the Achilles Tendon. Bone Jt. J. 2014, 96, 1344–1348. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, A.; Schilling, T.F. Tendon development and musculoskeletal assembly: Emerging roles for the extracellular matrix. Development 2015, 142, 4191–4204. [Google Scholar] [CrossRef] [PubMed]
- Thomopoulos, S.; Genin, G.M.; Galatz, L.M. The development and morphogenesis of the tendon-to-bone insertion—What development can teach us about healing. J. Musculoskelet. Neuronal Interact. 2010, 10, 35–45. [Google Scholar] [PubMed]
- Heinemeier, K.M.; Olesen, J.L.; Haddad, F.; Langberg, H.; Kjaer, M.; Baldwin, K.M.; Schjerling, P. Expression of collagen and related growth factors in rat tendon and skeletal muscle in response to specific contraction types. J. Physiol. 2007, 582, 1303–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marqueti, R.C.; Parizotto, N.A.; Chriguer, R.S.; Perez, S.E.A.; Selistre-de-Araujo, H.S. Androgenic-anabolic steroids associated with mechanical loading inhibit matrix metallopeptidase activity and affect the remodeling of the achilles tendon in rats. Am. J. Sports Med. 2006, 34, 1274–1280. [Google Scholar] [CrossRef] [PubMed]
- Marqueti, R.C.; Prestes, J.; Paschoal, M.; Ramos, O.H.P.; Perez, S.E.A.; Carvalho, H.F.; Selistre-de-Araujo, H.S. Matrix metallopeptidase 2 activity in tendon regions: Effects of mechanical loading exercise associated to anabolic-androgenic steroids. Eur. J. Appl. Physiol. 2008, 104, 1087–1093. [Google Scholar] [CrossRef]
- Maeda, E.; Hagiwara, Y.; Wang, J.H.C.; Ohashi, T. A new experimental system for simultaneous application of cyclic tensile strain and fluid shear stress to tenocytes in vitro. Biomed. Microdevices 2013, 15, 1067–1075. [Google Scholar] [CrossRef]
- Kongsgaard, M.; Reitelseder, S.; Pedersen, T.G.; Holm, L.; Aagaard, P.; Kjaer, M.; Magnusson, S.P. Region specific patellar tendon hypertrophy in humans following resistance training. Acta Physiol. 2007, 191, 111–121. [Google Scholar] [CrossRef]
- Carroll, C.C.; Whitt, J.A.; Peterson, A.; Gump, B.S.; Tedeschi, J.; Broderick, T.L. Influence of acetaminophen consumption and exercise on Achilles tendon structural properties in male Wistar rats. AJP Regul. Integr. Comp. Physiol. 2012, 302, 990–995. [Google Scholar] [CrossRef]
- Frizziero, A.; Salamanna, F.; Della Bella, E.; Vittadini, F.; Gasparre, G.; Nicoli Aldini, N.; Masiero, S.; Fini, M. The Role of Detraining in Tendon Mechanobiology. Front Aging Neurosci. 2016, 8, 43. [Google Scholar] [CrossRef]
- Frizziero, A.; Fini, M.; Salamanna, F.; Veicsteinas, A.; Maffulli, N.; Marini, M. Effect of training and sudden detraining on the patellar tendon and its enthesis in rats. BMC Musculoskelet. Disord. 2011, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Pope, H.G.; Wood, R.I.; Rogol, A.; Nyberg, F.; Bowers, L.; Bhasin, S. Adverse Health Consequences of Performance-Enhancing Drugs: An Endocrine Society Scientific Statement. Endocr. Rev. 2014, 35, 341–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanayama, G.; Deluca, J.; Meehan, W.P.; Hudson, J.I.; Isaacs, S.; Baggish, A.; Weiner, R.; Micheli, L.; Pope, H.G., Jr. Ruptured tendons in anabolic-androgenic steroid users. Am. J. Sports Med. 2015, 43, 2638–2644. [Google Scholar] [CrossRef]
- Jones, I.A.; Togashi, R.; Hatch, G.F.R.; Weber, A.E.; Vangsness, C.T. Anabolic steroids and tendons: A review of their mechanical, structural, and biologic effects. J. Orthop. Res. 2018, 36, 2830–2841. [Google Scholar] [CrossRef]
