Patellar Tendon Structural Difference Occurs in Female and Male Professional Basketball Players: 8 Months Follow-Up
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Inclusion Exclusion Criteria
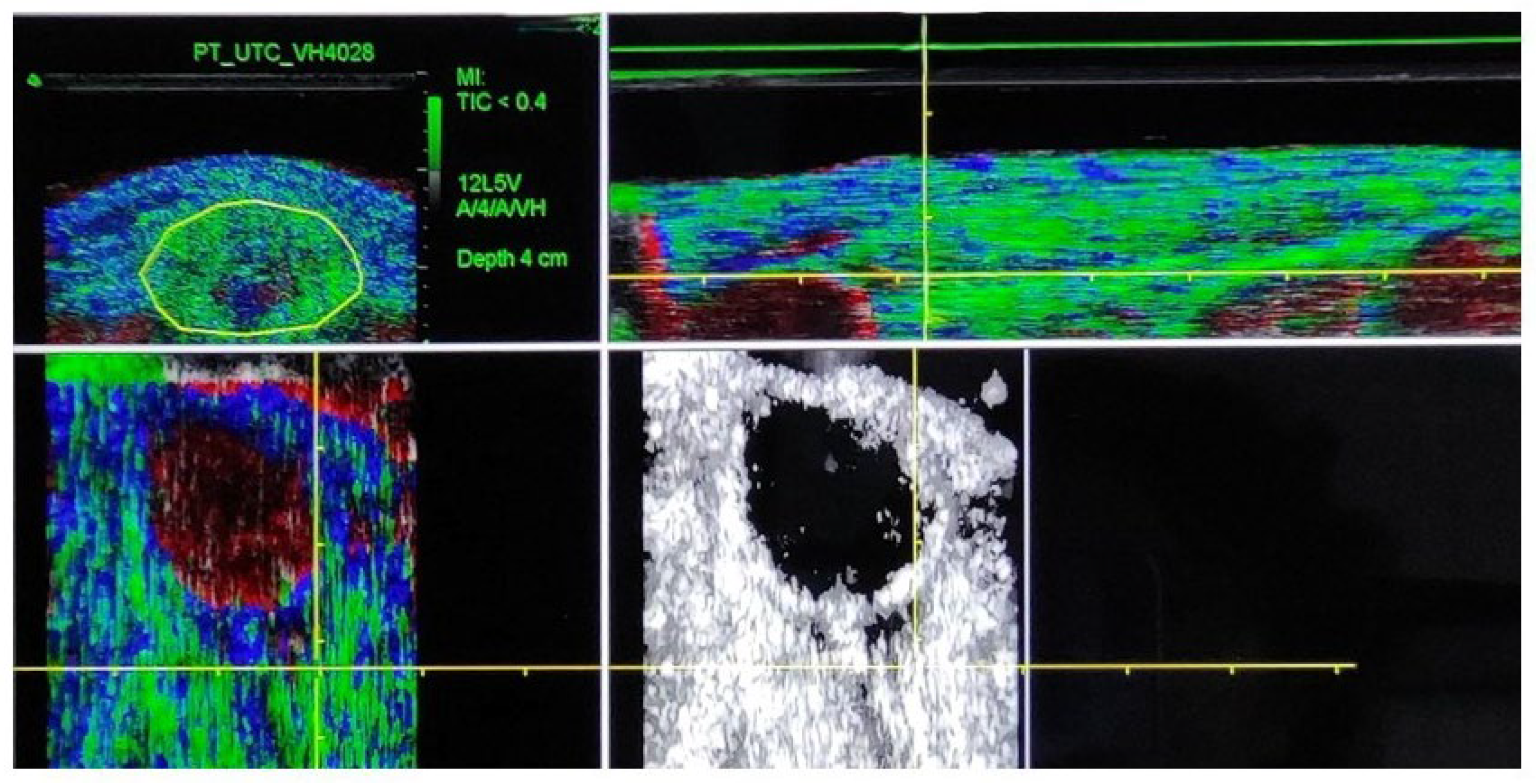
2.3. Procedure
2.4. Statistical Analysis
3. Results
3.1. Tendon Adaptations
3.2. Tendon Structure Differences Between Sexes
3.3. Tendon Structure Differences Between Jumping and Non-Jumping
3.4. Tendon Structure Differences Between Symptomatic and Asymptomatic
3.5. Tendon Structure Differences Between Season/Cohort
4. Discussion
- Sex Differences in Tendon Structure
- Other Factors: Jumping Leg and Pain
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cook, J.L.; Purdam, C.R. The challenge of managing tendinopathy in competing athletes. Br. J. Sports Med. 2014, 48, 506–509. [Google Scholar] [CrossRef]
- Schwartz, A.; Watson, J.N.; Hutchinson, M.R. Patellar Tendinopathy. Sports Health 2015, 7, 415–420. [Google Scholar] [CrossRef]
- Cook, J.; Rio, E.; Purdam, C.R.; Girdwood, M.; Ortega-Cebrian, S.; Docking, S.I. El continuum de la patología de tendón: Concepto actual e implicaciones clínicas. Apunt. Med. l’Esport 2017, 52, 61–69. [Google Scholar] [CrossRef]
- Ferretti, A.; Ippolito, E.; Mariani, P.; Puddu, G. Jumper’s knee. Am. J. Sports Med. 1983, 11, 58–62. [Google Scholar] [CrossRef]
- Blazina, M.E.; Kerlan, R.K.; Jobe, F.W.; Carter, V.S.; Carlson, G.J. Jumper’s Knee. Orthop. Clin. N. Am. 1973, 4, 665–678. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Ooi, C.C.; Connell, D. Tendinopathy: Is Imaging Telling Us the Entire Story? J. Orthop. Sport. Phys. Ther. 2015, 45, 842–852. [Google Scholar] [CrossRef]
- Conde, J.M.; Cabero Morán, M.T.; Pascual, C.M. Prospective epidemiological study of basketball injuries during one competitive season in professional and amateur Spanish basketball. Phys. Sportsmed. 2022, 50, 349–358. [Google Scholar] [CrossRef]
- Hutchison, M.K.; Houck, J.; Cuddeford, T.; Dorociak, R.; Brumitt, J. Prevalence of Patellar Tendinopathy and Patellar Tendon Abnormality in Male Collegiate Basketball Players: A Cross-Sectional Study. J. Athl. Train. 2019, 54, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Florit, D.; Pedret, C.; Casals, M.; Malliaras, P.; Sugimoto, D.; Rodas, G. Incidence of Tendinopathy in Team Sports in a Multidisciplinary Sports Club Over 8 Seasons. J. Sports Sci. Med. 2019, 18, 780–788. [Google Scholar] [PubMed]
- Russell, J.L.; McLean, B.D.; Impellizzeri, F.M.; Strack, D.S.; Coutts, A.J. Measuring Physical Demands in Basketball: An Explorative Systematic Review of Practices. Sports Med. 2021, 51, 81–112. [Google Scholar] [CrossRef]
