Abstract
Purpose: While peak oxygen uptake (O2peak) is the gold standard method for assessing exercise tolerance, there is a tendency for underestimation. Several other cardiopulmonary exercise testing (CPET) variables may provide additive prognostic value beyond O2peak alone. The aim of this study was to examine if alternative CPET indices of exercise tolerance are (a) impaired in people with T2D and (b) independently associated with measures of cardiovascular structure and function measured via echocardiography and cardiac MRI. Methods: Participants with type 2 diabetes (T2D) and healthy controls underwent cardiac magnetic resonance imaging, transthoracic echocardiography, and a CPET. Multiple linear regression was used to determine the relationship between indices of exercise tolerance and markers of cardiovascular structure and function. Results: A total of 84 people with T2D and 36 healthy volunteers were included in the analysis. All CPET outcomes were worse in those with T2D vs. the controls. Three CPET outcomes were associated with markers of cardiovascular structure and function: O2 recovery with mean aortic distensibility (β = 0.218, p = 0.049); heart rate recovery with early filling velocity on transmitral Doppler/early relaxation velocity (β = −0.270, p = 0.024), left ventricular mass/volume ratio (β = −0.248, p = 0.030) and mean aortic distensibility (β = 0.222, p = 0.029); and O2 at the ventilatory threshold with myocardial perfusion reserve (β = 0.273, p = 0.018). Perspective: These lesser-used CPET indices could be used to identify which people with T2D are at elevated risk of progression to symptomatic heart failure. However, larger longitudinal studies are required to confirm these findings and their potential clinical application.
1. Introduction
People with type 2 diabetes (T2D) have more than a two-fold increased risk of developing heart failure (HF) compared to those without T2D [1,2]. Prior to the development of symptomatic HF, up to half of people with T2D have asymptomatic left ventricle (LV) dysfunction [3,4], meeting the classification for stage B HF [5].
A reduced exercise tolerance is a hallmark of HF [6]. Numerous studies have evaluated the cardiopulmonary performance in response to physical exercise in T2D subjects and consistently observed a reduced peak oxygen uptake capacity (O2peak) in comparison to non-T2D subjects [7]. A reduced exercise tolerance, expressed either by a reduced peak workload or O2peak, is a powerful marker of an impaired global health status as well as a hallmark of HF [6]. People with T2D who are asymptomatic of any cardiovascular disease have been reported to have a 20–30% reduction in O2peak [8,9,10,11] and an early diabetes-related cardiopulmonary impairment has been postulated [12].
Cardiopulmonary exercise testing (CPET) is considered the gold standard non-invasive measure for assessing cardiorespiratory fitness and exercise tolerance [13]. CPET provides a simultaneous assessment of the cardiovascular, respiratory, muscular, and metabolic systems [14,15,16]. While O2peak bears strong prognostic value in several patient populations, including people with T2D [17], there is a tendency for O2peak to be underestimated because of reduced patient motivation and premature termination of the exercise test by the examiner, or by the patient, due to feeling uncomfortable and unfamiliar with the level of physical exertion. More recently, other CPET parameters have emerged that offer insight into multiorgan physiologic reserve capacity and provide better or additive prognostic value than O2peak [18,19,20]. This includes the following: the cardiorespiratory optimal point (COP) [21,22], HR reserve [23], the ventilatory threshold 24, oxygen uptake recovery (O2 recovery) [24], and HR recovery [25].
Given the tendency for O2peak to be underestimated alongside the more recently examined CPET parameters that offer insight into multiorgan physiologic reserve capacity and provide better or additive prognostic value than O2peak, there is a need to explore whether these alternative CPET indices of exercise tolerance are worse among people with T2D and obesity, and whether any are associated with markers of cardiovascular remodelling and dysfunction. The research gap this study aims to fill is whether there is any evidence that alternative CPET indices of exercise tolerance could hold any prognostic value for cardiovascular remodelling and dysfunction. By understanding whether these alternative CPET indices of exercise tolerance are worse in people with T2D and obesity, and whether there are any associations that exist between cardiovascular remodelling and dysfunction, further research can begin to explore whether these alternative CPET indices of exercise tolerance hold any possible prognostic value for people with T2D and obesity.
Therefore, the aim of this analysis was to examine if alternative parameters of exercise tolerance measured via CPET are (a) impaired in people with T2D without cardiovascular disease compared to age-, sex-, and ethnicity-matched healthy controls and (b) if they are independently associated with measures of cardiovascular structure and function (dependant variable), specifically early filling velocity on transmitral Doppler/early relaxation velocity [E/e′], LV mass/volume ratio, aortic distensibility [AoD], peak early diastolic strain rate [PEDSR], or myocardial perfusion reserve [MPR]. These markers of cardiovascular structure and function were selected because they have been reported to be abnormal in people with obesity and T2D without a history of and asymptomatic of any cardiovascular disease or dysfunction [26]. The hypothesis was that all alternative CPET indices of exercise tolerance measured would be worse in people with T2D and obesity compared to healthy matched controls, and that at least one of these alternative CPET indices of exercise tolerance would be associated with at least one measure of cardiovascular structure and function.
