Interrelationship Between Cardiopulmonary Exercise Testing Indices and Markers of Subclinical Cardiovascular Dysfunction in Those with Type 2 Diabetes—An Observational Cross-Sectional Analysis
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Assessments
2.3. Comprehensive Contrast-Enhanced, Stress and Rest Perfusion Cardiac Magnetic Resonance Imaging
2.4. Transthoracic Echocardiography
2.5. Symptom-Limited Cardiopulmonary Exercise Test
2.6. Statistical Analysis
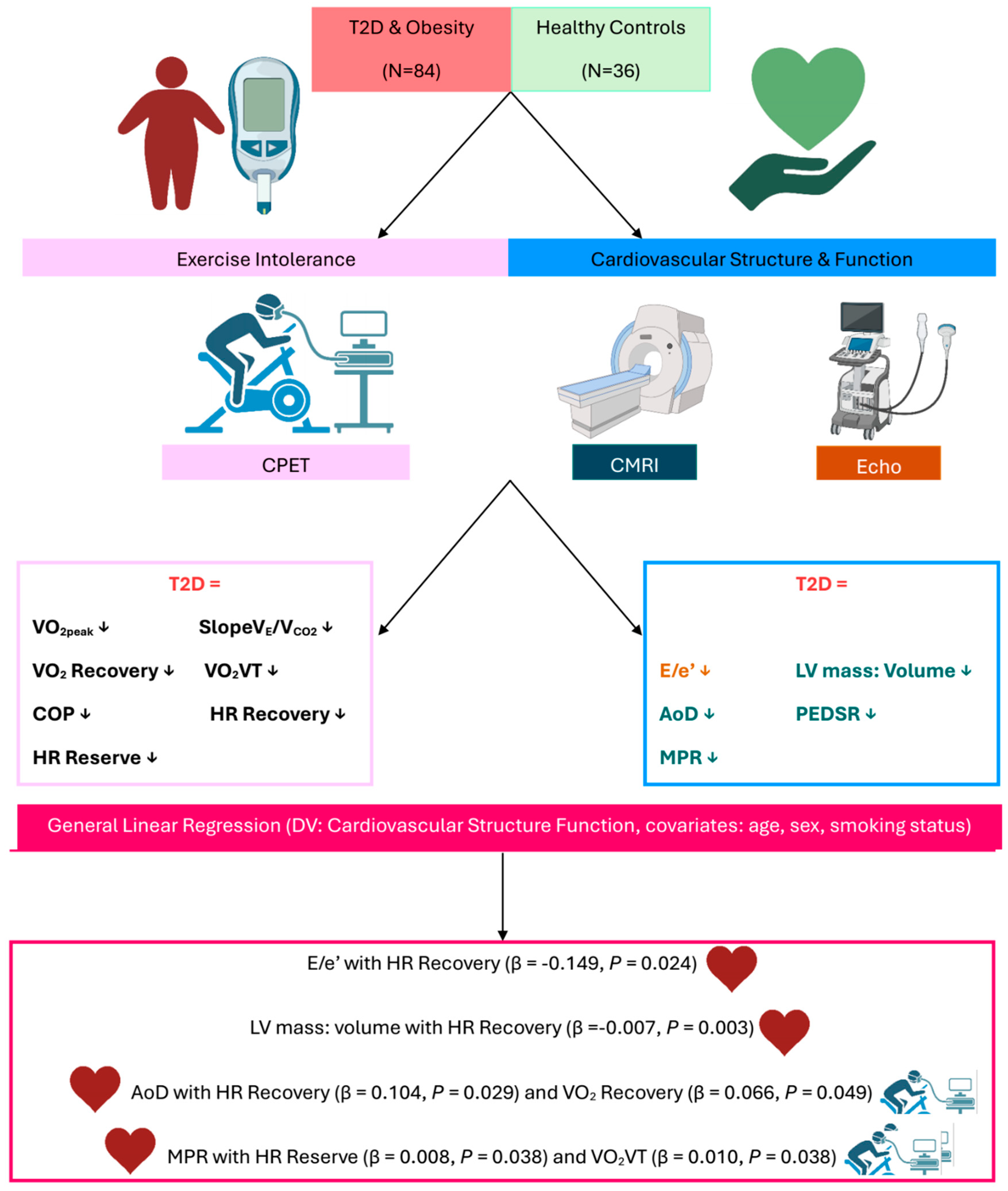
3. Results
3.1. Participant Characteristics
3.2. Cardiovascular Structure and Function
Cardiac Magnetic Resonance Imaging
3.3. Echocardiography
3.4. Indices of Exercise Tolerance
3.5. Associations with Markers of Cardiovascular Remodelling
4. Discussion
4.1. Key Findings
4.2. O2 at the Ventilatory Threshold
4.3. O2 Recovery
4.4. Heart Rate Recovery
4.5. Strengths and Limitations
5. Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nichols, G.A.; Hillier, T.A.; Erbey, J.R.; Brown, J.B. Congestive heart failure in type 2 diabetes: Prevalence, incidence, and risk factors. Diabetes Care 2001, 24, 1614–1619. [Google Scholar] [CrossRef]
- Dei Cas, A.; Khan, S.S.; Butler, J.; Mentz, R.J.; Bonow, R.O.; Avogaro, A.; Tschoepe, D.; Doehner, W.; Greene, S.J.; Senni, M.; et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015, 3, 136–145. [Google Scholar] [CrossRef]
- Faden, G.; Faganello, G.; De Feo, S.; Berlinghieri, N.; Tarantini, L.; Di Lenarda, A.; Faggiano, P.; Cioffi, G. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: Data from the SHORTWAVE study. Diabetes Res. Clin. Pract. 2013, 101, 309–316. [Google Scholar] [CrossRef]
- Lam, C.S. Diabetic cardiomyopathy: An expression of stage B heart failure with preserved ejection fraction. Diabetes Vasc. Dis. Res. 2015, 12, 234–238. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Classes of Heart Failure. 2022. Available online: https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure (accessed on 15 March 2023).
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Nesti, L.; Pugliese, N.R.; Sciuto, P.; Natali, A. Type 2 diabetes and reduced exercise tolerance: A review of the literature through an integrated physiology approach. Cardiovasc. Diabetol. 2020, 19, 134. [Google Scholar] [CrossRef]
- Reusch, J.E.; Bridenstine, M.; Regensteiner, J.G. Type 2 diabetes mellitus and exercise impairment. Rev. Endocr. Metab. Disord. 2013, 14, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.A.; Reusch, J.E.; Levi, M.; Regensteiner, J.G. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 2007, 30, 2880–2885. [Google Scholar] [CrossRef]
- Nojima, H.; Yoneda, M.; Watanabe, H.; Yamane, K.; Kitahara, Y.; Sekikawa, K.; Yamamoto, H.; Yokoyama, A.; Hattori, N.; Kohno, N.; et al. Eboshida Akira Hashimoto Yasuo Kawasaki Hiromi Kato Norihisa Kurihara Hidemi Ochi Mitsuo Ohtaki Megu Onari Kiyoshi Inamizu Tsutomu Asahara Toshimasa Harada Ryozo. Association between aerobic capacity and the improvement in glycemic control after the exercise training in type 2 diabetes. Diabetol. Metab. Syndr. 2017, 9, 63. [Google Scholar]
