Kinetics of Serum Myoglobin and Creatine Kinase Related to Exercise-Induced Muscle Damage and ACTN3 Polymorphism in Military Paratroopers Under Intense Exercise
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval and Sampling
2.2. Biological Samples and Physical Activity
2.3. Rating of Perceived Exertion and Clinical Analysis
2.4. The ACTN3 Polymorphism
2.5. Statistical Analysis
3. Results
3.1. Exercise and Rating of Perceived Exertion
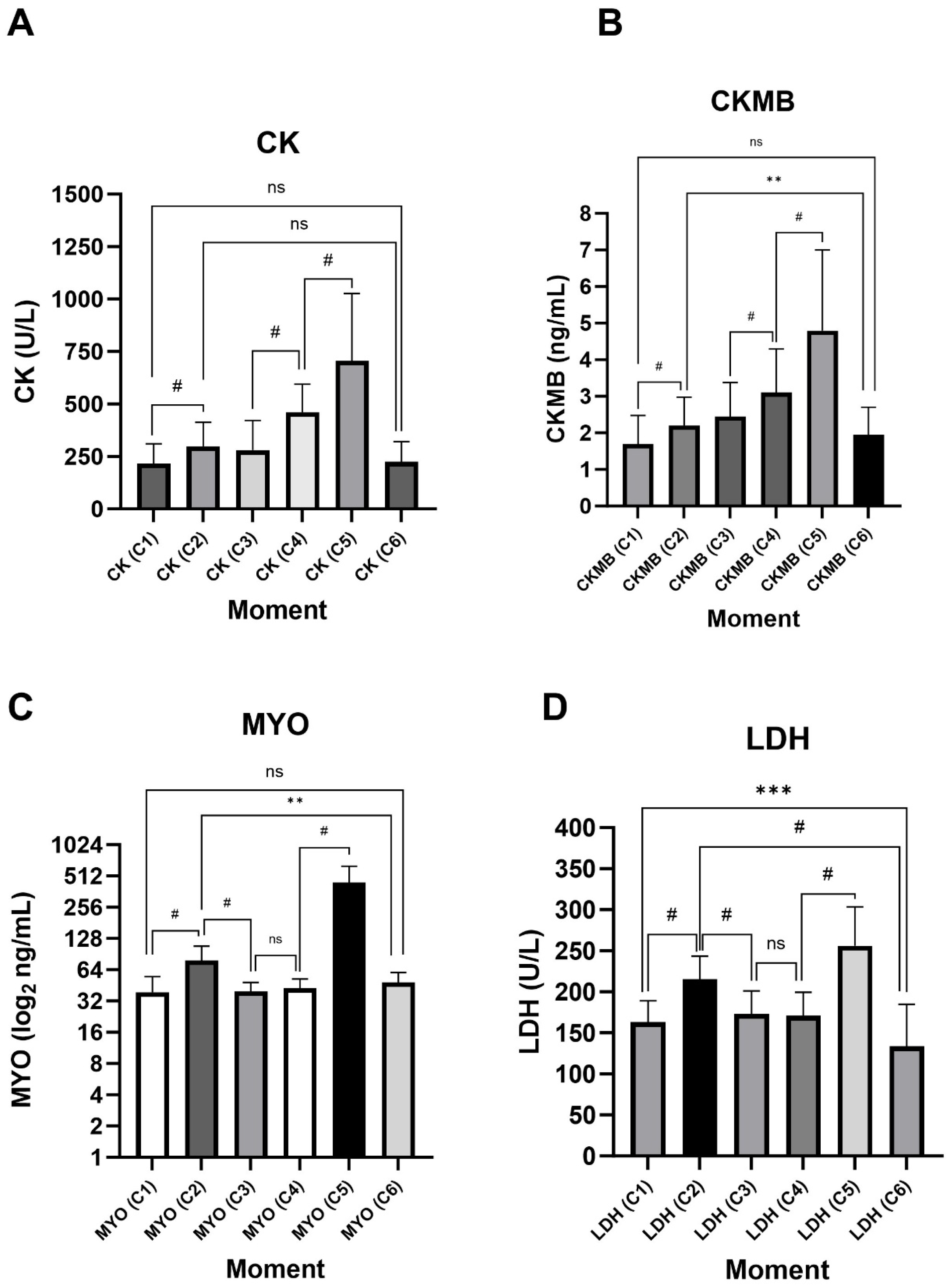
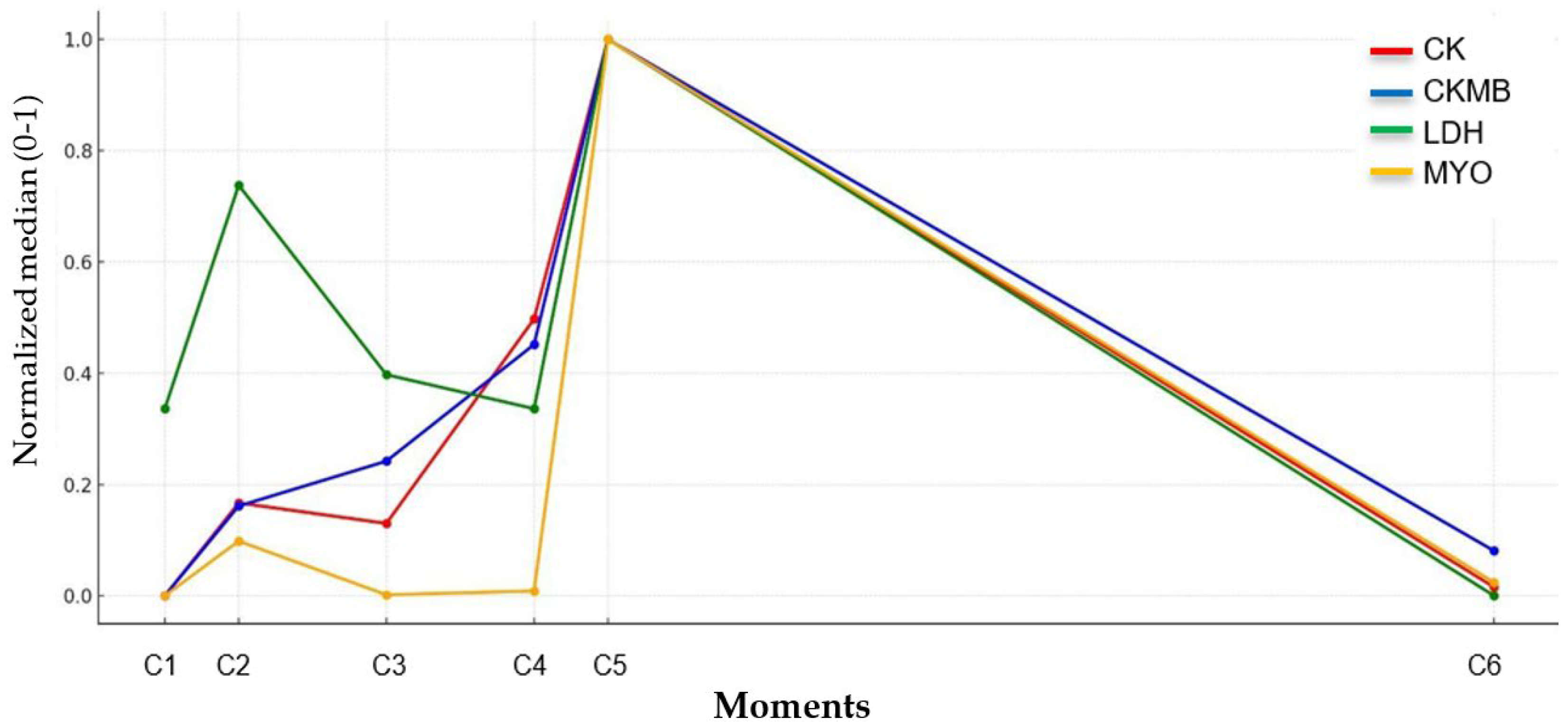
3.2. The Laboratory Markers
3.3. ACTN3 Gene SNP Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cowan, D.N.; Jones, B.H.; Shaffer, R.A. Musculoskeletal Injuries in the Military Training Environment. In Musculoskeletal Injuries in the Military; Cameron, K.L., Owens, B.D., Eds.; Springer: New York, NY, USA, 2016; pp. 195–210. ISBN 978-1-4939-2983-2. [Google Scholar]
- Hauschild, V.D.; DeGroot, D.W.; Hall, S.M.; Grier, T.L.; Deaver, K.D.; Hauret, K.G.; Jones, B.H. Fitness Tests and Occupational Tasks of Military Interest: A Systematic Review of Correlations. Occup. Environ. Med. 2017, 74, 144–153. [Google Scholar] [CrossRef]
- Markus, I.; Constantini, K.; Hoffman, J.R.; Bartolomei, S.; Gepner, Y. Exercise-Induced Muscle Damage: Mechanism, Assessment and Nutritional Factors to Accelerate Recovery. Eur. J. Appl. Physiol. 2021, 121, 969–992. [Google Scholar] [CrossRef]
- Del Coso, J.; Valero, M.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F. Optimum Polygenic Profile to Resist Exertional Rhabdomyolysis during a Marathon. PLoS ONE 2017, 12, e0172965. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Kim, S.; Ryu, H.Y.; Cha, K.S.; Sung, D.J. Exercise-Induced Rhabdomyolysis Mechanisms and Prevention: A Literature Review. J. Sport. Health Sci. 2016, 5, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Atias-Varon, D.; Sherman, H.; Yanovich, R.; Heled, Y. Rhabdomyolysis After Crawling Military Training. Mil. Med. 2017, 182, e1948–e1952. [Google Scholar] [CrossRef] [PubMed]
- Molloy, J.M.; Pendergrass, T.L.; Lee, I.E.; Chervak, M.C.; Hauret, K.G.; Rhon, D.I. Musculoskeletal Injuries and United States Army Readiness Part I: Overview of Injuries and Their Strategic Impact. Mil. Med. 2020, 185, e1461–e1471. [Google Scholar] [CrossRef]
- Teyhen, D.S.; Goffar, S.L.; Shaffer, S.W.; Kiesel, K.; Butler, R.J.; Tedaldi, A.-M.; Prye, J.C.; Rhon, D.I.; Plisky, P.J. Incidence of Musculoskeletal Injury in US Army Unit Types: A Prospective Cohort Study. J. Orthop. Sports Phys. Ther. 2018, 48, 749–757. [Google Scholar] [CrossRef]
- O’Connor, F.G.; Brennan, F.H.; Campbell, W.; Heled, Y.; Deuster, P. Return to Physical Activity after Exertional Rhabdomyolysis. Curr. Sports Med. Rep. 2008, 7, 328–331. [Google Scholar] [CrossRef]
- Torres, P.A.; Helmstetter, J.A.; Kaye, A.M.; Kaye, A.D. Rhabdomyolysis: Pathogenesis, Diagnosis, and Treatment. Ochsner J. 2015, 15, 58–69. [Google Scholar] [PubMed]
- Lippi, G.; Schena, F.; Ceriotti, F. Diagnostic Biomarkers of Muscle Injury and Exertional Rhabdomyolysis. Clin. Chem. Lab. Med. CCLM 2018, 57, 175–182. [Google Scholar] [CrossRef]
