One-Year Follow-Up Cognitive Decline After Hip Fracture Surgery: The Prognostic Role of NSE and S100B Biomarkers in Elderly Patients, a Multicentric Study
Abstract
1. Introduction
2. Materials and Methods
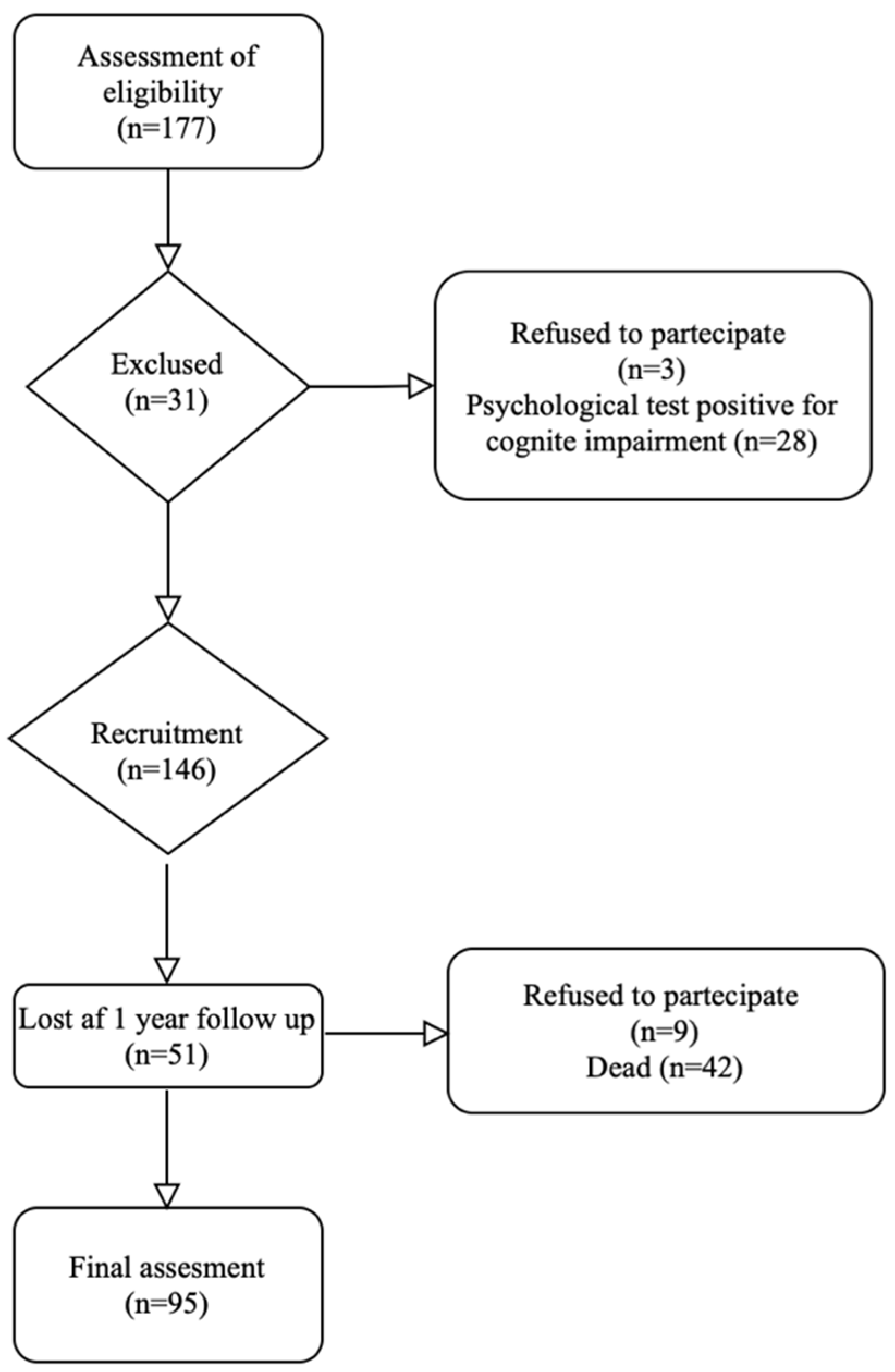
2.1. Patients and Study Design
2.2. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Q.; Wan, H.; Pan, H.; Xu, Y. Postoperative cognitive dysfunction-current research progress. Front. Behav. Neurosci. 2024, 18, 1328790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Huang, X.; Sun, S.; Wang, Y.; Han, L.; Zhang, T.; Zhang, T.; Chen, X. Recent Advances in the Mechanisms of Postoperative Neurocognitive Dysfunction: A Narrative Review. Biomedicines 2025, 13, 115. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Devlieger, B.K.; Rommens, P.M.; Baranowski, A.; Wagner, D. Early Hip Fracture Surgery in Patients Taking Direct Oral Anticoagulants Improves Outcome. J. Clin. Med. 2024, 13, 4707. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yin, W.Y.; Peng, T.; Guo, B.C.; Fan, C.C.; Xu, J.; Liu, X.M.; Li, X. Effects of parecoxib on postoperative cognitive dysfunction and serum levels of NSE and S100β in elderly patients undergoing surgery. Eur. Rev. Med. Pharmacol. Sci. 2024, 28, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, X.; Wu, F.; Liu, Y.; Yang, Y.; Chen, W.; Pan, Z.; Hu, W.; Zheng, F.; He, H. Relationship between postoperative biomarkers of neuronal injury and postoperative cognitive dysfunction: A meta-analysis. PloS ONE 2023, 18, e0284728. [Google Scholar] [CrossRef]
- van Munster, B.C.; Korse, C.M.; de Rooij, S.E.; Bonfrer, J.M.; Zwinderman, A.H.; Korevaar, J.C. Markers of cerebral damage during delirium in elderly patients with hip fracture. BMC Neurol. 2009, 9, 21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, Y.; Jiang, L.; Han, Y. Reduced Concentrations of NSE, S100β, Aβ, and Proinflammatory Cytokines in Elderly Patients Receiving Ultrasound-Guided Combined Lumbar Plexus-Sciatic Nerve Block during Hip Replacement. Genet. Res. 2022, 2022, 1384609. [Google Scholar] [CrossRef]