- Khan, H.; Kunutsor, S.; Rauramaa, R.; Savonen, K.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Butler, J.; Laukkanen, J.A. Cardiorespiratory fitness and risk of heart failure: A population-based follow-up study. Eur. J. Heart Fail. 2014, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Marx, N. Diabetes mellitus and heart failure. Am. J. Cardiol. 2017, 120, S37–S47. [Google Scholar] [CrossRef]
- Tran, D. Cardiopulmonary exercise testing. In Investigations of Early Nutrition Effects on Long-Term Health: Methods and Applications; Springer: New York, NY, USA, 2018; pp. 285–295. [Google Scholar]
- Herdy, A.H.; Uhlendorf, D. Reference values for cardiopulmonary exercise testing for sedentary and active men and women. Arq. Bras. Cardiol. 2011, 96, 54–59. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2012, 33, 2917–2927. [Google Scholar] [CrossRef]
- Corrà, U.; Piepoli, M.F. Summary statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction recommendations for performance and interpretation. Monaldi Arch. Chest Dis. 2007, 68, 6–12. [Google Scholar] [CrossRef]
- Church, T.S.; LaMonte, M.J.; Barlow, C.E.; Blair, S.N. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch. Intern. Med. 2005, 165, 2114–2120. [Google Scholar] [CrossRef]
- Guazzi, M.; Dickstein, K.; Vicenzi, M.; Arena, R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: A comparative analysis on clinical and prognostic insights. Circ. Heart Fail. 2009, 2, 549–555. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129 Pt 2, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Araújo, C.G.S. Cardiorespiratory optimal point during exercise testing as a predictor of all-cause mortality. Rev. Port. Cardiol. (Engl. Ed.) 2017, 36, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.A.; Filho, A.S.S.; Ramos, P.S.; Chiappa, A.M.G.; Aprigliano, V.; Oliveira-Silva, I.; Cunha, R.M.; Fajemiroye, J.O.; Vieira, R.P.; Ferrari, G.; et al. Exploring the Clinical Utility of Cardiorespiratory Optimal Point in Heart Failure Patients: Creating a New Research Gap. Appl. Sci. 2025, 15, 3495. [Google Scholar] [CrossRef]
- Santos, M.; West, E.; Skali, H.; Forman, D.E.; Junior, W.N.; Shah, A.M. Resting heart rate and chronotropic response to exercise: Prognostic implications in heart failure across the left ventricular ejection fraction spectrum. J. Card. Fail. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.S.; Wooster, L.T.; Buswell, M.; Patel, S.; Pappagianopoulos, P.P.; Bakken, K.; White, C.; Tanguay, M.; Blodgett, J.B.; Baggish, A.L.; et al. Post-exercise oxygen uptake recovery delay: A novel index of impaired cardiac reserve capacity in heart failure. JACC Heart Fail. 2018, 6, 329–339. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Forman, D.E.; Chase, P.; Guazzi, M.; Myers, J.; Bensimhon, D.; Peberdy, M.A.; Ashley, E.; West, E.; Arena, R. The prognostic significance of heart rate recovery is not dependent upon maximal effort in patients with heart failure. Int. J. Cardiol. 2013, 168, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Swarbrick, D.J.; Athithan, L.; Brady, E.M.; Henson, J.; Baldry, E.; Argyridou, S.; Jaicim, N.B.; Squire, G.; Walters, Y.; et al. Effects of low-energy diet or exercise on cardiovascular function in working-age adults with type 2 diabetes: A prospective, randomized, open-label, blinded end point trial. Diabetes Care 2020, 43, 1300–1310. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Brady, E.M.; Swarbrick, D.J.; Athithan, L.; Henson, J.; Baldry, E.; McAdam, J.; Marsh, A.-M.; Parke, K.S.; Wormleighton, J.V.; et al. Rationale, design and study protocol of the randomised controlled trial: Diabetes Interventional Assessment of Slimming or Training tO Lessen Inconspicuous Cardiovascular Dysfunction (the DIASTOLIC study). BMJ Open 2019, 9, e023207. [Google Scholar] [CrossRef]
- Wharton, G.; Steeds, R.; Allen, J.; Phillips, H.; Jones, R.; Kanagala, P.; Lloyd, G.; Masani, N.; Mathew, T.; Oxborough, D. A minimum dataset for a standard adult transthoracic echocardiogram: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2015, 2, G9–G24. [Google Scholar] [CrossRef]
- Nauta, J.F.; Hummel, Y.M.; van der Meer, P.; Lam, C.S.; Voors, A.A.; van Melle, J.P. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: A systematic review in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1303–1311. [Google Scholar]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J.; Froelicher, V.F. Principles of exercise testing and interpretation. J. Cardiopulm. Rehabil. Prev. 1987, 7, 189. [Google Scholar] [CrossRef]
- Look AHEADResearch Group Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef]
- Aneja, A.; Tang, W.W.; Bansilal, S.; Garcia, M.J.; Farkouh, M.E. Diabetic cardiomyopathy: Insights into pathogenesis, diagnostic challenges, and therapeutic options. Am. J. Med. 2008, 121, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Wasserman, K.; Kilkowski, C.; Kleemann, T.; Kilkowski, A.; Bangert, M.; Schneider, S.; Schwarz, A.; Senges, J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002, 106, 3079–3084. [Google Scholar] [CrossRef]
- Sari, I.; Soydinc, S.; Davutoglu, V.; Sezen, Y.; Aksoy, M. Uncomplicated diabetes mellitus is equivalent for coronary artery disease: New support from novel angiographic myocardial perfusion-myocardial blush. Int. J. Cardiol. 2008, 127, 262–265. [Google Scholar] [CrossRef]
- Larghat, A.M.; Swoboda, P.P.; Biglands, J.D.; Kearney, M.T.; Greenwood, J.P.; Plein, S. The microvascular effects of insulin resistance and diabetes on cardiac structure, function, and perfusion: A cardiovascular magnetic resonance study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1368–1376. [Google Scholar] [CrossRef]
- Sørensen, M.H.; Bojer, A.S.; Pontoppidan, J.R.; Broadbent, D.A.; Plein, S.; Madsen, P.L.; Gæde, P. Reduced myocardial perfusion reserve in type 2 diabetes is caused by increased perfusion at rest and decreased maximal perfusion during stress. Diabetes Care 2020, 43, 1285–1292. [Google Scholar] [CrossRef]
- Opherk, D.; Schwarz, F.; Mall, G.; Manthey, J.; Baller, D.; Kübler, W. Coronary dilatory capacity in idiopathic dilated cardiomyopathy: Analysis of 16 patients. Am. J. Cardiol. 1983, 51, 1657–1662. [Google Scholar] [CrossRef]