- Meister, J.; Reddy, K. Rhabdomyolysis: An Overview. Am. J. Nurs. 2002, 102, 75–79. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Franke, R.; Rodrigues, R.; Geremia, J.M.; Teixeira, B.C.; Boeno, F.; Rabello, R.; Baroni, B.M.; Lima, C.S. Moderate Intensity Cycling Is Better than Running on Recovery of Eccentric Exercise-Induced Muscle Damage. Phys. Ther. Sport 2021, 50, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A. Hormonal Responses and Adaptations to Resistance Exercise and Training. Sports Med. 2005, 35, 339–361. [Google Scholar] [CrossRef]
- Nelson, D.A.; Deuster, P.A.; Carter, R.; Hill, O.T.; Wolcott, V.L.; Kurina, L.M. Sickle Cell Trait, Rhabdomyolysis, and Mortality among U.S. Army Soldiers. N. Engl. J. Med. 2016, 375, 435–442. [Google Scholar] [CrossRef]
- Keltz, E.; Khan, F.Y.; Mann, G. Rhabdomyolysis. The Role of Diagnostic and Prognostic Factors. Muscles Ligaments Tendons J. 2013, 3, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Kodadek, L.; Carmichael, S.P.; Seshadri, A.; Pathak, A.; Hoth, J.; Appelbaum, R.; Michetti, C.P.; Gonzalez, R.P. Rhabdomyolysis: An American Association for the Surgery of Trauma Critical Care Committee Clinical Consensus Document. Trauma. Surg. Acute Care Open 2022, 7, e000836. [Google Scholar] [CrossRef]
- Kumar, V.; Kansal, R.; Kanojia, R.; Vaiphei, K.; Dhillon, M. Can We Use Creatine Kinase Muscle Type as a Potential Marker for Muscle Viability in Mangled Extremities? A Preliminary Evaluation of Its Applicability and a Literature Review. J. Musculoskelet. Surg. Res. 2019, 3, 254. [Google Scholar] [CrossRef]
- Callegari, G.A.; Novaes, J.S.; Neto, G.R.; Dias, I.; Garrido, N.D.; Dani, C. Creatine Kinase and Lactate Dehydrogenase Responses After Different Resistance and Aerobic Exercise Protocols. J. Hum. Kinet. 2017, 58, 65–72. [Google Scholar] [CrossRef]
- Schwiete, C.; Roth, C.; Mester, J.; Broich, H.; Behringer, M. Overlaps of Skeletal Muscle Fatigue and Skeletal Muscle Damage: The Muscle Injury Continuum. Sports Med. Open 2025, 11, 73. [Google Scholar] [CrossRef]
- Tietze, D.C.; Borchers, J. Exertional Rhabdomyolysis in the Athlete: A Clinical Review. Sports Health 2014, 6, 336–339. [Google Scholar] [CrossRef]
- Kenney, K.; Landau, M.E.; Gonzalez, R.S.; Hundertmark, J.; O’Brien, K.; Campbell, W.W. Serum Creatine Kinase after Exercise: Drawing the Line between Physiological Response and Exertional Rhabdomyolysis. Muscle Nerve 2012, 45, 356–362. [Google Scholar] [CrossRef] [PubMed]
- Park, C.-H.; Kim, K.-B.; Han, J.; Ji, J.-G.; Kwak, Y.-S. Cardiac Damage Biomarkers Following a Triathlon in Elite and Non-Elite Triathletes. Korean J. Physiol. Pharmacol. 2014, 18, 419. [Google Scholar] [CrossRef]
- Sharifzadeh, H.; Monazami, A.A.; Azizi, M. Effects of Acute Resistance Training on Biochemical Markers of Myocardial Injury (CTnT, CTnI, CK-MB) in Non-Athlete Women. J. Kermanshah Univ. Med. Sci. 2019, 23, e84103. [Google Scholar] [CrossRef]
- Park, C.H.; Kwak, Y.-S. Changes of Cardiac Biomarkers after Ultradistance and Standard-Distance Triathlon. J. Exerc. Rehabil. 2019, 15, 254–257. [Google Scholar] [CrossRef]
- Chavez, L.O.; Leon, M.; Einav, S.; Varon, J. Beyond Muscle Destruction: A Systematic Review of Rhabdomyolysis for Clinical Practice. Crit. Care 2016, 20, 135. [Google Scholar] [CrossRef] [PubMed]