- Safavynia, S.A.; Goldstein, P.A. The Role of Neuroinflammation in Postoperative Cognitive Dysfunction: Moving from Hypothesis to Treatment. Front. Psychiatry 2019, 9, 752. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Majewski, P.; Zegan-Barańska, M.; Karolak, I.; Kaim, K.; Żukowski, M.; Kotfis, K. Current Evidence Regarding Biomarkers Used to Aid Postoperative Delirium Diagnosis in the Field of Cardiac Surgery—Review. Medicina 2020, 56, 493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wallin, A.; Nordlund, A.; Jonsson, M.; Blennow, K.; Zetterberg, H.; Öhrfelt, A.; Stålhammar, J.; Eckerström, M.; Carlsson, M.; Olsson, E.; et al. Alzheimer’s disease--subcortical vascular disease spectrum in a hospital-based setting: Overview of results from the Gothenburg MCI and dementia studies. J. Cereb. Blood Flow Metab. 2016, 36, 95–113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Evered, L.; Silbert, B.; Scott, D.A.; Ames, D.; Maruff, P.; Blennow, K. Cerebrospinal Fluid Biomarker for Alzheimer Disease Predicts Postoperative Cognitive Dysfunction. Anesthesiology 2016, 124, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.L.; Slongo, T.F.; Agel, J.; Broderick, J.S.; Creevey, W.; DeCoster, T.A.; Prokuski, L.; Sirkin, M.S.; Ziran, B.; Henley, B.; et al. Fracture and dislocation classification compendium—2007: Orthopaedic Trauma Association classification, database and outcomes committee. J. Orthop. Trauma 2007, 21, S1–S133. [Google Scholar] [CrossRef] [PubMed]
- Coviello, M.; Ippolito, F.; Abate, A.; Zavattini, G.; Zaccari, D.; Leone, A.; Noia, G.; Caiaffa, V.; Maccagnano, G. Computer-assisted navigation for intramedullary nailing of intertrochanteric femur fractures: A preliminary result. Med. Glas. 2023, 1, 20. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Xi, C.H.; An, Y.F.; Dong, W.H.; Zhou, M. Perioperative inflammatory response and protein S-100β concentrations—Relationship with post-operative cognitive dysfunction in elderly patients. Acta Anaesthesiol. Scand. 2012, 56, 595–600. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, E. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc. 1975, 23, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Foderaro, G.; Isella, V.; Mazzone, A.; Biglia, E.; Di Gangi, M.; Pasotti, F.; Sansotera, F.; Grobberio, M.; Raimondi, V.; Mapelli, C.; et al. Brand new norms for a good old test: Northern Italy normative study of MiniMental State Examination. Neurol. Sci. 2022, 43, 3053–3063. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Şişman, A.; Avci, Ö.; Çepni, S.K.; Batar, S.; Polat, Ö. Risk factors for cut-out in intertrochanteric fractures treated with proximal femoral nail of double proximal screw design. J. Clin. Orthop. Trauma 2022, 28, 101832. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wolf, H.; Krall, C.; Pajenda, G.; Leitgeb, J.; Bukaty, A.J.; Hajdu, S.; Sarahrudi, K. Alterations of the biomarker S-100B and NSE in patients with acute vertebral spine fractures. Spine J. 2014, 14, 2918–2922. [Google Scholar] [CrossRef] [PubMed]
- Jones, E.L.; Gauge, N.; Nilsen, O.B.; Lowery, D.; Wesnes, K.; Katsaiti, E.; Arden, J.; Amoako, D.; Prophet, N.; Purushothaman, B.; et al. Analysis of neuron-specific enolase and S100B as biomarkers of cognitive decline following surgery in older people. Dement. Geriatr. Cogn. Disord. 2012, 34, 307–311. [Google Scholar] [CrossRef] [PubMed]
- Makovec, M.; Skitek, M.; Šimnovec, L.; Jerin, A. Neuron-Specific Enolase and S100B as Biomarkers of Ischemic Brain Injury During Surgery. Clin. Pract. 2025, 15, 74. [Google Scholar] [CrossRef]
- Smith, G.T.; Chen, T.J.; Shah, N.M.; Agrest, B.; Grotticelli, J. Anesthesia-mediated neuroinflammatory sequelae in post operative cognitive dysfunction: Mechanisms and therapeutic implications. Anesthesiology 2025, 3, 1281034. [Google Scholar] [CrossRef]
- Devinney, M.J.; Wong, M.K.; Wright, M.C.; Marcantonio, E.R.; Terrando, N.; Browndyke, J.N.; Whitson, H.E.; Cohen, H.J.; Nackley, A.G.; Klein, M.E.; et al. Role of Blood-Brain Barrier Dysfunction in Delirium following Non-cardiac Surgery in Older Adults. Ann. Neurol. 2023, 94, 1024–1035. [Google Scholar] [CrossRef]
- Feng, X.; Hu, J.; Hua, F.; Zhang, J.; Zhang, L.; Xu, G. The correlation of intraoperative hypotension and postoperative cognitive impairment: A meta-analysis of randomized controlled trials. BMC Anesthesiol. 2020, 20, 193. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Zhang, J.; He, X.; Huang, J. S100B in postoperative cognitive impairment: Systematic review and meta-analysis. Clin. Chim. Acta 2025, 576, 120380. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.W.Y.; Fan, S.; Li, W.Y.; Thangavelu, V.; Saripella, A.; Englesakis, M.; Yan, E.; Chung, F. Prevalence of postoperative neurocognitive disorders in older non-cardiac surgical patients: A systematic review and meta-analysis. J. Clin. Anesth. 