2. Methods
This is a secondary analysis of the “Diabetes Interventional Assessment of Slimming or Training to Lessen Inconspicuous Cardiovascular Dysfunction” (the DIASTOLIC trial) [26]. The methods of the DIASTOLIC trial have been previously described in detail [26]. In summary, the DIASTOLIC trial was a single-centre study, which comprised a baseline cross-sectional case–control analysis in adults with T2D compared to age-, sex-, and ethnicity-matched non-T2D controls.
2.1. Participants
Participants with T2D were eligible if they were working-age adults (≥18 and ≤65 years) with established T2D and a body mass index (BMI) > 30 kg/m2 (or >27 kg/m2 if South Asian), but had no signs, symptoms, or history of any cardiovascular disease or dysfunction (asymptomatic of any cardiovascular disease or disorder). Ethical approval was granted by the National Research Ethics Service. The study’s sponsor was the University of Leicester (Research Ethics Committee West Midlands–Coventry, reference: 15/WM/0222, 7 July 2015). The study was conducted in accordance with the International Conference on Harmonisation-Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided written informed consent in advance of entering the study.
2.2. Assessments
All participants underwent the following assessments: demographics, medical history, anthropometric measures, fasting blood samples, comprehensive contrast-enhanced, stress and rest perfusion cardiac magnetic resonance imaging (CMR), transthoracic echocardiography, and a symptom-limited cardiopulmonary exercise test (CPET).
2.3. Comprehensive Contrast-Enhanced, Stress and Rest Perfusion Cardiac Magnetic Resonance Imaging
CMR scanning was performed using a standardised protocol on a Siemens scanner (Erlangen, Germany) at 1.5T (Siemens Aera) with an 18-channel cardiac coil and retrospective electrocardiographic (ECG) gating. This standardised scanning protocol has been described in detail previously [27]. Patients were advised to abstain from caffeine-containing products at least 12 h prior to CMR scanning. CMR images were analysed offline while blinded to all patient details and treatment groups. Cardiac chamber volume, function, and strain were assessed by a single experienced observer (G.S.G.) using cmr42 version 5 (Circle Cardiovascular Imaging, Calgary, Alberta, Canada). Aortic distensibility was analysed by two experienced operators using Java Image Manipulation version 6 (Xinapse Software, Essex, UK).
2.4. Transthoracic Echocardiography
Comprehensive transthoracic echocardiography was performed according to the British Society of Echocardiography guidelines [28] by one of three accredited operators. Each participant was assessed for early diastolic transmittal flow velocity (E) and early diastolic mitral annular velocity (e′) to estimate LV filling pressures via Doppler echocardiography as E/e′ is a non-invasive surrogate for LV filling pressure [29].
2.5. Symptom-Limited Cardiopulmonary Exercise Test
Participants underwent a symptom-limited maximum incremental exercise test on a stationary bicycle (electromagnetically-braked cycle ergometer) with expired gas analysis to determine O2peak. The exercise test began with a 3 min warm-up phase with 0 watts of resistance. Participants’ resistance then increased each minute by a pre-determined workload which had been calculated based on participants’ age, sex, height and weight using the following calculation [30]:
Before commencing the test, the air conditioning temperature was set to 19 degrees Celsius. Prior to test initiation, a brief description of the test was given to participants. It was emphasised to participants that they were in charge and could stop if they felt distressed, but that it was important for them to exercise to their maximum level. Participants were also taught to use a ‘thumb up’ signal to signify that everything was satisfactory and a ‘thumb down’ if they were experiencing any difficulty but did not wish to stop. Participants were also advised to point to the site of discomfort, such as the chest or legs.
Participants were then prepared for testing; the skin was prepped to attach the ECG electrodes, and a blood pressure cuff and oximeter were attached. It was explained to participants that they should aim for 60–80 revolutions per minute, and the process of the incremental increase in resistance was explained. It was re-emphasised to participants that they should exercise until they felt that they were unable to continue.
Once the participant was confident with the protocol, their position on the bike was checked to ensure comfort and correct technique, and the face mask was placed on the participant’s face. Once the participant was comfortable, the exercise test was initiated.
Once the participant indicated that they were at maximal exertion and that they were unable to continue, the test was terminated.
The raw data from each CPET test were exported to an excel sheet and were then later used for the manual calculation of each of the several CPET variables. A description of how each CPET variable was calculated is presented in Table 1.

Table 1.
Calculations for CPET indices of exercise tolerance.