- Lippi, G.; Favaloro, E.J.; Guidi, G.C. The Genetic Basis of Human Athletic Performance. Why Are Psychological Components so Often Overlooked? J. Physiol. 2008, 586, 3017, author reply 3019-20. [Google Scholar] [CrossRef]
- Liang, X.; Liu, L.; Fu, T.; Zhou, Q.; Zhou, D.; Xiao, L.; Liu, J.; Kong, Y.; Xie, H.; Yi, F.; et al. Exercise Inducible Lactate Dehydrogenase B Regulates Mitochondrial Function in Skeletal Muscle. J. Biol. Chem. 2016, 291, 25306–25318. [Google Scholar] [CrossRef]
- Leite, C.D.F.C.; Zovico, P.V.C.; Rica, R.L.; Barros, B.M.; Machado, A.F.; Evangelista, A.L.; Leite, R.D.; Barauna, V.G.; Maia, A.F.; Bocalini, D.S. Exercise-Induced Muscle Damage after a High-Intensity Interval Exercise Session: Systematic Review. Int. J. Environ. Res. Public Health 2023, 20, 7082. [Google Scholar] [CrossRef] [PubMed]
- Seto, J.T.; Roeszler, K.N.; Meehan, L.R.; Wood, H.D.; Tiong, C.; Bek, L.; Lee, S.F.; Shah, M.; Quinlan, K.G.R.; Gregorevic, P.; et al. ACTN3 Genotype Influences Skeletal Muscle Mass Regulation and Response to Dexamethasone. Sci. Adv. 2021, 7, eabg0088. [Google Scholar] [CrossRef]
- Vincent, B.; Windelinckx, A.; Nielens, H.; Ramaekers, M.; Van Leemputte, M.; Hespel, P.; Thomis, M.A. Protective Role of α-Actinin-3 in the Response to an Acute Eccentric Exercise Bout. J. Appl. Physiol. 2010, 109, 564–573. [Google Scholar] [CrossRef]
- Ogura, Y.; Naito, H.; Kakigi, R.; Akema, T.; Sugiura, T.; Katamoto, S.; Aoki, J. Different Adaptations of Alpha-Actinin Isoforms to Exercise Training in Rat Skeletal Muscles. Acta Physiol. 2009, 196, 341–349. [Google Scholar] [CrossRef]
- El Ouali, E.M.; Barthelemy, B.; Del Coso, J.; Hackney, A.C.; Laher, I.; Govindasamy, K.; Mesfioui, A.; Granacher, U.; Zouhal, H. A Systematic Review and Meta-Analysis of the Association Between ACTN3 R577X Genotypes and Performance in Endurance Versus Power Athletes and Non-Athletes. Sports Med. Open 2024, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Moreno, V.; Areces, F.; Ruiz-Vicente, D.; Ordovás, J.M.; Del Coso, J. Influence of the ACTN3 R577X Genotype on the Injury Epidemiology of Marathon Runners. PLoS ONE 2020, 15, e0227548. [Google Scholar] [CrossRef]
- Eynon, N.; Hanson, E.D.; Lucia, A.; Houweling, P.J.; Garton, F.; North, K.N.; Bishop, D.J. Genes for Elite Power and Sprint Performance: ACTN3 Leads the Way. Sports Med. 2013, 43, 803–817. [Google Scholar] [CrossRef]
- de Lima, L.C.R.; Bueno Junior, C.R.; de Oliveira Assumpção, C.; de Menezes Bassan, N.; Barreto, R.V.; Cardozo, A.C.; Greco, C.C.; Denadai, B.S. The Impact of ACTN3 Gene Polymorphisms on Susceptibility to Exercise-Induced Muscle Damage and Changes in Running Economy Following Downhill Running. Front. Physiol. 2021, 12, 769971. [Google Scholar] [CrossRef]
- de Almeida, K.Y.; Cetolin, T.; Marrero, A.R.; Aguiar Junior, A.S.; Mohr, P.; Kikuchi, N. A Pilot Study on the Prediction of Non-Contact Muscle Injuries Based on ACTN3 R577X and ACE I/D Polymorphisms in Professional Soccer Athletes. Genes 2022, 13, 2009. [Google Scholar] [CrossRef]