2025, 103, 111830. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yang, W.; Xue, J.; Chen, J.; Liu, S.; Zhang, S.; Zhang, X.; Gu, X.; Dong, Y.; Qiu, P. Neuroinflammation: The central enabler of postoperative cognitive dysfunction. Biomed. Pharmacother. 2023, 167, 115582. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, J.; Ling, J.; Wu, Y.; Yang, P.; Liu, X.; Liu, J.; Zhang, D.; Yin, X.; Yu, P.; et al. The association between diabetes mellitus and postoperative cognitive dysfunction: A systematic review and meta-analysis. Int. J. Surg. 2025, 111, 2633–2650. [Google Scholar] [CrossRef] [PubMed]
- Elias, M.H.; Abdul Hamid, N.; Maluin, S.M.; Aris, S.; Kadiman, S.; Muhammad Hafidz, K.; Juliana, N. Preoperative Protein Profiling Among Postoperative Cognitive Dysfunction (POCD) Patients Following Open-Heart Surgery: A Systematic Review and Integrated Bioinformatic Analysis. Int. J. Mol. Sci. 2024, 25, 12238. [Google Scholar] [CrossRef]
- Zhou, W.; Zhu, B.; Weng, Y.; Chen, C.; Ni, J.; Shen, W.; Lan, W.; Wang, J. The Combination of Presurgical Cortical Gray Matter Volumetry and Cerebral Perfusion Improves the Efficacy of Predicting Postoperative Cognitive Impairment of Elderly Patients. Tomography 2024, 10, 1379–1396. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, T.; Li, J.; Jiang, C.; Yuan, L.; Wu, J.; Mazaheri, A.; Wang, M.; Jin, S.; Myles, P.S.; Yao, Y.; et al. Incidence of 12-month postoperative cognitive decline following regional vs. general anaesthesia in older patients undergoing hip fracture surgery: Follow-up of the RAGA trial. Anaesthesia 2025, 80, 771–780. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van der Feltz-Cornelis, C.; Turk, F.; Sweetman, J.; Khunti, K.; Gabbay, M.; Shepherd, J.; Montgomery, H.; Strain, W.D.; Lip, G.Y.H.; Wootton, D.; et al. Prevalence of mental health conditions and brain fog in people with long COVID: A systematic review and meta-analysis. Gen. Hosp. Psychiatry 2024, 88, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Evered, L.A.; Silbert, B.S. Postoperative Cognitive Dysfunction and Noncardiac Surgery. Anesth. Analg. 2018, 127, 496–505. [Google Scholar] [CrossRef] [PubMed]