- Scanlan, A.T.; Dascombe, B.J.; Reaburn, P.; Dalbo, V.J. The physiological and activity demands experienced by Australian female basketball players during competition. J. Sci. Med. Sport. 2012, 15, 341–347. [Google Scholar] [CrossRef]
- Stojanović, E.; Stojiljković, N.; Scanlan, A.T.; Dalbo, V.J.; Berkelmans, D.M.; Milanović, Z. The Activity Demands and Physiological Responses Encountered During Basketball Match-Play: A Systematic Review. Sports Med. 2018, 48, 111–135. [Google Scholar] [CrossRef]
- Torres-Ronda, L.; Ric, A.; Llabres-Torres, I.; de Las Heras, B.; Schelling IDel Alcazar, X. Position-Dependent Cardiovascular Response and Time-Motion Analysis During Training Drills and Friendly Matches in Elite Male Basketball Players. J. Strength Cond. Res. 2016, 30, 60–70. [Google Scholar] [CrossRef]
- Malliaras, P.; Cook, J.; Purdam, C.; Rio, E. Patellar Tendinopathy: Clinical Diagnosis, Load Management, and Advice for Challenging Case Presentations. J. Orthop. Sport. Phys. Ther. 2015, 45, 887–898. [Google Scholar] [CrossRef]
- van der Worp, H.; van Ark, M.; Roerink, S.; Pepping, G.-J.; van den Akker-Scheek, I.; Zwerver, J. Risk factors for patellar tendinopathy: A systematic review of the literature. Br. J. Sports Med. 2011, 45, 446–452. [Google Scholar] [CrossRef]
- Hernández, G.; Dominguez, D.; Moreno, J.; Til Perez, L.; Ortís, L.; Pedret, C.; van Schie, H.; Rodas, G. Caracterización por Ultrasound Tissue Characterization de los tendones rotulianos de jugadores de baloncesto; comparación entre profesionales versus formativos y asintomáticos versus sintomáticos. Apunts. Med. l’Esport 2017, 52, 45–52. [Google Scholar] [CrossRef]
- Rudavsky, A.; Cook, J.; Docking, S. Quantifying proximal patellar tendon changes during adolescence in elite ballet dancers, a 2-year study. Scand. J. Med. Sci. Sports 2018, 28, 2369–2374. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Cook, J. Pathological tendons maintain sufficient aligned fibrillar structure on ultrasound tissue characterization (UTC). Scand. J. Med. Sci. Sports 2016, 26, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, A.; Stewart, A.M.; Hopkins, W.G.; Elias, G.P.; Aughey, R.J. Effects of Training Load and Leg Dominance on Achilles and Patellar Tendon Structure. Int. J. Sports Physiol. Perform. 2017, 12, S2122–S2126. [Google Scholar] [CrossRef] [PubMed]
- van Schie, H.T.M.; de Vos, R.J.; de Jonge, S.; Bakker, E.M.; Heijboer, M.P.; Verhaar, J.A.N.; Tol, J.L.; Weinans, H. Ultrasonographic tissue characterisation of human Achilles tendons: Quantification of tendon structure through a novel non-invasive approach. Br. J. Sports Med. 2010, 44, 1153–1159. [Google Scholar] [CrossRef]