- Nitenberg, A.; Foult, J.M.; Blanchet, F.; Zouioueche, S. Multifactorial determinants of reduced coronary flow reserve after dipyridamole in dilated cardiomyopathy. Am. J. Cardiol. 1985, 55, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.O., III; Cunnion, R.E.; Parrillo, J.E.; Palmeri, S.T.; Tucker, E.E.; Schenke, W.H.; Epstein, S.E. Dynamic limitation of coronary vasodilator reserve in patients with dilated cardiomyopathy and chest pain. J. Am. Coll. Cardiol. 1987, 10, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Treasure, C.B.; AVita, J.; ACox, D.; Fish, R.D.; Gordon, J.B.; Mudge, G.H.; Colucci, W.S.; Sutton, M.G.; Selwyn, A.P.; Alexander, R.W. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation 1990, 81, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sakai, Y.; Morooka, S.; Hayashi, T.; Takayanagi, K.; Yamanaka, T.; Kakoi, H.; Takabatake, Y. Coronary flow reserve in patients with dilated cardiomyopathy. Am. Heart J. 1993, 125, 93–98. [Google Scholar] [CrossRef]
- Inoue, T.; Sakai, Y.; Morooka, S.; Hayashi, T.; Takayanagi, K.; Yamaguchi, H.; Kakoi, H.; Takabatake, Y. Vasodilatory capacity of coronary resistance vessels in dilated cardiomyopathy. Am. Heart J. 1994, 127, 376–381. [Google Scholar] [CrossRef]
- Arena, R.; Guazzi, M.; Myers, J.; Peberdy, M.A. Prognostic value of heart rate recovery in patients with heart failure. Am. Heart J. 2006, 151, 851-e7–851-e13. [Google Scholar] [CrossRef]
- Fortin, M.; Turgeon, P.-Y.; Nadreau, É.; Grégoire, P.; Maltais, L.-G.; Sénéchal, M.; Provencher, S.; Maltais, F. Prognostic value of oxygen kinetics during recovery from cardiopulmonary exercise testing in patients with chronic heart failure. Can. J. Cardiol. 2015, 31, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, A.; Laperche, T.; Morvan, D.; Geneves, M.; Caviezel, B.; Gourgon, R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure: Analysis with gas exchange measurements and NMR spectroscopy. Circulation 1995, 91, 2924–2932. [Google Scholar] [CrossRef]
- Nanas, S.; Nanas, J.; Kassiotis, C.; Nikolaou, C.; Tsagalou, E.; Sakellariou, D.; Terovitis, I.; Papazachou, O.; Drakos, S.; Papamichalopoulos, A.; et al. Early recovery of oxygen kinetics after submaximal exercise test predicts functional capacity in patients with chronic heart failure. Eur. J. Heart Fail. 2001, 3, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Karagodin, I.; Aba-Omer, O.; Sparapani, R.; Strande, J.L. Aortic stiffening precedes onset of heart failure with preserved ejection fraction in patients with asymptomatic diastolic dysfunction. BMC Cardiovasc. Disord. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.D.; Levy, D.; Dannenberg, A.L.; Garrison, R.J.; Castelli, W.P. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study). Am. J. Cardiol. 1990, 65, 371–376. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Swarbrick, D.J.; Hunt, W.H.; Levelt, E.; Graham-Brown, M.P.; Parke, K.S.; Wormleighton, J.V.; Lai, F.Y.; Yates, T.; Wilmot, E.G.; et al. Relation of aortic stiffness to left ventricular remodeling in younger adults with type 2 diabetes. Diabetes 2018, 67, 1395–1400. [Google Scholar] [CrossRef]