- Peña-Vázquez, O.; Enriquez-del Castillo, L.A.; González-Chávez, S.A.; Güereca-Arvizuo, J.; Candia Lujan, R.; Carrasco Legleu, C.E.; Cervantes Hernández, N.; Pacheco-Tena, C. Prevalence of Polymorphism and Post-Training Expression of ACTN3 (R/X) and ACE (I/D) Genes in CrossFit Athletes. Int. J. Environ. Res. Public Health 2023, 20, 4404. [Google Scholar] [CrossRef]
- Guilherme, J.P.L.F.; Oliveira, E.M. Increased Prevalence of the Null Allele of the p.Arg577Ter Variant in the ACTN3 Gene in Brazilian Long-distance Athletes: A Retrospective Study. Ann. Hum. Genet. 2024, 88, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Pimenta, E.M.; Coelho, D.B.; Veneroso, C.E.; Barros Coelho, E.J.; Cruz, I.R.; Morandi, R.F.; De Pussieldi, G.A.; Carvalho, M.R.S.; Garcia, E.S.; De Paz Fernández, J.A. Effect of ACTN3 Gene on Strength and Endurance in Soccer Players. J. Strength Cond. Res. 2013, 27, 3286–3292. [Google Scholar] [CrossRef] [PubMed]
- Del Coso, J.; Salinero, J.J.; Lara, B.; Gallo-Salazar, C.; Areces, F.; Puente, C.; Herrero, D. ACTN3 X-Allele Carriers Had Greater Levels of Muscle Damage during a Half-Ironman. Eur. J. Appl. Physiol. 2017, 117, 151–158. [Google Scholar] [CrossRef]
- Massidda, M.; Bachis, V.; Corrias, L.; Piras, F.; Scorcu, M.; Culigioni, C.; Masala, D.; Calò, C.M. ACTN3 R577X Polymorphism Is Not Associated with Team Sport Athletic Status in Italians. Sports Med. Open 2015, 1, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Campos, L.C.B.; Campos, F.A.D.; Franchini, E.; Ribeiro, A.G.S.V.; Pellegrinotti, I.L.; Verlengia, R. Effects of Two 8-Week Physical Training Models (Traditional and Specific) on Improved Military Physical Performance. Eur. J. Phys. Educ. Sport. Sci. 2023, 10, 110–130. [Google Scholar] [CrossRef]
- Borg, G. Perceived Exertion as an Indicator of Somatic Stress. Scand. J. Rehabil. Med. 1970, 2, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Shieh, G.; Jan, S.-L.; Randles, R.H. Power and Sample Size Determinations for the Wilcoxon Signed-Rank Test. J. Stat. Comput. Simul. 2007, 77, 717–724. [Google Scholar] [CrossRef]
- Cabral, L.L.; Lopes, P.B.; Wolf, R.; Stefanello, J.M.F.; Pereira, G. A systematic review of cross-cultural adaptation and validation of borg’s rating of perceived exertion scale. J. Phys. Educ. 2017, 28, e2853. [Google Scholar] [CrossRef]
- Ojanen, T.; Häkkinen, K.; Hanhikoski, J.; Kyröläinen, H. Effects of Task-Specific and Strength Training on Simulated Military Task Performance in Soldiers. Int. J. Environ. Res. Public Health 2020, 17, 8000. [Google Scholar] [CrossRef]
- Baird, M.F.; Graham, S.M.; Baker, J.S.; Bickerstaff, G.F. Creatine-Kinase- and Exercise-Related Muscle Damage Implications for Muscle Performance and Recovery. J. Nutr. Metab. 2012, 2012, 960363. [Google Scholar] [CrossRef]
- Becker, M.; Sperlich, B.; Zinner, C.; Achtzehn, S. Intra-Individual and Seasonal Variation of Selected Biomarkers for Internal Load Monitoring in U-19 Soccer Players. Front. Physiol. 2020, 11, 838. [Google Scholar] [CrossRef]
- Chalchat, E.; Charlot, K.; Garcia-Vicencio, S.; Hertert, P.; Baugé, S.; Bourdon, S.; Bompard, J.; Farges, C.; Martin, V.; Bourrilhon, C.; et al. Circulating MicroRNAs after a 24-h Ultramarathon Run in Relation to Muscle Damage Markers in Elite Athletes. Scand. J. Med. Sci. Sports 2021, 31, 1782–1795. [Google Scholar] [CrossRef]