- Diane, A.; Allouch, A.; Mu-U-Min, R.B.A.; Al-Siddiqi, H.H. Endoplasmic reticulum stress in pancreatic β-cell dysfunctionality and diabetes mellitus: A promising target for generation of functional hPSC-derived β-cells in vitro. Front. Endocrinol. 2024, 15, 1386471. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Travica, N.; Lotfaliany, M.; Marriott, A.; Safavynia, S.A.; Lane, M.M.; Gray, L.; Veronese, N.; Berk, M.; Skvarc, D.; Aslam, H.; et al. Peri-Operative Risk Factors Associated with Post-Operative Cognitive Dysfunction (POCD): An Umbrella Review of Meta-Analyses of Observational Studies. J. Clin. Med. 2023, 12, 1610. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Juliana, N.; Abd Aziz, N.A.S.; Maluin, S.M.; Abu Yazit, N.A.; Azmani, S.; Kadiman, S.; Hafidz, K.M.; Mohd Fahmi Teng, N.I.; Das, S. Nutritional Status and Post-Cardiac Surgery Outcomes: An Updated Review with Emphasis on Cognitive Function. J. Clin. Med. 2024, 13, 4015. [Google Scholar] [CrossRef] [PubMed]
- Alalawi, R.; Yasmeen, N. Postoperative Cognitive Dysfunction in the Elderly: A Review Comparing the Effects of Desflurane and Sevflurane. J. PeriAnesth. Nurs. 2018, 33, 732–740. [Google Scholar] [CrossRef]
| Preoperative Features | (n = 146) |
|---|---|
| Age (year) | 83.17 ± 8.21 |
| Gender (female) | 88 (60.2%) |
| BMI (Kg/m 2) | 27.30 ± 4.92 |
| Side (right) | 69 (47.3%) |
| Type of fracture | |
| Intracapsular | 50 (34.2%) |
| Extracapsular | 96 (65.8%) |
| Surgical Time (min) | 25.17 ± 9.91 |
| ASA | |
| ≤2 | 57 (39.1%) |
| >3 | 89 (60.9%) |
| Hypertension | 65 (44.5%) |
| History of smoking | 69 (47.3%) |
| Hypercholesterolemia | 60 (41.1%) |
| Diabetes mellitus | 27 (18.4%) |
| Peripheral vascular disease | 5 (3.4%) |
| History of myocardial infarct | 5 (3.4%) |
| NSE (μg/L) | 15.41 ± 4.90 |
| S-100B (μg/L) | 0.51 ± 0.22 |
| POCD n = 30 (20.5%) | Non-POCD n = 116 (79.5%) | p-Value | |
|---|---|---|---|
| NSE at recruitment | 15.88 ± 5.47 | 15.27 ± 4.86 | 0.31 |
| NSE 3d | 16.57 ± 5.63 | 15.91 ± 5.18 | 0.33 |
| S-100B at recruitment | 0.49 ± 0.21 | 0.52 ± 0.22 | 0.35 |
| S-100B 3d | 0.56 ± 0.15 | 0.52 ± 0.21 | 0.65 |
| MMSE at recruitment | 18.37 ± 5.89 | 21.97 ± 5.08 | 0.04 |
| PS at recruitment | 4.97 ± 0.28 | 3.53 ± 0.69 | 0.02 |
| POCD n = 36 (37.9%) | Non-POCD n = 59 (62.1%) | p-Value | |
|---|---|---|---|