- Tsitsilonis, S.; Panayiotis, C.E.; Athanasios, M.S.; Stavros, K.K.; Ioannis, V.S.; George, A.; Konstantinos, F.; Despina, P.N.; Aristides, Z.B. Anabolic androgenic steroids reverse the beneficial effect of exercise on tendon biomechanics: An experimental study. Foot Ankle Surg. 2014, 20, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Marqueti, R.C.; Prestes, J.; Wang, C.C.; Ramos, O.H.P.; Perez, S.E.A.; Nakagaki, W.R.; Carvalho, H.F.; Selistre-de-Araujo, H.S. Biomechanical responses of different rat tendons to nandrolone decanoate and load exercise. Scand. J. Med. Sci. Sports. 2011, 21, 91–99. [Google Scholar] [CrossRef]
- Marqueti, R.C.; Paulino, M.G.; Fernandes, M.N.; de Oliveira, E.M.; Selistre-de-Araujo, H.S. Tendon structural adaptations to load exercise are inhibited by anabolic androgenic steroids. Scand. J. Med. Sci. Sports 2014, 24, 39–51. [Google Scholar] [CrossRef]
- Pope, H.G.; Katz, D.L. Affective and psychotic symptoms associated with anabolic steroid use. Am. J. Psychiatr. 1988, 145, 487–490. [Google Scholar]
- Weibel, E.R. Stereological Principles for Morphometry in Electron Microscopic Cytology. Int. Rev. Cytol. 1969, 26, 235–302. [Google Scholar]
- Kjær, M.; Langberg, H.; Heinemeier, K.; Bayer, M.L.; Hansen, M.; Holm, L.; Doessing, S.; Kongsgaard, M.; Krogsgaard, M.R.; Magnusson, S.P. From mechanical loading to collagen synthesis, structural changes and function in human tendon. Scand. J. Med. Sci. Sports 2009, 19, 500–510. [Google Scholar] [CrossRef] [Green Version]
- Olesen, J.L.; Heinemeier, K.M.; Gemmer, C.; Kjær, M.; Flyvbjerg, A.; Langberg, H. Exercise-dependent IGF-I, IGFBPs, and type I collagen changes in human peritendinous connective tissue determined by microdialysis. J. Appl. Physiol. 2007, 102, 214–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinemeier, K.M.; Kjaer, M. In vivo investigation of tendon responses to mechanical loading. J. Musculoskelet. Neuronal Interact. 2011, 11, 115–123. [Google Scholar] [PubMed]
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Malheiro, O.C.D.M.; Giacomini, C.T.; Justulin, L.A.; Delella, F.K.; Dal-Pai-Silva, M.; Felisbino, S.L. Calcaneal tendon regions exhibit different MMP-2 activation after vertical jumping and treadmill running. Anat. Rec. 2009, 292, 1656–1662. [Google Scholar] [CrossRef] [PubMed]
- Michna, H. Tendon injuries induced by exercise and anabolic steroids in experimental mice. Int. Orthop. 1987, 11, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, T.; Kubota, M.; Saito, M.; Hattori, H.; Kimura, T.; Marumo, K. Quantitative and qualitative change of collagen of achilles tendons in rats with systemic administration of glucocorticoids. Foot Ankle Int. 2016, 37, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Marqueti, R.d.C.; Heinemeier, K.M.; Durigan, J.L.Q.; De Andrade Perez, S.E.; Schjerling, P.; Kjaer, M.; Carvalho, H.F.; Selistre-de-Araujo, H.S. Gene expression in distinct regions of rat tendons in response to jump training combined with anabolic androgenic steroid administration. Eur. J. Appl. Physiol. 2012, 112, 1505–1515. [Google Scholar] [CrossRef]
- Flück, M.; Ruoss, S.; Möhl, C.B.; Valdivieso, P.; Benn, M.C.; von Rechenberg, B.; Laczko, E.; Hu, J.; Wieser, K.; Meyer, D.C.; et al. Genomic and lipidomic actions of nandrolone on detached rotator cuff muscle in sheep. J. Steroid Biochem. Mol. Biol. 2017, 165, 382–395. [Google Scholar] [CrossRef] [Green Version]
- Parssinen, M.; Karila, T.; Kovanen, V.; Seppälä, T. The effect of supraphysiological doses of anabolic androgenic steroids on collagen metabolism. Int. J. Sports Med. 2000, 21, 406–411. [Google Scholar] [CrossRef]
- Evans, N.A. Current Concepts in Anabolic-Androgenic Steroids. Am. J. Sports Med. 2004, 32, 534–542. [Google Scholar] [CrossRef]