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine Kinase Monitoring in Sport Medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Banfi, G.; Colombini, A.; Lombardi, G.; Lubkowska, A. Metabolic Markers in Sports Medicine. Adv. Clin. Chem. 2012, 56, 1–54. [Google Scholar]
- Brooks, G.A. The Science and Translation of Lactate Shuttle Theory. Cell Metab. 2018, 27, 757–785. [Google Scholar] [CrossRef] [PubMed]
- Nalcakan, G.R. The Effects of Sprint Interval vs. Continuous Endurance Training on Physiological and Metabolic Adaptations in Young Healthy Adults. J. Hum. Kinet. 2014, 44, 97–109. [Google Scholar] [CrossRef]
- Morales, A.P.; Maciel, R.N.; Jorge, F.S.; Arêas Neto, N.T.; Cordeiro, D.D.C.; Viana, M.A.S.; Oliveira, C.J.L. Alterações Dos Níveis Séricos de Creatinina, Ácido Úrico, Creatina Kinase e Da Taxa de Filtração Glomerular Em Corredores de “Rua”. Rev. Bras. Cineantropometria Desempenho Hum. 2013, 15, 71–81. [Google Scholar] [CrossRef]
- Fernandes, J.; Lamb, K.; Twist, C. Exercise-Induced Muscle Damage and Recovery in Young and Middle-Aged Males with Different Resistance Training Experience. Sports 2019, 7, 132. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Z. Neuromuscular injury method in different strength sports damage. Rev. Bras. Med. Esporte 2021, 27, 767–769. [Google Scholar] [CrossRef]
- Noakes, T.D.; Kotzenberg, G.; McArthur, P.S.; Dykman, J. Elevated Serum Creatine Kinase MB and Creatine Kinase BB-Isoenzyme Fractions after Ultra-Marathon Running. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 52, 75–79. [Google Scholar] [CrossRef]
- Eynon, N.; Ruiz, J.R.; Femia, P.; Pushkarev, V.P.; Cieszczyk, P.; Maciejewska-Karlowska, A.; Sawczuk, M.; Dyatlov, D.A.; Lekontsev, E.V.; Kulikov, L.M.; et al. The ACTN3 R577X Polymorphism across Three Groups of Elite Male European Athletes. PLoS ONE 2012, 7, e43132. [Google Scholar] [CrossRef] [PubMed]
- Norman, B.; Esbjörnsson, M.; Rundqvist, H.; Österlund, T.; Glenmark, B.; Jansson, E. ACTN3 Genotype and Modulation of Skeletal Muscle Response to Exercise in Human Subjects. J. Appl. Physiol. 2014, 116, 1197–1203. [Google Scholar] [CrossRef]
- Yang, N.; MacArthur, D.G.; Wolde, B.; Onywera, V.O.; Boit, M.K.; Lau, S.Y.M.-A.; Wilson, R.H.; Scott, R.A.; Pitsiladis, Y.P.; North, K. The ACTN3 R577X Polymorphism in East and West African Athletes. Med. Sci. Sports Exerc. 2007, 39, 1985–1988. [Google Scholar] [CrossRef]
- Pimenta, E.M.; Coelho, D.B.; Cruz, I.R.; Morandi, R.F.; Veneroso, C.E.; De Azambuja Pussieldi, G.; Carvalho, M.R.S.; Silami-Garcia, E.; De Paz Fernández, J.A. The ACTN3 Genotype in Soccer Players in Response to Acute Eccentric Training. Eur. J. Appl. Physiol. 2012, 112, 1495–1503. [Google Scholar] [CrossRef] [PubMed]
- Tharabenjasin, P.; Pabalan, N.; Jarjanazi, H. Association of the ACTN3 R577X (Rs1815739) Polymorphism with Elite Power Sports: A Meta-Analysis. PLoS ONE 2019, 14, e0217390. [Google Scholar] [CrossRef]
- Pickering, C.; Kiely, J. ACTN3: More than Just a Gene for Speed. Front. Physiol. 2017, 8, 1080. [Google Scholar] [CrossRef]