2.6. Statistical Analysis
Statistical analyses were performed using a commercially available software package (IBM SPSS Statistics; version 26. Hampshire, UK). Normality of the outcome measures was assessed using histograms, the Shapiro–Wilk test, and Q-Q plots. To compare CPET indices of exercise tolerance between people with obesity and T2D and age-, sex-, and ethnicity-matched healthy controls, independent samples t-tests or Mann–Whitney tests were employed depending on data distribution. To examine the association between CPET indices of exercise tolerance and markers of cardiovascular structure and function, a multiple linear regression model was used to assess whether each CPET variable was associated with each marker of cardiovascular structure and function. This model was adjusted for age, sex, and smoking status. These parameters were adjusted for as they were reported to be major determinants of subclinical cardiovascular dysfunction in this cohort of working-age adults with T2D [26]. An exploratory analysis which included adjusted for BMI was also run to assess the impact of body mass (Supplementary Material Table S1). This exploratory analysis was performed on both people with T2D and healthy volunteers. Results for the associations between the CPET variables and cardiovascular structure/function were further adjusted for multiple testing (Bonferroni correction). A p-value of 0.05 was considered statistically significant.
3. Results
3.1. Participant Characteristics
A total of 84 people with T2D and obesity and 36 healthy volunteers had completed a CPET and were therefore included in the case vs. control baseline analysis of CPET indices of exercise tolerance. The mean T2D duration for those with T2D was 62.4 ± 38.2 months. The healthy volunteers (controls) and T2D with obesity group (cases) were similar for age, sex, and ethnicity distribution; however, as expected, the control group had lower overall body weight, BMI, blood pressure, HR, and HbA1c (Table 2).

Table 2.
Participant characteristics.
3.2. Cardiovascular Structure and Function
Cardiac Magnetic Resonance Imaging
The values for the baseline CMR and echocardiographic imaging in those with T2D vs. controls are shown in Table 1 and have been described in detail previously [26]. Briefly, the CMR revealed that compared to controls, those with T2D had a significantly lower LV peak early diastolic strain rate (1.10 ± 0.16 vs. 1.01 ± 0.19, respectively, p = 0.02), smaller indexed LV volumes, higher LV ejection fraction, higher LV mass, increased concentric LV remodelling (LV mass: volume, 0.71 ± 0.10 vs. 0.82 ± 0.12 g/mL, respectively, p < 0.001) and lower mean aortic distensibility (6.56 ± 2.02 vs. 4.16 ± 2.05 mmHg−1 × 10−3, respectively, p < 0.001). Those with T2D also had a lower myocardial perfusion reserve.
3.3. Echocardiography
Compared to healthy matched controls, those with T2D had a significantly lower mean E/A (1.21 ± 0.25 vs. 0.95 ± 0.21, respectively, p < 0.001) and a significantly higher E/e′ (6.2 [5.0–7.8] vs. 8.1 [6.2–9.6], respectively, p < 0.001) (Table 2).
3.4. Indices of Exercise Tolerance
All assessed CPET outcomes were significantly impaired in those with T2D compared to controls (Table 3). HR reserve was >50% higher in controls (p < 0.001), meaning that healthy volunteers had the ability to increase their HR by more than 50% compared to people with obesity and T2D. O2 at the ventilatory threshold (O2VT), a marker of aerobic capacity, was 38% lower in people with T2D (p < 0.001) demonstrating that the ventilatory threshold is achieved earlier in people with obesity and T2D compared to their healthy counterparts. SlopeVE/VCO2 was 10% (p = 0.008) and COP was 6% lower in controls (p = 0.009) compared to T2D. O2 recovery was 14% faster in healthy volunteers (p < 0.001), demonstrating a delay in O2 recovery in people with obesity and T2D. HR recovery was 4% faster in healthy volunteers (p < 0.001).

Table 3.
CPET variables in T2D vs. healthy volunteers.
3.5. Associations with Markers of Cardiovascular Remodelling
The relationship between the three alternative parameters of exercise tolerance and five cardiovascular structure/function on CMR in people with obesity and T2D, with adjustment for multiple testing, are provided in Table 4 and described here. O2 recovery was significantly positively associated with mean aortic distensibility (β = 0.218, p = 0.049), demonstrating that a faster O2 recovery was associated with less aortic stiffness. HR recovery had a significant inverse association with E/e′ (β = −0.270, p = 0.024), indicating that a slower HR recovery was associated with elevated E/e′ (higher LV filling pressures). HR recovery had a significant negative association with LV mass/volume ratio (β = −0.248, p = 0.030), suggesting a slower HR recovery was associated with elevated LV mass/volume ratio (more concentric remodelling). HR recovery had a significant positive association with mean aortic distensibility (β = 0.222, p = 0.029) indicating that a faster HR recovery was associated with increased aortic distensibility (less aortic stiffening). Finally, O2VT was significantly positively associated with MPR (β = 0.273, p = 0.018), thus suggesting that a higher O2VT (the ventilatory threshold occurs later in the exercise course) was associated with a higher MPR, representing less microvascular dysfunction.