- Wasmund, S.L.; Owan, T.; Yanowitz, F.G.; Adams, T.D.; Hunt, S.C.; Hamdan, M.H.; Litwin, S.E. Improved heart rate recovery after marked weight loss induced by gastric bypass surgery: Two-year follow up in the Utah Obesity Study. Heart Rhythm 2011, 8, 84–90. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Nishime, E.O.; Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Lauer, M.S. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 2000, 284, 1392–1398. [Google Scholar] [CrossRef]
- Cole, C.R.; Foody, J.M.; Blackstone, E.H.; Lauer, M.S. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann. Intern. Med. 2000, 132, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Morshedi-Meibodi, A.; Larson, M.G.; Levy, D.; O’Donnell, C.J.; Vasan, R.S. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). Am. J. Cardiol. 2002, 90, 848–852. [Google Scholar] [CrossRef]
- Adabag, A.S.; Grandits, G.A.; Prineas, R.J.; Crow, R.S.; Bloomfield, H.E.; Neaton, J.D.; MRFIT Research Group. Relation of heart rate parameters during exercise test to sudden death and all-cause mortality in asymptomatic men. Am. J. Cardiol. 2008, 101, 1437–1443. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Vetrovec, G.W.; Gorelik, D.; Froelicher, V.F. The importance of heart rate recovery in patients with heart failure or left ventricular systolic dysfunction. J. Card. Fail. 2005, 11, 624–630. [Google Scholar] [CrossRef]
- Ben Driss, A.; Tabet, J.-Y.; Meurin, P.; Weber, H.; Dumaine, R.; Renaud, N.; Grosdemouge, A.; Beauvais, F.; Solal, A.C. Heart rate recovery identifies high risk heart failure patients with intermediate peak oxygen consumption values. Int. J. Cardiol. 2011, 149, 284–285. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. The prognostic value of the heart rate response during exercise and recovery in patients with heart failure: Influence of beta-blockade. Int. J. Cardiol. 2010, 138, 166–173. [Google Scholar] [CrossRef]
- Guazzi, M.; Myers, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Arena, R. Heart rate recovery predicts sudden cardiac death in heart failure. Int. J. Cardiol. 2010, 144, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.M.; Horton, E.S.; Bahnson, J.; Gregg, E.W.; Jakicic, J.M.; Regensteiner, J.G.; Ribisl, P.M.; Soberman, J.E.; Stewart, K.J.; Espeland, M.A.; et al. Prevalence and predictors of abnormal cardiovascular responses to exercise testing among individuals with type 2 diabetes: The Look AHEAD (Action for Health in Diabetes) study. Diabetes Care 2010, 33, 901–907. [Google Scholar] [CrossRef]
- Zafrir, B.; Azencot, M.; Dobrecky-Mery, I.; Lewis, B.S.; Flugelman, M.Y.; Halon, D.A. Resting heart rate and measures of effort-related cardiac autonomic dysfunction predict cardiovascular events in asymptomatic type 2 diabetes. Eur. J. Prev. Cardiol. 2016, 23, 1298–1306. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Lauer, M.S.; Earnest, C.P.; Church, T.S.; Kampert, J.B.; Gibbons, L.W.; Blair, S.N. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care 2003, 26, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M. Ventricular remodeling in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2013, 10, 341–349. [Google Scholar] [CrossRef] [PubMed]
| CPET Indices of Exercise Tolerance | Calculation |
|---|---|