- Baltazar-Martins, G.; Gutiérrez-Hellín, J.; Aguilar-Navarro, M.; Ruiz-Moreno, C.; Moreno-Pérez, V.; López-Samanes, Á.; Domínguez, R.; Del Coso, J. Effect of ACTN3 Genotype on Sports Performance, Exercise-Induced Muscle Damage, and Injury Epidemiology. Sports 2020, 8, 99. [Google Scholar] [CrossRef]
- Sierra, A.P.R.; Oliveira, R.A.; Silva, E.D.; Lima, G.H.O.; Benetti, M.P.; Kiss, M.A.P.; Sierra, C.A.; Ghorayeb, N.; Seto, J.T.; Pesquero, J.B.; et al. Association between Hematological Parameters and Iron Metabolism Response after Marathon Race and ACTN3 Genotype. Front. Physiol. 2019, 10, 697. [Google Scholar] [CrossRef] [PubMed]
- Belli, T.; Crisp, A.; Verlengia, R. Greater Muscle Damage in Athletes with ACTN3 R577X (RS1815739) Gene Polymorphism after an Ultra-Endurance Race: A Pilot Study. Biol. Sport. 2017, 2, 105–110. [Google Scholar] [CrossRef]
- Venckunas, T.; Skurvydas, A.; Brazaitis, M.; Kamandulis, S.; Snieckus, A.; Moran, C.N. Human Alpha-Actinin-3 Genotype Association with Exercise-Induced Muscle Damage and the Repeated-Bout Effect. Appl. Physiol. Nutr. Metab. 2012, 37, 1038–1046. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, M.; Homma, H.; de Almeida, K.Y.; Kozuma, A.; Saito, M.; Tsuchiya, Y.; Kouzaki, K.; Ochi, E.; Okamoto, T.; Nakazato, K.; et al. Effect of the ACTN3 R577X Polymorphism on Serum Creatine Kinase and Interleukin-6 Levels After Maximal Eccentric Exercise. Am. J. Phys. Med. Rehabil. 2025, 104, 415–421. [Google Scholar] [CrossRef]
- Cruz, A.; Gomes, D.; Verdan, C.; Branquinho, J.; Xavier, M.; Kirsztajn, G.; Guedes Miranda Dos Santos, C.; Pesquero, J.B.; Carneiro, A. ACE I/D and AGT Met235Thr Polymorphisms Distinctly Affect Biomarker Levels and Risk of AKI and Exertional Rhabdomyolysis After Intense Exercise. Mil. Med. 2025, usaf041, ahead of print. [Google Scholar] [CrossRef]

| Day | Duration | Activity |
|---|---|---|
| D1 | 25 min | - 5 km run in 25 min, with camouflage pants and combat boots |
| D2 | 2.5 min Free | - 50 m of swimming - 4 m of rope climb test without feet |
| D3 | 50 min | - 5 × 400 m running routines - 100 kangaroo flexions between running routines - 10 min break |
| 50 min | - 2 × 400 m running routines - 20 kangaroo flexions followed by 10 push-ups in 3 min - Interspersed, 150 kangaroo flexions and 150 jumping jacks in sets of 10 repetitions in 25 min - 10 min break | |
| 50 min | - 150 kangaroo push-ups were performed interspersed with 200 regular push-ups in sets of 10 exercises |
| Variable | Median |
|---|---|
| Age | 23.0 ± 2.0 |
| Borg (C2) | 11.0 ± 2.0 |
| Borg (C5) | 16.5 ± 3.0 ** |
| Biomarker | TT (XX) | CT (RX) | CC (RR) | p | Allele R | Allele X | p |
|---|---|---|---|---|---|---|---|
| Frequency “n” (%) | 5 (15.6) | 15 (46.9) | 12 (37.5) | 0.085 | 27 (84.4) | 5 (15.6) | <0.001 * |
| Last Cooper’s test (m) | 3115 ± 158 | 3262 ± 181 | 3200 ± 150 | 0.080 | 3200 ± 150 | 3115 ± 158 | 0.070 |
| RPE POST 5KMRUN (C2) | 11.5± 2.0 | 11.0± 2.0 | 11.0± 5.0 | 0.940 | 11.0± 3.0 | 11.5± 2.0 | 0.9352 |
| RPE POST HELMET (C5) | 15.0± 3.0 | 16.0± 3.0 | 17.0± 3.0 | 0.514 | 16.5± 3.0 | 15.0± 3.0 | 0.620 |
| BIOMARKER | All (n = 32) | TT (XX) | CT (RX) | CC (RR) | p (Genotype Model) | RR + RX | XX | p (Recessive Model) |