Table 4.
Associations between novel CPET indices and cardiovascular structure/function.
Healthy volunteers showed no association between alternative parameters of exercise tolerance and the five cardiovascular structure/function on CMR (Supplementary Table S1).
4. Discussion
To our knowledge, this is the first study to compare alternative parameters of exercise tolerance markers derived from CPET in people with obesity and T2D with evidence of subclinical cardiovascular structural and functional abnormalities that align with stage B HF vs. healthy controls. In addition, this is also the first study to assess the association between indices of exercise tolerance, beyond O2peak and cardiovascular structure/function in this patient population.
4.1. Key Findings
This secondary analysis of the DIASTOLIC trial [26] revealed two key findings: Firstly, all the CPET indices of exercise tolerance assessed, which included ventilatory efficiency (SlopeVE/VCO2), O2 recovery, HR recovery, HR reserve, O2VT, and COP, are impaired in people with obesity and T2D compared to age-, sex-, and ethnicity-matched healthy controls. Secondly, O2 recovery, HR recovery, and O2VT all revealed significant associations with markers of cardiovascular structure/function independent of age, sex, and smoking status. A visual summary of the key findings is presented in Figure 1.
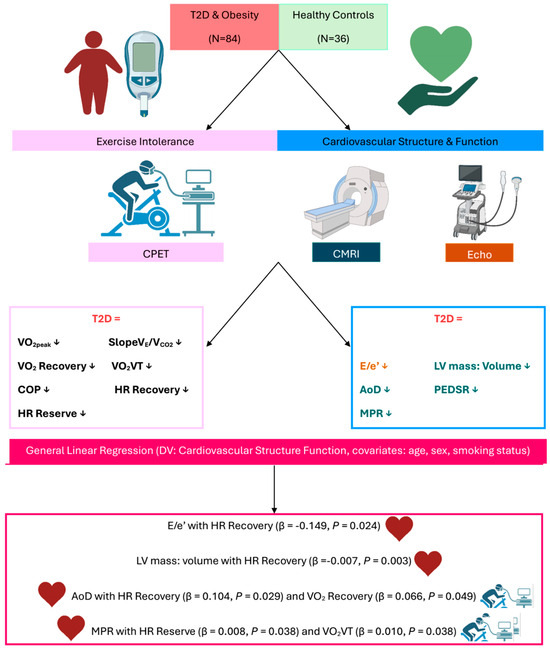
Figure 1.
Visual summary of key findings. A visual summary of the key findings. Abbreviations: T2D: Type-2 diabetes; CPET: Cardiopulmonary exercise test; CMRI: Cardiac magnetic resonance imaging; Echo: Echocardiography; DV: dependant variable; VE: minute ventilation; VCO2: Carbon Dioxide Output; VE/VCO2: ventilatory efficiency; O2: oxygen uptake; HR: heart rate; VT: ventilatory threshold; COP: cardiorespiratory optimal point; E/e′: Mitral inflow velocity/ mitral annular early diastolic velocity; LV: left ventricular.
While it is well recognised that people with T2D have a reduced exercise capacity [31], this has almost exclusively been demonstrated using O2peak as the marker of exercise tolerance, but there is an array of CPET outcomes which have shown prognostic power in HF, yet exploration into how these outcomes compare in people with T2D, and whether these indices are associated with abnormal cardiovascular structure and function, for the most part, has been neglected. These alternative indices offer an opportunity to potentially detect early determinants of progression to HF in people with T2D that could be modifiable [32].
4.2. O2 at the Ventilatory Threshold
It has been suggested that an early ventilatory threshold is associated with a higher risk of death in people with HF [33], with a ventilatory threshold of <11 O2 mL/kg/min suggested as the threshold for those with a high risk of HF-related mortality. O2VT has also demonstrated greater prognostic strength for predicting 6-month mortality than O2peak [33]. The mean O2VT in the present investigation in those with obesity and T2D was 9.1 mL/kg/min and, on an individual level, 62 (73%) of people with obesity and T2D fell below the threshold of a O2VT < 11, while only 7 (19%) of healthy volunteers met this criterion. Thus, this suggests that people with obesity and T2D, despite being asymptomatic of any cardiovascular dysfunction, may be at increased risk of HF-related mortality.
Linear regression modelling revealed an association between O2VT and MPR. O2VT showed a positive relationship with MPR (a measure of microvascular function) which is defined as the maximal increase in myocardial blood flow above rest expressed as a ratio to resting blood flow. A reduced MPR has previously been shown to associate with LV diastolic dysfunction [34] and has frequently been reported as being reduced in people with T2D [34,35,36]. Given that diminished MPR has also been frequently observed in patients with chronic HF [37,38,39,40,41,42], one may postulate that those who are at higher risk of developing symptomatic HF may be identified using O2VT from CPET, but longitudinal studies are required to confirm this hypothesis.