- Abate, M.; Salini, V.; Andia, I. How Obesity Affects Tendons? Springer: Cham, Switzerland, 2016; pp. 167–177. [Google Scholar]
- Bi, Y.; Ehirchiou, D.; Kilts, T.M.; Inkson, C.A.; Embree, M.C.; Sonoyama, W.; Li, L.; Leet, A.I.; Seo, B.M.; Zhang, L.; et al. Identification of tendon stem/progenitor cells and the role of the extracellular matrix in their niche. Nat. Med. 2007, 13, 1219–1227. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yuan, T.; Wang, J.H. Moderate treadmill running exercise prior to tendon injury enhances wound healing in aging rats. Oncotarget 2016, 7, 8498. [Google Scholar] [CrossRef] [PubMed]
- De Mos, M.; Koevoet, W.J.; Jahr, H.; Verstegen, M.M.; Heijboer, M.P.; Kops, N.; Van Leeuwen, J.P.; Weinans, H.; Verhaar, J.A.; van Osch, G.J. Intrinsic differentiation potential of adolescent human tendon tissue: An in-vitro cell differentiation study. BMC Musculoskelet. Disord. 2007, 8, 16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.-C. The Effects of Mechanical Loading on Tendons-An In Vivo and In Vitro Model Study. PLoS ONE 2013, 8, 71740. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.; Zwerver, J.; Grewal, N.; De Sa, A.; Alktebi, T.; Granville, D.J.; Hart, D.A. Lipids, adiposity and tendinopathy: Is there a mechanistic link? Critical review. Br. J. Sports Med. 2015, 49, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Ackerman, J.E.; Geary, M.B.; Orner, C.A.; Bawany, F.; Loiselle, A.E. Obesity/Type II diabetes alters macrophage polarization resulting in a fibrotic tendon healing response. PLoS ONE 2017, 12, e0181127. [Google Scholar] [CrossRef] [PubMed]
- Gaida, J.E.; Ashe, M.C.; Bass, S.L.; Cook, J.L. Is adiposity an under-recognized risk factor for tendinopathy? A systematic review. Arthritis Care Res. 2009, 61, 840–849. [Google Scholar] [CrossRef] [Green Version]
- Józsa, L.; Kannus, P. Histopathological findings in spontaneous tendon ruptures. Scand. J. Med. Sci. Sports 2007, 7, 113–118. [Google Scholar] [CrossRef]
- Egerbacher, M.; Arnoczky, S.P.; Caballero, O.; Lavagnino, M.; Gardner, K.L. Loss of Homeostatic Tension Induces Apoptosis in Tendon Cells: An In Vitro Study. Clin. Orthop. Relat. Res. 2008, 466, 1562–1568. [Google Scholar] [CrossRef]
- Benson, R.T.; McDonnell, S.M.; Knowles, H.J.; Rees, J.L.; Carr, A.J.; Hulley, P.A. Tendinopathy and tears of the rotator cuff are associated with hypoxia and apoptosis. J. Bone Jt. Surg. 2010, 92, 448–453. [Google Scholar] [CrossRef] [Green Version]
- Lundgreen, K.; Lian, Ø.B.; Engebretsen, L.; Scott, A. Tenocyte apoptosis in the torn rotator cuff: A primary or secondary pathological event? Br. J. Sports Med. 2011, 45, 1035–1039. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, M.; de Cássia Marqueti, R.; Perez, S.; Selistre-de-Araujo, H.S. Nandrolone inhibits VEGF mRNA in rat muscle. Int. J. Sports Med. 2009, 30, 775–778. [Google Scholar] [CrossRef] [PubMed]
- Petersen, W.; Unterhauser, F.; Pufe, T.; Zantop, T.; Südkamp, N.; Weiler, A. The angiogenic peptide vascular endothelial growth factor (VEGF) is expressed during the remodeling of free tendon grafts in sheep. Arch. Orthop. Trauma Surg. 2003, 123, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Mujika, I.; Padilla, S. Detraining: Loss of Training-Induced Physiological and Performance Adaptations. Part I. Sport Med. 2000, 30, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Van Roie, E.; Walker, S.; Van Driessche, S.; Baggen, R.; Coudyzer, W.; Bautmans, I.; Delecluse, C. Training load does not affect detraining’s effect on muscle volume, muscle strength and functional capacity among older adults. Exp. Gerontol. 2017, 98, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Gondin, J.; Guette, M.; Ballay, Y.; Martin, A. Neural and muscular changes to detraining after electrostimulation training. Eur. J. Appl. Physiol. 2006, 97, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Kubo, K.; Ikebukuro, T.; Maki, A.; Yata, H.; Tsunoda, N. Time course of changes in the human Achilles tendon properties and metabolism during training and detraining in vivo. Eur. J. Appl. Physiol. 2012, 112, 2679–2691. [Google Scholar] [CrossRef] [PubMed]