| NSE at recruitment | 15.28 ± 6.06 | 15.85 ± 4.81 | 0.82 |
| NSE 3d | 15.71 ± 5.07 | 15.76 ± 5.18 | 0.76 |
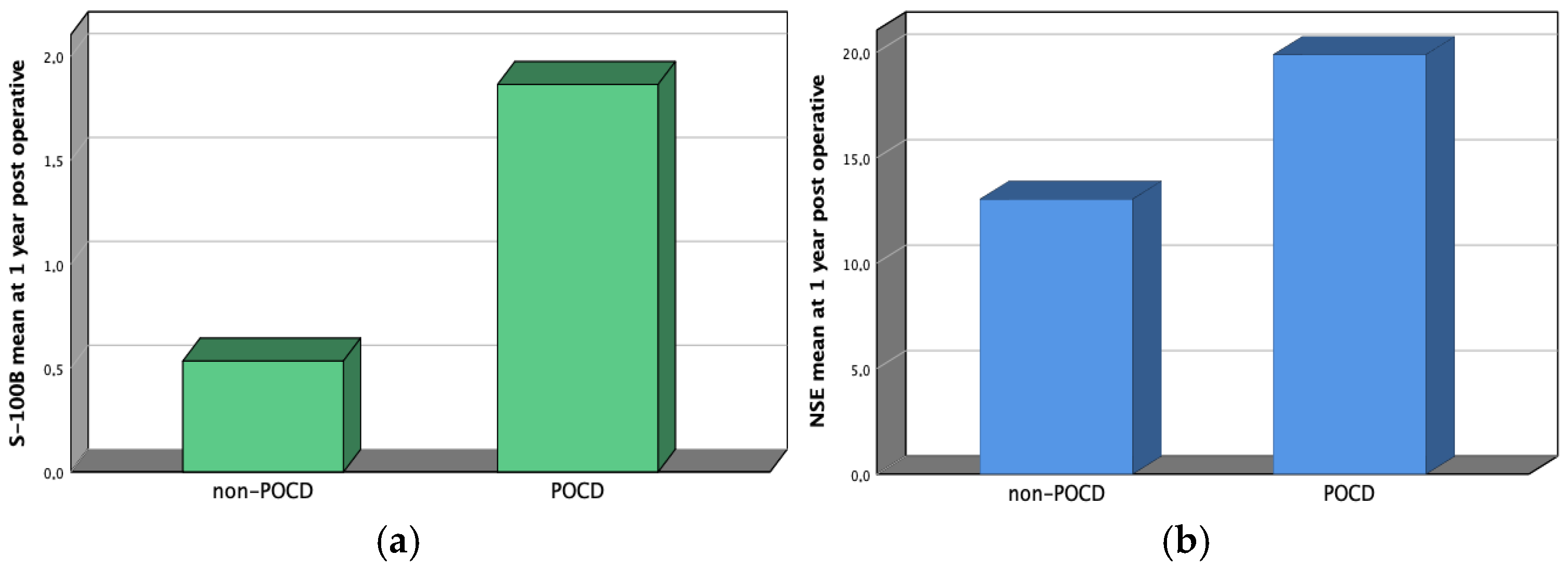
| NSE 1y | 19.88 ± 4.03 | 16.69 ± 4.67 | 0.01 |
| S-100B at recruitment | 0.51 ± 0.24 | 0.53 ± 0.19 | 0.89 |
| S-100B 3d | 0.54 ± 0.23 | 0.55 ± 0.31 | 0.77 |
| S-100B 1y | 1.86 ± 0.9 | 0.78 ± 0.45 | 0.01 |
| MMSE at recruitment | 15.01 ± 2.64 | 24.76 ± 4.87 | 0.01 |
| PS at recruitment | 3.94 ± 0.23 | 3.46 ± 0.72 | 0.03 |
| Factor | p-Value | OR | 95% CI for OR |
|---|---|---|---|
| Age | 0.01 | 1.24 | 1.13–1.34 |
| Diabetes mellitus | 0.01 | 4.41 | 1.48–13.16 |
| MMSE at recruitment | 0.01 | 0.25 | 0.10–0.60 |
| PS at recruitment | 0.01 | 9.81 | 2.27–42.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coviello, M.; Barone, D.; Abate, A.; Geronimo, A.; Cassano, G.D.; Caiaffa, V.; Solarino, G.; Maccagnano, G. One-Year Follow-Up Cognitive Decline After Hip Fracture Surgery: The Prognostic Role of NSE and S100B Biomarkers in Elderly Patients, a Multicentric Study. J. Funct. Morphol. Kinesiol. 2025, 10, 380. https://doi.org/10.3390/jfmk10040380
Coviello M, Barone D, Abate A, Geronimo A, Cassano GD, Caiaffa V, Solarino G, Maccagnano G. One-Year Follow-Up Cognitive Decline After Hip Fracture Surgery: The Prognostic Role of NSE and S100B Biomarkers in Elderly Patients, a Multicentric Study. Journal of Functional Morphology and Kinesiology. 2025; 10(4):380. https://doi.org/10.3390/jfmk10040380
Chicago/Turabian StyleCoviello, Michele, Delia Barone, Antonella Abate, Alessandro Geronimo, Giuseppe Danilo Cassano, Vincenzo Caiaffa, Giuseppe Solarino, and Giuseppe Maccagnano. 2025. "One-Year Follow-Up Cognitive Decline After Hip Fracture Surgery: The Prognostic Role of NSE and S100B Biomarkers in Elderly Patients, a Multicentric Study" Journal of Functional Morphology and Kinesiology 10, no. 4: 380. https://doi.org/10.3390/jfmk10040380
APA StyleCoviello, M., Barone, D., Abate, A., Geronimo, A., Cassano, G. D., Caiaffa, V., Solarino, G., & Maccagnano, G. (2025). One-Year Follow-Up Cognitive Decline After Hip Fracture Surgery: The Prognostic Role of NSE and S100B Biomarkers in Elderly Patients, a Multicentric Study. Journal of Functional Morphology and Kinesiology, 10(4), 380. https://doi.org/10.3390/jfmk10040380