4.3. O2 Recovery
Oxygen uptake and HR recovery following exercise both predict adverse outcomes in HF [43,44]. O2 recovery in the 60 s following exercise in the present study was reduced in people with obesity and T2D compared to age-, sex-, and ethnicity-matched healthy controls, demonstrating a delayed O2 recovery in obese T2D participants. Abnormally prolonged O2 recovery following exercise has been observed in patients with HF [45,46] and was linked to higher mortality rates in 200 chronic HF patients [44]. The current study adds to the depth of evidence on reduced O2 kinetics in T2D, but more specifically in patients with stage A and B HF.
It was also observed that O2 recovery had a positive association with mean AoD. Associations between proximal aortic stiffening, as indicated by decreased aortic distensibility, and HF development have been observed in patients with asymptomatic diastolic dysfunction [47]. LV mass and aortic stiffening are well established predictors of adverse cardiovascular outcomes [48]. We have previously demonstrated that AoD is independently associated with concentric LV remodelling in younger asymptomatic adults with T2D [49]. This supports the postulation that one mechanism through which aortic stiffening drives adverse cardiovascular outcomes in the context of T2D is via adverse LV remodelling, which increases the risk of subsequent HF [49]. Notably, our T2D participants had a 20% increase in concentric LV remodelling compared to matched healthy controls [49]. Targeting aortic stiffness (and by proxy LV hypertrophy) could potentially prevent progression to overt, symptomatic HF, which is of particular importance in younger T2D subjects, who have the highest lifetime risk of adverse cardiovascular outcomes, and in whom aortic stiffening and cardiac remodelling is likely to be reversible. However, in this present analysis, O2 recovery did not associate with LV mass/volume ratio (a marker of concentric remodelling).
4.4. Heart Rate Recovery
In the present study, HR recovery was also reduced in people with obesity and T2D. HR recovery was also associated with a reduced AoD, along with a higher LV mass/volume ratio and elevated E/e′. Reduced HR recovery is a very powerful predictor of increased mortality in obesity [50] in apparently healthy people [51,52,53,54,55] and in people with HF [25,43,56,57,58,59]. Impaired HR recovery is highly prevalent in asymptomatic T2D subjects [60] and has been linked to adverse cardiovascular outcomes and increased all-cause mortality [61,62]. Reduced HR recovery after exercise may be a manifestation of cardiac autonomic neuropathy, which is a common complication of T2D. HR recovery was associated with an increased LV mass: volume (a marker of concentric remodelling) ratio and elevated E/e′ (an index to evaluate LV filling pressure/assess LV diastolic function) [63,64]. Concentric remodelling has been associated with impaired diastolic function [65]. HR recovery, therefore, appears to be an important CPET outcome in people with T2D that could be used to indicate which T2D patients are likely to develop significant cardiovascular structural/functional abnormalities.
4.5. Strengths and Limitations
This is the first study to investigate the associations between alternative parameters of exercise tolerance derived from CPET in people with T2D and obesity, and cardiovascular abnormalities aligned with stage B HF. This, however, is a very early suggestion and requires a significantly larger evidence base before we can make more substantial claims about the use of CPET for risk stratification within T2D patient cohorts. This investigation was in a relatively small cohort and had more than twice as many people with T2D compared to healthy volunteers. This investigation also looked at CPET indices during exercise and explored how they associate with cardiovascular structure and function at rest (except myocardial blood flow). Furthermore, the results of this study are purely observational, and therefore, no causality can be inferred or assumed from the results.
5. Perspective
Overall, it is also evident from the present investigation that there is an array of indices of exercise tolerance that can be obtained from CPET which demonstrate impaired exercise tolerance in people with obesity and T2D when compared to age-, sex-, and ethnicity-matched healthy controls, which offer additive or improved prognostic power compared to O2peak. These findings may inform risk stratification approaches to the identification of those at elevated risk of progressing to stage C or D HF. However, larger longitudinal studies are required to confirm these findings and their potential clinical application. Future research on case–control comparisons should include larger sample sizes with even more matching (e.g., for weight and blood pressure).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jfmk10040371/s1. Table S1. Exploratory analysis with BMI as a covariate and the analysis also run on healthy matched controls; Table S2. Medication usage of those with type-2 diabetes and obesity.