- Van Schie, H.; Docking, S.; Daffy, J.; Praet, S.; Rosengarten, S.; Cook, J.L. Ultrasound tissue characterization, an innovative technique for injury-prevention and monitoring of tendinopathy. Br. J. Sports Med. 2013, 47, e2. [Google Scholar] [CrossRef]
- Ark, M.; Docking, S.; Akker-Scheek, I.; Rudavsky, A.; Rio, E.; Zwerver, J.; Cook, J.L. Does the adolescent patellar tendon respond to 5 days of cumulative load during a volleyball tournament?: Response of the patellar tendon to load. Scand. J. Med. Sci. Sports 2015, 26, 188–196. [Google Scholar]
- Rabello, M.; Zwerver, J.; Stewart, R.; van der Akker-Scheek, I.; Brink, M.S. Patellar tendon structure responds to load over a 7-week preseason in elite male volleyball players. Scand. J. Med. Sci. Sports 2019, 29, 992–999. [Google Scholar] [CrossRef]
- Ortega-Cebrián, S.; Navarro, R.; Seda, S.; Salas, S.; Guerra-Balic, M. Patellar tendon structural adaptations occur during pre-season and first competitive cycle in male professional handball players. Int. J. Environ. Res. Public Health 2021, 18, 12156. [Google Scholar] [CrossRef]
- Steinberg, N.; Funk, S.; Svorai-Band, S.; Yavnai, N.; Pantanowitz, M.; Zeev, A.; Dar, G. The Influence of a 14-Week Infantry Commanders Courses on the Achilles Tendon and Patellar Tendon Structure. Mil. Med. 2022, 187, e377–e386. [Google Scholar] [CrossRef] [PubMed]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Rabello, L.; Albers, I.; Ark, M.; Diercks, R.; Akker-Scheek, I.; Zwerver, J. Running a Marathon—Its Influence on Achilles Tendon Structure. J. Athl. Train. 2020, 55, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Ark, M.; Rabello, L.; Hoevenaars, D.; Meijerink, J.; Gelderen, N.; Zwerver, J.; van den Akker-Scheek, I. Inter- and intra-rater reliability of ultrasound tissue characterization (UTC) in patellar tendons. Scand. J. Med. Sci. Sports 2019, 29, 1205–1211. [Google Scholar] [CrossRef]
- Rabello Ml Dams, O.; Akker-Scheek, I.; Zwerver, J.; O’Neill, S. Substantiating the Use of Ultrasound Tissue Characterization in the Analysis of Tendon Structure: A Systematic Review. Clin. J. Sport Med. 2019, 31, e161–e175. [Google Scholar] [CrossRef]
- Rabello, M. The influence of load on tendons and tendinopathy The influence of load on tendons and tendinopathy Studying Achilles and patellar tendons using UTC. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 2019. [Google Scholar]