|---|---|---|---|---|---|---|---|---|
| CK (C1) | 217.0 ± 139.0 | 175.0 ± 62.0 | 217.0 ± 117.0 | 244.5 ± 112.0 | 0.284 | 228.0 ± 141.0 | 175.0 ± 62.0 | 0.166 |
| CK (C2) | 298.5 ± 181.0 | 257.0 ± 76.0 | 298.0 ± 195.0 | 324.5 ± 192.0 | 0.249 | 324.0 ± 177.0 | 257.0 ± 76.0 | 0.109 |
| CK (C3) | 280.5 ± 196.0 | 243.0 ± 114.0 | 277.0 ± 214.0 | 349.5 ± 242.0 | 0.310 | 283.0 ± 202.0 | 243.0 ± 114.0 | 0.263 |
| CK (C4) | 460.0 ± 221.0 | 422.0 ± 206.0 | 442.0 ± 172.0 | 498.0 ± 312.0 | 0.164 | 465.0 ± 211.0 | 422.0 ± 206.0 | 0.310 |
| CK (C5) | 705.5 ± 421.0 | 598.0 ± 299.0 | 708.0 ± 290.0 | 848.5 ± 445.0 | 0.090 (ES = 0.097) | 711.0 ± 451.0 | 598.0 ± 299.0 | 0.077 (ES = 0.320) |
| CK (C6) | 224.5 ± 163.0 | 208.0 ± 1315.0 | 229.0 ± 175.0 | 235.0 ± 139.0 | 0.681 | 229.0 ± 145.0 | 208.0 ± 1315.0 | 0.999 |
| CK-MB (C1) | 1.7 ± 1.1 | 2.2 ± 1.1 | 1.7 ± 0.7 | 1.6 ± 1.2 | 0.694 | 1.6 ± 1.0 | 2.2 ± 1.0 | 0.448 |
| CK-MB (C2) | 2.2 ± 1.1 | 2.9 ± 1.1 | 2.0 ± 1.0 | 2.1 ± 1.9 | 0.771 | 2.0 ± 1.0 | 2.9 ± 1.0 | 0.545 |
| CK-MB (C3) | 2.4 ± 1.6 | 3.0 ± 2.2 | 2.3 ± 1.5 | 2.4 ± 2.1 | 0.558 | 2.3 ± 2.0 | 3.0 ± 2.0 | 0.545 |
| CK-MB (C4) | 3.1 ± 2.0 | 4.3 ± 3.5 | 3.0 ± 2.0 | 3.1 ± 1.8 | 0.274 | 3.0 ± 2.0 | 4.3 ± 3.0 | 0.285 |
| CK-MB (C5) | 4.8 ± 3.3 | 5.9 ± 3.7 | 4.5 ± 2.8 | 4.5 ± 6.7 | 0.893 | 4.5 ± 4.0 | 5.9 ± 4.0 | 0.658 |
| CK-MB (C6) | 1.9 ± 1.5 | 2.3 ± 1.9 | 1.6 ± 0.9 | 2.1 ± 1.5 | 0.171 | 1.6 ± 1.0 | 2.3 ± 2.0 | 0.999 |
| MYO (C1) | 38.9 ± 25.1 | 28.6 ± 26.6 | 39.6 ± 25.6 | 39.5 ± 31.8 | 0.220 | 39.6 ± 22.0 | 28.6 ± 27.0 | 0.087 (ES = 0.310) |
| MYO (C2) | 78.4 ± 46.0 | 66.1 ± 22.8 | 79.4 ± 42.0 | 94.4 ± 57.8 | 0.170 | 79.7 ± 56.0 | 66.1 ± 23.0 | 0.068 (ES = 0.326) |
| MYO (C3) | 39.6 ± 13.5 | 34.7 ± 18.2 | 40.0 ± 9.2 | 39.8 ± 18.8 | 0.473 | 40.5 ± 15.0 | 34.7 ± 18.0 | 0.241 |
| MYO (C4) | 42.4 ± 14.2 | 40.9 ± 10.2 | 45.6 ± 15.5 | 40.7 ± 13.4 | 0.627 | 44.9 ± 16.0 | 40.9 ± 10.0 | 0.650 |
| MYO (C5) | 441.3 ± 359.1 | 228.3 ± 215.1 | 457.8 ± 351.5 | 497.3 ± 379.2 | 0.057 (ES = 0.129) | 489.1 ± 345.0 | 228.3 ± 215.0 | 0.019 (ES = 0.409) |
| MYO (C6) | 48.5 ± 23.4 | 41.4 ± 99.5 | 46.5 ± 19.6 | 50.9 ± 36.8 | 0.910 | 49.9 ± 21.0 | 41.4 ± 100.0 | 0.880 |
| LDH (C1) | 166.0 ± 38.0 | 164.2 ± 73.0 | 157.4 ± 24.0 | 170.5 ± 37.0 | 0.421 | 163.2 ± 33.0 | 164.2 ± 73.0 | 0.954 |
| LDH (C2) | 210.0 ± 36.0 | 222.6 ± 78.0 | 202.5 ± 31.0 | 225.7 ± 47.0 | 0.126 | 211.4 ± 33.0 | 222.6 ± 78.0 | 0.676 |
| LDH (C3) | 174.0 ± 40.0 | 179.4 ± 78.0 | 169.0 ± 41.0 | 177.2 ± 26.0 | 0.600 | 172.4 ± 37.0 | 179.4 ± 78.0 | 0.713 |
| LDH (C4) | 166.0 ± 33.0 | 177.8 ± 73.0 | 162.4 ± 38.0 | 178.6 ± 36.0 | 0.297 | 169.6 ± 34.0 | 177.8 ± 73.0 | 0.669 |
| LDH (C5) | 240.0 ± 80.0 | 243.0 ± 95.0 | 242.5 ± 51.0 | 278.9 ± 82.0 | 0.109 | 258.1 ± 83.0 | 243.0 ± 95.0 | 0.590 |
| LDH (C6) | 127.0 ± 43.0 | 152.6 ± 140.0 | 115.3 ± 66.0 | 148.0 ± 41.0 | 0.248 | 130.5 ± 41.0 | 152.6 ± 150.0 | 0.796 |
| BIOMARKER | All (n = 32) | TT (XX) | CT (RX) | CC (RR) | p (Genotype Model) | RR + RX | XX | p (Recessive Model) |
|---|---|---|---|---|---|---|---|---|
| CK Δ5KMRUN | 70.5 ± 54.0 | 59.0 ± 25.0 | 76.0 ± 78.0 | 73.0 ± 49.0 | 0.205 | 73.0 ± 58.0 | 59.0 ± 25.0 | 0.077 |