Author Contributions
Conceptualisation: G.W.M.W., G.P.M., E.M.B.; methodology: G.W.M.W., G.S.G., J.H., T.Y., M.J.D., G.P.M., E.M.B.; formal analysis: G.W.M.W., G.P.M., E.M.B.; investigation: G.S.G., J.H., S.A., K.S.P.; data curation: G.W.M.W.; writing—original draft preparation: G.W.M.W.; writing—review and editing: G.S.G., J.H., S.A., K.S.P., T.Y., M.J.D., G.P.M., E.M.B.; visualisation: G.W.M.W., G.S.G., J.H., T.Y., M.J.D., G.P.M., E.M.B.; supervision: G.P.M., E.M.B.; project administration: G.W.M.W.; funding acquisition: G.S.G., G.P.M. All authors have read and agreed to the published version of the manuscript.
Funding
The DIASTOLIC study was funded by the National Institute for Health Research, Research Trainees and Associates Coordinating Centre through a career development fellowship to G.P.M. (CDF 2014-07-045). G.S.G was funded by the British Heart Foundation through a Clinical Research Training Fellowship (FS/16/47/32190). This report is independent research supported by the National Institute for Health Research (NIHR Research Professorship, G.M.P (RP-2017-08-ST2-007) which also funds E.M.B). Further support was provided by the NIHR Leicester Biomedical Research Centre, and the study utilised the NIHR Leicester Clinical Research Facility. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
Institutional Review Board Statement
The study was conducted in accordance with the International Conference on Harmonisation-Good Clinical Practice guidelines and the Declaration of Helsinki. All participants provided written informed consent in advance of entering the study. Ethical approval was granted by the National Research Ethics Service. The study sponsor was the University of Leicester (Research Ethics Committee West Midlands–Coventry, reference: 15/WM/0222).
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Acknowledgments
The authors declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation, and declare that the results of the present study do not constitute endorsement by the American College of Sports Medicine.
Conflicts of Interest
The authors have no conflicts of interest to disclose. The DIASTOLIC study was funded by the National Institute for Health Research, Research Trainees and Associates Coordinating Centre through a career development fellowship to G.P.M. (CDF 2014-07-045). G.S.G was funded by the British Heart Foundation through a Clinical Research Training Fellowship (FS/16/47/32190). This report is independent research supported by the National Institute for Health Research (NIHR Research Professorship, G.M.P (RP-2017-08-ST2-007) which also funds E.M.B). Further support was provided by the NIHR Leicester Biomedical Research Centre, and the study utilised the NIHR Leicester Clinical Research Facility. The views expressed in this publication are those of the author(s) and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
References
- Nichols, G.A.; Hillier, T.A.; Erbey, J.R.; Brown, J.B. Congestive heart failure in type 2 diabetes: Prevalence, incidence, and risk factors. Diabetes Care 2001, 24, 1614–1619. [Google Scholar] [CrossRef]
- Dei Cas, A.; Khan, S.S.; Butler, J.; Mentz, R.J.; Bonow, R.O.; Avogaro, A.; Tschoepe, D.; Doehner, W.; Greene, S.J.; Senni, M.; et al. Impact of diabetes on epidemiology, treatment, and outcomes of patients with heart failure. JACC Heart Fail. 2015, 3, 136–145. [Google Scholar] [CrossRef]
- Faden, G.; Faganello, G.; De Feo, S.; Berlinghieri, N.; Tarantini, L.; Di Lenarda, A.; Faggiano, P.; Cioffi, G. The increasing detection of asymptomatic left ventricular dysfunction in patients with type 2 diabetes mellitus without overt cardiac disease: Data from the SHORTWAVE study. Diabetes Res. Clin. Pract. 2013, 101, 309–316. [Google Scholar] [CrossRef]
- Lam, C.S. Diabetic cardiomyopathy: An expression of stage B heart failure with preserved ejection fraction. Diabetes Vasc. Dis. Res. 2015, 12, 234–238. [Google Scholar] [CrossRef] [PubMed]
- American Heart Association. Classes of Heart Failure. 2022. Available online: https://www.heart.org/en/health-topics/heart-failure/what-is-heart-failure/classes-of-heart-failure (accessed on 15 March 2023).