| SlopeVE/VCO2 slope | The linear regression slope of VE and VCO2 from the start of the exercise to the VT. Presented as the gradient of the slope. |
| O2 Recovery | How much O2 falls from peak to 60 s post-exercise cessation. Presented as the percentage decrease from peak to 60 s post-exercise cessation. |
| Heart rate recovery | How much heart rate falls from peak to 60 s post-exercise cessation. Presented as the percentage decrease in heart rate from peak to 60 s post-exercise cessation. |
| Heart rate reserve | How much the heart rate increases from resting to peak exercise. Presented as the percentage increase from resting to peak exercise |
| Oxygen uptake at the ventilatory threshold | The O2 (mL/kg/min) value at which the ventilatory threshold (VT) occurs. The VT was calculated using the V-Slope method. |
| Cardiorespiratory optimal point | The lowest value of VE/VCO2 in a given minute during an incremental exercise test. VE/VCO2 was averaged over every minute during the CPET and the lowest is the COP. |
| T2D | Healthy Controls | p-Value | |
|---|---|---|---|
| N = 84 | N = 36 | ||
| Demographics and anthropometry | |||
| Age (years) | 50.5 ± 6.3 | 48.6 ± 6.2 | 0.124 |
| Sex (N [%] female) | 34 [40%] | 17 [47%] | 0.466 |
| Weight (kg) | 102.2 ± 14.9 | 70.4 ± 10.8 | <0.001 |
| BMI (kg/m2) | 36.5 ± 5.2 | 24.5 ± 2.4 | <0.001 |
| SBP (mmHg) | 140.0 ± 16.5 | 120.94 ± 13.24 | <0.001 |
| DBP (mmHg) | 87.7 ± 9.1 | 76.44 ± 7.15 | <0.001 |
| Resting HR (BPM) | 73.9 ± 9.4 | 61.7 ± 9.8 | <0.001 |
| Smoking Status (N [Never/Ex/Current]) | 44/22/14 | 27/08/2001 | 0.027 |
| Ethnicity (N [% BAME]) | 34 [40%] | 12 [33%] | 0.494 |
| T2D Duration (months) | 64.3 ± 38.7 | N/A | N/A |
| Cardiovascular Structure/Function | |||
| E/e′ | 8.3 ± 2.4 | 6.4 ± 1.5 | <0.001 |
| LV mass: volume (g/mL) | 0.8 ± 0.1 | 0.7 ± 0.1 | <0.001 |
| Aortic Distensibility (mmHg−1 × 103) | 4.1 ± 2.1 | 6.6 ± 2.0 | <0.001 |
| Peak Early Diastolic Strain Rate (S−1) | 1.0 ± 0.2 | 1.1 ± 0.2 | 0.008 |
| Myocardial Perfusion Reserve | 3.0 ± 0.9 | 3.9 ± 0.01 | <0.001 |
| T2D Control | |||
| HbA1c (mmol/mol) | 56 ± 11 | 35 ± 3 | <0.001 |
| HbA1c (%) | 7.3 ± 10 | 5.4 ± 0.2 | <0.001 |
| T2D | Healthy Controls | ||
|---|---|---|---|
| CPET Variable | N = 84 | N = 36 | p-value |
| O2peak (L/min) | 1.7 ± 0.5 | 1.9 ± 0.7 | 0.012 |
| O2peak (mL/kg/min) | 16.6 ± 4.0 | 27.5 ± 8.6 | <0.001 |
| SlopeVE/VCO2 | 28.8 ± 5.4 | 26.1 ± 4.3 | 0.008 |
| O2 Recovery (%) | 17.7 ± 6.7 | 20.4 ± 7.2 | <0.001 |
| O2VT (mL/kg/min) | 9.1 ± 2.6 | 13.3 ± 4.2 | <0.001 |
| Percentage of peak O2 at VT (%) | 54.9 ± 10.7 | 49.7 ± 9.5 | 0.012 |
| COP | 28.4 ± 3.1 | 26.7 ± 3.7 | 0.009 |
| HR Reserve (%) | 103.6 ± 32.6 | 154.9 ± 38.6 | <0.001 |
| HR Recovery (%) | 12.3 ± 4.4 | 16.3 ± 5.5 | <0.001 |
| O2 at VT < 11 (N [%]) | 62 [73] | 12 [33] | <0.001 |
| VE/VCO2 (<29.9) (N [%]) | 35 [41] | 7 [19] | 0.022 |
| SlopeVE/VCO2 | HR Recovery (%) | HR Reserve (%) | O2VT (mL/kg/min) | O2 Recovery (%) | COP | |
|---|---|---|---|---|---|---|
| N = 84 | E/e′ | |||||
| β | 0.023 | −0.149 | 0.004 | −0.027 | −0.033 | 0.016 |
| 95% CI | −0.087, 0.133 | −0.277, −0.02 | −0.014, 0.022 | −0.244, 0.189 | −0.117, 0.05 | −0.179, 0.211 |
| p-Value | 0.681 | 0.024 | 0.641 | 0.801 | 0.43 | 0.869 |