- Scott, A.; Docking, S.; Vicenzino, B.; Alfredson, H.; Zwerver, J.; Lundgreen, K.; Finlay, O.; Pollock, N.; Cook, J.L.; Fearon, A.; et al. Sports and exercise-related tendinopathies: A review of selected topical issues by participants of the second International Scientific Tendinopathy Symposium (ISTS) Vancouver 2012. Br. J. Sports Med. 2013, 47, 536–544, Erratum in Br. J. Sports Med. 2013, 47, 774. [Google Scholar] [CrossRef]
- Rio, E.; Kidgell, D.; Lorimer Moseley, G.; Gaida, J.; Docking, S.; Purdam, C.; Cook, J. Tendon neuroplastic training: Changing the way we think about tendon rehabilitation: A narrative review. Br. J. Sports Med. 2016, 50, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Malliaras, P.; Kamal, B.; Nowell, A.; Farley, T.; Dhamu, H.; Simpson, V.; Morrissey, D.; Langberg, H.; Maffulli, N.; Reeves, N.D. Patellar tendon adaptation in relation to load-intensity and contraction type. J. Biomech. 2013, 46, 1893–1899. [Google Scholar] [CrossRef] [PubMed]
- Rio, E.; Moseley, L.; Purdam, C.; Samiric, T.; Kidgell, D.; Pearce, A.J.; Jaberzadeh, S.; Cook, J. The pain of tendinopathy: Physiological or pathophysiological? Sports Med. 2014, 44, 9–23. [Google Scholar] [CrossRef] [PubMed]
- de Vries, A.J.; van der Worp, H.; Diercks, R.L.; van den Akker-Scheek, I.; Zwerver, J. Risk factors for patellar tendinopathy in volleyball and basketball players: A survey-based prospective cohort study. Scand. J. Med. Sci. Sports 2015, 25, 678–684. [Google Scholar] [CrossRef]
| Gender (n) | Female (23) | Male (20) |
|---|---|---|
| Height (Kg) (mean ± SD) | 180.91 ± 9.56 | 199.76 ± 7.98 * |
| Weight (cm) (mean ± SD) | 74.7 ± 6.82 | 89.22 ± 7.11 * |
| Position (n/%) | ||
| Shooting Guard | 5 (21.73%) | 4 (20%) |
| Pivot Wing | 5 (21.73%) | 6 (30%) |
| Small Forwards | 6 (26.08%) | 5 (25%) |
| Point Guard | 4 (17.39%) | 4 (20%) |
| Center | 3 (13.04%) | 1 (5%) |
| Jumping leg (n/%) | ||
| Right | 19 (82.6%) | 16 (80%) |
| Left | 3 (13.04%) | 4 (20%) |
| Patellar Tendon Pain (n/%) | ||
| Right (Baseline) | 4 (17.3%) | 5 (25%) |
| Right (1st Half) | 2 (8.6%) | 1 (5%) |
| Right (All Season) | 3 (13.04%) | 3 (15%) |
| Left (Baseline) | 2 (8.6%) | 2 (10%) |
| Left (1st Half) | 1 (4.3%) | 2 (10%) |
| Left (All Season) | 2 (8.6%) | 3 (15%) |
| Exposition (h) | ||
| 1st Half | 77.47 (35.98) | 94.33 (27.34) * |
| All Season | 221.54 (48.12) | 258.33 (41.27) |
| Time of Season | Tendon Length (%) | Echo-Type | Side | Median (IQR) | 95% CI | p-Value | Cohen’s d | SEM | MDC | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sup Lim | Inf Lim | |||||||||
| 4 months | Prox Tendon | Type I | L | 9.15 (14.34) | 10.29 | −3.49 | 0.43 | 0.65 | 0.25 | 2.61 |