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmunds, L.H., Jr.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Nesti, L.; Pugliese, N.R.; Sciuto, P.; Natali, A. Type 2 diabetes and reduced exercise tolerance: A review of the literature through an integrated physiology approach. Cardiovasc. Diabetol. 2020, 19, 134. [Google Scholar] [CrossRef]
- Reusch, J.E.; Bridenstine, M.; Regensteiner, J.G. Type 2 diabetes mellitus and exercise impairment. Rev. Endocr. Metab. Disord. 2013, 14, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.A.; Reusch, J.E.; Levi, M.; Regensteiner, J.G. Skeletal muscle deoxygenation after the onset of moderate exercise suggests slowed microvascular blood flow kinetics in type 2 diabetes. Diabetes Care 2007, 30, 2880–2885. [Google Scholar] [CrossRef]
- Nojima, H.; Yoneda, M.; Watanabe, H.; Yamane, K.; Kitahara, Y.; Sekikawa, K.; Yamamoto, H.; Yokoyama, A.; Hattori, N.; Kohno, N.; et al. Eboshida Akira Hashimoto Yasuo Kawasaki Hiromi Kato Norihisa Kurihara Hidemi Ochi Mitsuo Ohtaki Megu Onari Kiyoshi Inamizu Tsutomu Asahara Toshimasa Harada Ryozo. Association between aerobic capacity and the improvement in glycemic control after the exercise training in type 2 diabetes. Diabetol. Metab. Syndr. 2017, 9, 63. [Google Scholar]
- Khan, H.; Kunutsor, S.; Rauramaa, R.; Savonen, K.; Kalogeropoulos, A.P.; Georgiopoulou, V.V.; Butler, J.; Laukkanen, J.A. Cardiorespiratory fitness and risk of heart failure: A population-based follow-up study. Eur. J. Heart Fail. 2014, 16, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Marx, N. Diabetes mellitus and heart failure. Am. J. Cardiol. 2017, 120, S37–S47. [Google Scholar] [CrossRef]
- Tran, D. Cardiopulmonary exercise testing. In Investigations of Early Nutrition Effects on Long-Term Health: Methods and Applications; Springer: New York, NY, USA, 2018; pp. 285–295. [Google Scholar]
- Herdy, A.H.; Uhlendorf, D. Reference values for cardiopulmonary exercise testing for sedentary and active men and women. Arq. Bras. Cardiol. 2011, 96, 54–59. [Google Scholar] [CrossRef]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Eur. Heart J. 2012, 33, 2917–2927. [Google Scholar] [CrossRef]
- Corrà, U.; Piepoli, M.F. Summary statement on cardiopulmonary exercise testing in chronic heart failure due to left ventricular dysfunction recommendations for performance and interpretation. Monaldi Arch. Chest Dis. 2007, 68, 6–12. [Google Scholar] [CrossRef]
- Church, T.S.; LaMonte, M.J.; Barlow, C.E.; Blair, S.N. Cardiorespiratory fitness and body mass index as predictors of cardiovascular disease mortality among men with diabetes. Arch. Intern. Med. 2005, 165, 2114–2120. [Google Scholar] [CrossRef]
- Guazzi, M.; Dickstein, K.; Vicenzi, M.; Arena, R. Six-minute walk test and cardiopulmonary exercise testing in patients with chronic heart failure: A comparative analysis on clinical and prognostic insights. Circ. Heart Fail. 2009, 2, 549–555. [Google Scholar] [CrossRef]
- Malhotra, R.; Bakken, K.; D’Elia, E.; Lewis, G.D. Cardiopulmonary exercise testing in heart failure. JACC Heart Fail. 2016, 4, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129 Pt 2, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Araújo, C.G.S. Cardiorespiratory optimal point during exercise testing as a predictor of all-cause mortality. Rev. Port. Cardiol. (Engl. Ed.) 2017, 36, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Silva, W.A.; Filho, A.S.S.; Ramos, P.S.; Chiappa, A.M.G.; Aprigliano, V.; Oliveira-Silva, I.; Cunha, R.M.; Fajemiroye, J.O.; Vieira, R.P.; Ferrari, G.; et al. Exploring the Clinical Utility of Cardiorespiratory Optimal Point in Heart Failure Patients: Creating a New Research Gap. Appl. Sci. 2025, 15, 3495. [Google Scholar] [CrossRef]
- Santos, M.; West, E.; Skali, H.; Forman, D.E.; Junior, W.N.; Shah, A.M. Resting heart rate and chronotropic response to exercise: Prognostic implications in heart failure across the left ventricular ejection fraction spectrum. J. Card. Fail. 2018, 24, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Bailey, C.S.; Wooster, L.T.; Buswell, M.; Patel, S.; Pappagianopoulos, P.P.; Bakken, K.; White, C.; Tanguay, M.; Blodgett, J.B.; Baggish, A.L.; et al. Post-exercise oxygen uptake recovery delay: A novel index of impaired cardiac reserve capacity in heart failure. JACC Heart Fail. 2018, 6, 329–339. [Google Scholar] [CrossRef]