- Salamanna, F.; Frizziero, A.; Pagani, S.; Giavaresi, G.; Curzi, D.; Falcieri, E.; Marini, M.; Abruzzo, P.M.; Martini, L.; Fini, M. Metabolic and cytoprotective effects of in vivo peri-patellar hyaluronic acid injections in cultured tenocytes. Connect. Tissue Res. 2015, 56, 35–43. [Google Scholar] [CrossRef]
| S | T | SAAS | TAAS | ||||||
|---|---|---|---|---|---|---|---|---|---|
| IA (Vv%) Mean (SEM) | 6W (Vv%) Mean (SEM) | IA (Vv%) Mean (SEM) | 6W (Vv%) Mean (SEM) | IA (Vv%) Mean (SEM) | 6W (Vv%) Mean (SEM) | IA (Vv%) Mean (SEM) | 6W (Vv%) Mean (SEM) | ||
| Proximal Region | Adipocytes | 6.19 (11.13) | 10.42 (14.05) | 4.32 (10.11) | 9.64 (9.33) * | 7.92 (13.49) | 7.55 (11.18) | 8.24 (13.36) | 3.93 (7.97) |
| Blood Vessels | 4.29 (3.05) | 4.00 (2.68) | 3.29 (4.06) | 4.59 (3.39) | 3.53 (2.54) | 3.80 (3.95) | 2.51 (2.77) | 2.59 (1.93) | |
| Peritendinous Sheath Cells | 3.59 (2.63) | 4.92 (2.68) | 4.17 (3.46) | 4.92 (2.45) | 2.55 (2.08) | 4.38 (3.23) ** | 2.68 (2.32) | 2.86 (1.75) | |
| Tendon Proper Cells | 2.31 (1.15) | 2.79 (0.98) | 2.84 (1.26) | 2.98 (1.70) | 3.95 (2.81) | 2.24 (1.06) ** | 3.89 (1.99) | 2.83 (1.20) * | |
| Distal Region | Adipocytes | 5.12 (10.88) | 4.97 (14.01) | 4.84 (9.50) | 2.75 (7.35) | 0.97 (3.76) | 14.58 (21.35) *** | 0.04 (0.19) | 0.87 (1.92) * |
| Blood Vessels | 2.43 (2.01) | 2.86 (2.38) | 4.51 (3.59) | 2.16 (1.62) *** | 2.95 (2.11) | 2.49 (2.68) | 1.63 (1.40) | 2.44 (1.82) * | |
| Peritendinous Sheath Cells | 3.40 (2.38) | 6.10 (2.56) *** | 5.57 (3.68) | 4.80 (2.02) | 2.76 (1.98) | 4.00 (2.47) ** | 2.45 (2.20) | 4.31 (2.84) ** | |
| Tendon Proper Cells | 2.56 (1.09) | 2.97 (0.81) | 3.29 (2.63) | 4.59 (2.40) * | 2.92 (1.62) | 3.84 (1.91) | 2.19 (1.10) | 4.87 (2.77) *** | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santana Oliveira, A.J.; Batista e Silva, L.L.; Barin, F.R.; Leite Pereira, E.C.; Selistre-de-Araujo, H.S.; De Cássia Marqueti, R. Detraining and Anabolic-Androgenic Steroid Discontinuation Change Calcaneal Tendon Morphology. J. Funct. Morphol. Kinesiol. 2019, 4, 1. https://doi.org/10.3390/jfmk4010001
Santana Oliveira AJ, Batista e Silva LL, Barin FR, Leite Pereira EC, Selistre-de-Araujo HS, De Cássia Marqueti R. Detraining and Anabolic-Androgenic Steroid Discontinuation Change Calcaneal Tendon Morphology. Journal of Functional Morphology and Kinesiology. 2019; 4(1):1. https://doi.org/10.3390/jfmk4010001
Chicago/Turabian StyleSantana Oliveira, Anderson José, Lívia Larissa Batista e Silva, Fabrício Reichert Barin, Elaine Cristina Leite Pereira, Heloisa Sobreiro Selistre-de-Araujo, and Rita De Cássia Marqueti. 2019. "Detraining and Anabolic-Androgenic Steroid Discontinuation Change Calcaneal Tendon Morphology" Journal of Functional Morphology and Kinesiology 4, no. 1: 1. https://doi.org/10.3390/jfmk4010001
APA StyleSantana Oliveira, A. J., Batista e Silva, L. L., Barin, F. R., Leite Pereira, E. C., Selistre-de-Araujo, H. S., & De Cássia Marqueti, R. (2019). Detraining and Anabolic-Androgenic Steroid Discontinuation Change Calcaneal Tendon Morphology. Journal of Functional Morphology and Kinesiology, 4(1), 1. https://doi.org/10.3390/jfmk4010001