| R | 7.97 (17.01) | 0.26 | −0.86 | 0.63 | 0.52 | 0.16 | 3.10 | |||
| Type II | L | −5.04 (16.82) | 0.36 | −0.13 | 0.39 | −0.86 | 0.23 | 3.90 | ||
| R | −1.1 (4.3) | 0.43 | 0.86 | 0.24 | −0.69 | 0.82 | 0.78 | |||
| Type III | L | 0 (0.86) | 0.42 | 0.24 | 0.39 | 0.44 | 0.92 | 0.82 | ||
| R | 0 (0.41) | 0.66 | −0.66 | 0.76 | −0.38 | 0.69 | 0.76 | |||
| Type IV | L | 0 (0.87) | 0.41 | 0.98 | 0.81 | −0.42 | 0.57 | 0.16 | ||
| R | 0 (0.61) | 0.93 | −0.24 | 0.89 | 0.58 | 0.29 | 0.04 | |||
| Mid-Tendon | Type I | L | 6.41 (17.62) | 0.10 | −0.29 | 0.70 | 1.00 | 0.63 | 0.58 | |
| R | 9.93 (26.84) | 0.87 | −0.34 | 0.92 | 0.77 | 0.55 | 4.60 | |||
| Type II | L | −8.88 (15.64) | 0.37 | −0.04 | 0.04 * | −0.13 | 0.02 | 2.15 | ||
| R | −4.15 (13.86) | 0.90 | −0.91 | 0.18 | −0.11 | 0.14 | 0.29 | |||
| Type III | L | 0 (0.13) | 0.76 | 0.70 | 0.76 | 0.37 | 0.72 | 0.64 | ||
| R | 0 (0.1) | 0.45 | 0.82 | 0.55 | −0.34 | 0.80 | 0.61 | |||
| Type IV | L | 0 (0.69) | 0.40 | −0.48 | 0.55 | 0.26 | 0.99 | 0.86 | ||
| R | 0 (0.52) | 0.37 | 0.97 | 0.16 | −0.86 | 0.54 | 1.82 | |||
| 8 months | Prox Tendon | Type I | L | 5.05 (28.93) | 0.61 | −0.94 | 0.05 * | 1.14 | 0.77 | 2.54 |
| R | 8.31 (13.97) | 0.88 | −0.76 | 0.88 | −0.18 | 0.75 | 1.79 | |||
| Type II | L | −5.31 (25.64) | 0.36 | 0.93 | 0.02 * | −0.35 | 0.92 | 2.31 | ||
| R | −1.53 (12.3) | 0.15 | 0.08 | 0.62 | 0.33 | 0.67 | 0.48 | |||
| Type III | L | 0 (0.07) | 0.16 | −0.10 | 0.06 | 0.71 | 0.09 | 0.23 | ||
| R | 0 (0.26) | 0.11 | 0.64 | 0.28 | −0.17 | 0.77 | 0.08 | |||
| Type IV | L | 0 (0.12) | 0.63 | 0.06 | 0.89 | −0.13 | 0.54 | 0.06 | ||
| R | 0 (0.69) | 0.71 | −0.28 | 0.23 | 0.83 | 0.36 | 1.36 | |||
| Mid-Tendon | Type I | L | 2.83 (25.93) | 0.91 | −0.37 | 0.09 | 0.60 | 0.77 | 1.75 | |
| R | 6.95 (23.01) | 0.09 | 0.21 | 0.03 * | −1.08 | 0.59 | 2.70 | |||
| Type II | L | −1.51 (17.78) | 0.38 | −0.84 | 0.01 * | 2.16 | 0.95 | 1.24 | ||
| R | −4.61 (19.42) | 0.64 | 0.36 | 0.01 * | −1.35 | 0.65 | 0.31 | |||
| Type III | L | 0 (0.31) | 0.17 | −0.01 | 0.64 | 0.31 | 0.36 | 0.59 | ||
| R | 0 (0.48) | 0.34 | 0.17 | 0.04 * | −1.04 | 0.04 | 0.15 | |||
| Type IV | L | 0 (0.57) | 0.31 | −0.40 | 0.91 | 0.16 | 0.81 | 0.27 | ||
| R | 0 (0.87) | 0.42 | −0.16 | 0.01 * | −0.15 | 0.02 | 0.05 | |||
| Male | Female | ||||||
|---|---|---|---|---|---|---|---|
| Echo-Type | Median (IQR) | 95% CI (Sup to Inf) | Median (IQR) | 95% CI (Sup to Inf) | Effect Size | ||
| Baseline | Proximal Tendon | Type I | −2.7 (22.2) | (7.07 to −25.47) | −4.4 (37.7) | (12.44 to 0.01) | −0.17 |