| CK ΔHELMET | 274.5 ± 222.0 | 235.0 ± 162.0 | 330.0 ± 214.0 | 261.0 ± 380.0 | 0.241 | 306.0 ± 314.0 | 235.0 ± 162.0 | 0.098 |
| CK ΔRECOVER | 476.0 ± 286.0 | 371.0 ± 1026.0 | 525.0 ± 224.0 | 483.0 ± 439.0 | 0.042 | 505.0 ± 348.0 | 371.0 ± 1026.0 | 0.009 |
| CK-MB Δ5KMRUN | 0.5 ± 0.5 | 0.6 ± 0.0 | 0.4 ± 0.0 | 0.5 ± 1.0 | 0.951 | 0.5 ± 1.0 | 0.6 ± 0.0 | 0.960 |
| CK-MB ΔHELMET | 1.7 ± 1.7 | 1.5 ± 2.0 | 1.9 ± 1.0 | 1.4 ± 3.0 | 0.648 | 1.7 ± 2.0 | 1.5 ± 2.0 | 0.696 |
| CK-MB ΔRECOVER | 3.3 ± 2.3 | 3.6 ± 2.0 | 3.0 ± 2.0 | 3.2 ± 4.0 | 0.915 | 3.2 ± 3.0 | 3.6 ± 2.0 | 0.793 |
| MYO Δ5KMRUN | 38.7 ± 30.9 | 30.1 ± 34.0 | 38.4 ± 27.0 | 39.4 ± 56.0 | 0.397 | 39.4 ± 40.0 | 30.1 ± 34.0 | 0.241 |
| MYO ΔHELMET | 390.1 ± 349.8 | 187.8 ± 205.0 | 412.6 ± 322.0 | 492.2 ± 410.0 | 0.056 | 436.8 ± 339.0 | 187.8 ± 205.0 | 0.019 |
| MYO ΔRECOVER | 370.5 ± 356.8 | 191.0 ± 186.0 | 417.5 ± 327.0 | 391.0 ± 408.0 | 0.057 | 402.6 ± 341.0 | 191.0 ± 186.0 | 0.016 |
| LDH Δ5KMRUN | 49.9 ± 20.1 | 58.4 ± 27.0 | 45.1 ± 27.0 | 52.0 ± 37.0 | 0.504 | 50.9 ± 20.0 | 58.4 ± 27.0 | 0.380 |
| LDH ΔHELMET | 84.6 ± 33.5 | 65.2 ± 64.0 | 80.0 ± 33.0 | 99.2 ± 63.0 | 0.089 | 89.1 ± 48.0 | 65.2 ± 64.0 | 0.218 |
| LDH ΔRECOVER | 123.3 ± 45.8 | 102.5 ± 83.0 | 127.2 ± 93.0 | 127.9 ± 57.0 | 0.499 | 128.9 ± 85.0 | 102.5 ± 83.0 | 0.271 |
| Population | Total | ||
|---|---|---|---|
| RR | RX | XX | |
| Military participants | 37.5% | 46.9% | 15.6% |
| European | 31.0% | 51.1% | 17.9% |
| Africans | 77.5% | 22.1% | 0.4% |
| American | 16.1% | 53.3% | 30.5% |
| East Asian | 30.8% | 49.8% | 19.4% |
| South Asian | 15.7% | 51.1% | 33.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Augusto, R.d.S.; Dill, A.; Souza, E.; Nogueira, T.L.S.; Gomes, D.V.; Paiva, J.; Dornelas-Ribeiro, M.; Santos, C.G.M. Kinetics of Serum Myoglobin and Creatine Kinase Related to Exercise-Induced Muscle Damage and ACTN3 Polymorphism in Military Paratroopers Under Intense Exercise. J. Funct. Morphol. Kinesiol. 2025, 10, 381. https://doi.org/10.3390/jfmk10040381
Augusto RdS, Dill A, Souza E, Nogueira TLS, Gomes DV, Paiva J, Dornelas-Ribeiro M, Santos CGM. Kinetics of Serum Myoglobin and Creatine Kinase Related to Exercise-Induced Muscle Damage and ACTN3 Polymorphism in Military Paratroopers Under Intense Exercise. Journal of Functional Morphology and Kinesiology. 2025; 10(4):381. https://doi.org/10.3390/jfmk10040381
Chicago/Turabian StyleAugusto, Rachel de S., Adrieli Dill, Eliezer Souza, Tatiana L. S. Nogueira, Diego V. Gomes, Jorge Paiva, Marcos Dornelas-Ribeiro, and Caleb G. M. Santos. 2025. "Kinetics of Serum Myoglobin and Creatine Kinase Related to Exercise-Induced Muscle Damage and ACTN3 Polymorphism in Military Paratroopers Under Intense Exercise" Journal of Functional Morphology and Kinesiology 10, no. 4: 381. https://doi.org/10.3390/jfmk10040381
APA StyleAugusto, R. d. S., Dill, A., Souza, E., Nogueira, T. L. S., Gomes, D. V., Paiva, J., Dornelas-Ribeiro, M., & Santos, C. G. M. (2025). Kinetics of Serum Myoglobin and Creatine Kinase Related to Exercise-Induced Muscle Damage and ACTN3 Polymorphism in Military Paratroopers Under Intense Exercise. Journal of Functional Morphology and Kinesiology, 10(4), 381. https://doi.org/10.3390/jfmk10040381