| Effect size | 0.003 | 0.072 | 0.003 | 0.001 | 0.009 | <0.001 |
| LV mass: volume (g/mL) | ||||||
| β | 0.001 | −0.007 | −0.06 | −0.031 | −0.001 | −0.002 |
| 95% CI | −0.005, 0.007 | −0.014, −0.001 | −0.001, 0.001 | −0.011, 0.011 | −0.006, 0.004 | −0.012, 0.008 |
| p-Value | 0.738 | 0.030 | 0.818 | 0.988 | 0.695 | 0.671 |
| Effect size | 0.001 | 0.058 | 0.001 | <0.001 | 0.002 | 0.002 |
| Mean Aortic Distensibility (mmHg−1 × 103) | ||||||
| β | 0.041 | 0.104 | 0.008 | −0.086 | 0.066 | 0.127 |
| 95% CI | −0.039, 0.121 | 0.011, 0.197 | −0.005, 0.021 | −0.244, 0.072 | 0.00, 0.132 | −0.011, 0.266 |
| p-Value | 0.315 | 0.029 | 0.24 | 0.281 | 0.049 | 0.071 |
| Effect size | 0.013 | 0.059 | 0.017 | 0.015 | 0.05 | 0.041 |
| Peak Early Diastolic Strain Rate (S−1) | ||||||
| β | 0.002 | 0.009 | −0.096 | −0.009 | −0.002 | 0.012 |
| 95% CI | −0.006, 0.009 | −0.009, 0.009 | −0.001, 0.002 | −0.024, 0.006 | −0.009, 0.004 | −0.001, 0.025 |
| p-Value | 0.667 | 0.933 | 0.378 | 0.236 | 0.481 | 0.065 |
| Effect size | 0.002 | <0.001 | 0.01 | 0.018 | 0.006 | 0.042 |
| Myocardial Perfusion Reserve | ||||||
| β | 0.001 | 0.036 | 0.008 | 0.1 | −0.016 | −0.035 |
| 95% CI | −0.043, 0.046 | −0.017, 0.089 | 0.00, 0.016 | 0.017, 0.183 | −0.054, 0.021 | −0.114, 0.045 |
| p-Value | 0.949 | 0.182 | 0.038 | 0.018 | 0.388 | 0.386 |
| Effect size | <0.001 | 0.026 | 0.063 | 0.08 | 0.011 | 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walters, G.W.M.; Gulsin, G.S.; Henson, J.; Argyridou, S.; Parke, K.S.; Yates, T.; Davies, M.J.; McCann, G.P.; Brady, E.M. Interrelationship Between Cardiopulmonary Exercise Testing Indices and Markers of Subclinical Cardiovascular Dysfunction in Those with Type 2 Diabetes—An Observational Cross-Sectional Analysis. J. Funct. Morphol. Kinesiol. 2025, 10, 371. https://doi.org/10.3390/jfmk10040371
Walters GWM, Gulsin GS, Henson J, Argyridou S, Parke KS, Yates T, Davies MJ, McCann GP, Brady EM. Interrelationship Between Cardiopulmonary Exercise Testing Indices and Markers of Subclinical Cardiovascular Dysfunction in Those with Type 2 Diabetes—An Observational Cross-Sectional Analysis. Journal of Functional Morphology and Kinesiology. 2025; 10(4):371. https://doi.org/10.3390/jfmk10040371
Chicago/Turabian StyleWalters, Grace W. M., Gaurav S. Gulsin, Joseph Henson, Stavroula Argyridou, Kelly S. Parke, Thomas Yates, Melanie J. Davies, Gerry P. McCann, and Emer M. Brady. 2025. "Interrelationship Between Cardiopulmonary Exercise Testing Indices and Markers of Subclinical Cardiovascular Dysfunction in Those with Type 2 Diabetes—An Observational Cross-Sectional Analysis" Journal of Functional Morphology and Kinesiology 10, no. 4: 371. https://doi.org/10.3390/jfmk10040371
APA StyleWalters, G. W. M., Gulsin, G. S., Henson, J., Argyridou, S., Parke, K. S., Yates, T., Davies, M. J., McCann, G. P., & Brady, E. M. (2025). Interrelationship Between Cardiopulmonary Exercise Testing Indices and Markers of Subclinical Cardiovascular Dysfunction in Those with Type 2 Diabetes—An Observational Cross-Sectional Analysis. Journal of Functional Morphology and Kinesiology, 10(4), 371. https://doi.org/10.3390/jfmk10040371