| Type II | 0.4 (24.7) | (20.32 to −2.32) | −3.6 (22.2) | (2.933 to −4.4) | 0.21 * | ||
| Type III | 0 (0.5) | (2.77 to −0.37) | 0.04 (2.24) | (1.797 to −0.2) | 0.01 | ||
| Type IV | 0 (0.3) | (0.5 to 0.02) | 0.2 (0.8) | (0.934 to 0.07) | −0.04 | ||
| Mid-Tendon | Type I | −3.5 (27.1) | (2.38 to −22.72) | 4.6 (24.3) | (13.92 to −0.6) | −0.16 * | |
| Type II | 3.4 (27.9) | (22.25 to −2.38) | −0.24 (23.74) | (4.141 to −6.3) | 0.11 | ||
| Type III | 0 (0.05) | (0.2 to −0.02) | 0.2 (2.6) | (1.124 to −0.2) | −0.04 | ||
| Type IV | 0 (0) | (0.04 to 0) | 0.04 (0.7) | (0.346 to 0) | −0.07 | ||
| 4 months | Proximal Tendon | Type I | −3.45 (34.2) | (23.45 to −22.34) | 20 (24.8) | (13.33 to 1.34) | −0.1 |
| Type II | 2.25 (26) | (5.34 to −3.48) | −8.74 (22.84) | (3.929 to −13) | 0.02 | ||
| Type III | 0.2 (2.4) | (2.77 to −0.73) | −2 (2.4) | (−0.33 to −2.3) | 0.19 * | ||
| Type IV | 0 (0.3) | (0.8 to −0.08) | −0.34 (0.4) | (−0.27 to −1) | 0.24 * | ||
| Mid-Tendon | Type I | −0.4 (35.05) | (22.27 to −22.83) | 22.74 (22.97) | (16.77 to 4.66) | −0.16 | |
| Type II | 0.05 (20.36) | (4.5 to −22.22) | −22.34 (8.2) | (−4.12 to −14) | 0.23 | ||
| Type III | 0.05 (0.75) | (0.75 to 0.22) | −0.44 (2.2) | (−0.32 to −1.7) | 0.23 * | ||
| Type IV | 0 (0.3) | (0.28 to 0.02) | −0.2 (0.6) | (−0.14 to −0.7) | 0.19 * | ||
| 8 months | Proximal Tendon | Type I | −2.4 (20.5) | (4.28 to −22.32) | 22.8 (23.4) | (20.94 to 6.79) | −0.25 * |
| Type II | 2.4 (23.25) | (7.58 to −2.72) | −9.3 (22.4) | (1.133 to −14) | 0.11 * | ||
| Type III | 0.3 (2.7) | (2.24 to 0.37) | −0.74 (2.2) | (−0.33 to −0.9) | 0.57 * | ||
| Type IV | 0.2 (2.2) | (2.07 to 0.27) | −0.04 (0.3) | (−0.03 to −0.2) | 0.56 * | ||
| Mid-Tendon | Type I | −5.4 (25.75) | (5.42 to −25.3) | 23.4 (24.04) | (23 to 9.69) | −0.28 | |
| Type II | 3.2 (29.9) | (7.77 to −4.2) | −20.64 (23.24) | (−4.62 to −16) | 0.34 * | ||
| Type III | 0.25 (0.95) | (0.73 to 0.27) | −0.4 (0.9) | (−0.37 to −1) | 0.35 * | ||
| Type IV | 0.05 (0.25) | (0.2 to 0.04) | −0.2 (0.3) | (−0.04 to −0.3) | 0.28 * | ||
| PT Structural Difference (4 Months-Baseline and 8 Months-Baseline) | PT Structural Differences Between Male and Female | PT Structure Differences Between Jumping and Non-Jumping Leg | PT Structural Differences Between Symptomatic and Asymptomatic | PT Structural Differences Between Season/Cohort | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Proximal Tendon | Mid-Tendon | Proximal Tendon | Mid-Tendon | Proximal Tendon | Mid-Tendon | Proximal Tendon | Mid-Tendon | Proximal Tendon | Mid-Tendon | |
| Baseline | Female players showed greater disorganized tendon in echo-type II | Female players showed more aligned structural fibers in echo-type I | No differences | No differences | No different | Symptomatic tendons showed higher disorganized of echo-type II | No different | No different | ||
| 4 months | No different | Left tendon: different in echo-type II | Female players show fewer disorganized echo-type IV and greater organization of echo-type III than male | Female players show greater organization of echo-type III and fewer disorganized tendon of echo-type IV than male | Greater disorganized echo-type III and IV at the non-jumping leg | No different | No different | Symptomatic players showed higher disorganized fiber of echo-type I | No different | No different |
| 8 months | Left tendon: disorganized echo-type II and increased echo-type I | Disorganized echo-type II | Female players showed more aligned echo-type I, III and IV than male | Female players show fewer disorganized tendon of echo-type II and IV and greater organization of echo-type III than males | Greater disorganized echo-type III and IV at the non-jumping leg | No different | No different | Symptomatic players showed higher disorganized fiber of echo-type I | No different | No different |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Cebrián, S.; Bagur-Calafat, C.; Adillón, C.; Treviño, S.; Martin, C.; Ruiz, J.; Urbano, D.; Rodas, G. Patellar Tendon Structural Difference Occurs in Female and Male Professional Basketball Players: 8 Months Follow-Up. J. Funct. Morphol. Kinesiol. 2025, 10, 420. https://doi.org/10.3390/jfmk10040420
Ortega-Cebrián S, Bagur-Calafat C, Adillón C, Treviño S, Martin C, Ruiz J, Urbano D, Rodas G. Patellar Tendon Structural Difference Occurs in Female and Male Professional Basketball Players: 8 Months Follow-Up. Journal of Functional Morphology and Kinesiology. 2025; 10(4):420. https://doi.org/10.3390/jfmk10040420
Chicago/Turabian StyleOrtega-Cebrián, Silvia, Caritat Bagur-Calafat, Cristina Adillón, Silvia Treviño, Carles Martin, Javier Ruiz, David Urbano, and Gil Rodas. 2025. "Patellar Tendon Structural Difference Occurs in Female and Male Professional Basketball Players: 8 Months Follow-Up" Journal of Functional Morphology and Kinesiology 10, no. 4: 420. https://doi.org/10.3390/jfmk10040420
APA StyleOrtega-Cebrián, S., Bagur-Calafat, C., Adillón, C., Treviño, S., Martin, C., Ruiz, J., Urbano, D., & Rodas, G. (2025). Patellar Tendon Structural Difference Occurs in Female and Male Professional Basketball Players: 8 Months Follow-Up. Journal of Functional Morphology and Kinesiology, 10(4), 420. https://doi.org/10.3390/jfmk10040420