- Cahalin, L.P.; Forman, D.E.; Chase, P.; Guazzi, M.; Myers, J.; Bensimhon, D.; Peberdy, M.A.; Ashley, E.; West, E.; Arena, R. The prognostic significance of heart rate recovery is not dependent upon maximal effort in patients with heart failure. Int. J. Cardiol. 2013, 168, 1496–1501. [Google Scholar] [CrossRef] [PubMed]
- Gulsin, G.S.; Swarbrick, D.J.; Athithan, L.; Brady, E.M.; Henson, J.; Baldry, E.; Argyridou, S.; Jaicim, N.B.; Squire, G.; Walters, Y.; et al. Effects of low-energy diet or exercise on cardiovascular function in working-age adults with type 2 diabetes: A prospective, randomized, open-label, blinded end point trial. Diabetes Care 2020, 43, 1300–1310. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Brady, E.M.; Swarbrick, D.J.; Athithan, L.; Henson, J.; Baldry, E.; McAdam, J.; Marsh, A.-M.; Parke, K.S.; Wormleighton, J.V.; et al. Rationale, design and study protocol of the randomised controlled trial: Diabetes Interventional Assessment of Slimming or Training tO Lessen Inconspicuous Cardiovascular Dysfunction (the DIASTOLIC study). BMJ Open 2019, 9, e023207. [Google Scholar] [CrossRef]
- Wharton, G.; Steeds, R.; Allen, J.; Phillips, H.; Jones, R.; Kanagala, P.; Lloyd, G.; Masani, N.; Mathew, T.; Oxborough, D. A minimum dataset for a standard adult transthoracic echocardiogram: A guideline protocol from the British Society of Echocardiography. Echo Res. Pract. 2015, 2, G9–G24. [Google Scholar] [CrossRef]
- Nauta, J.F.; Hummel, Y.M.; van der Meer, P.; Lam, C.S.; Voors, A.A.; van Melle, J.P. Correlation with invasive left ventricular filling pressures and prognostic relevance of the echocardiographic diastolic parameters used in the 2016 ESC heart failure guidelines and in the 2016 ASE/EACVI recommendations: A systematic review in patients with heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2018, 20, 1303–1311. [Google Scholar]
- Wasserman, K.; Hansen, J.E.; Sue, D.Y.; Whipp, B.J.; Froelicher, V.F. Principles of exercise testing and interpretation. J. Cardiopulm. Rehabil. Prev. 1987, 7, 189. [Google Scholar] [CrossRef]
- Look AHEADResearch Group Wing, R.R.; Bolin, P.; Brancati, F.L.; Bray, G.A.; Clark, J.M.; Coday, M.; Crow, R.S.; Curtis, J.M.; Egan, C.M.; Espeland, M.A.; et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N. Engl. J. Med. 2013, 369, 145–154. [Google Scholar] [CrossRef]
- Aneja, A.; Tang, W.W.; Bansilal, S.; Garcia, M.J.; Farkouh, M.E. Diabetic cardiomyopathy: Insights into pathogenesis, diagnostic challenges, and therapeutic options. Am. J. Med. 2008, 121, 748–757. [Google Scholar] [CrossRef] [PubMed]
- Gitt, A.K.; Wasserman, K.; Kilkowski, C.; Kleemann, T.; Kilkowski, A.; Bangert, M.; Schneider, S.; Schwarz, A.; Senges, J. Exercise anaerobic threshold and ventilatory efficiency identify heart failure patients for high risk of early death. Circulation 2002, 106, 3079–3084. [Google Scholar] [CrossRef]
- Sari, I.; Soydinc, S.; Davutoglu, V.; Sezen, Y.; Aksoy, M. Uncomplicated diabetes mellitus is equivalent for coronary artery disease: New support from novel angiographic myocardial perfusion-myocardial blush. Int. J. Cardiol. 2008, 127, 262–265. [Google Scholar] [CrossRef]
- Larghat, A.M.; Swoboda, P.P.; Biglands, J.D.; Kearney, M.T.; Greenwood, J.P.; Plein, S. The microvascular effects of insulin resistance and diabetes on cardiac structure, function, and perfusion: A cardiovascular magnetic resonance study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 1368–1376. [Google Scholar] [CrossRef]
- Sørensen, M.H.; Bojer, A.S.; Pontoppidan, J.R.; Broadbent, D.A.; Plein, S.; Madsen, P.L.; Gæde, P. Reduced myocardial perfusion reserve in type 2 diabetes is caused by increased perfusion at rest and decreased maximal perfusion during stress. Diabetes Care 2020, 43, 1285–1292. [Google Scholar] [CrossRef]
- Opherk, D.; Schwarz, F.; Mall, G.; Manthey, J.; Baller, D.; Kübler, W. Coronary dilatory capacity in idiopathic dilated cardiomyopathy: Analysis of 16 patients. Am. J. Cardiol. 1983, 51, 1657–1662. [Google Scholar] [CrossRef]
- Nitenberg, A.; Foult, J.M.; Blanchet, F.; Zouioueche, S. Multifactorial determinants of reduced coronary flow reserve after dipyridamole in dilated cardiomyopathy. Am. J. Cardiol. 1985, 55, 748–754. [Google Scholar] [CrossRef] [PubMed]
- Cannon, R.O., III; Cunnion, R.E.; Parrillo, J.E.; Palmeri, S.T.; Tucker, E.E.; Schenke, W.H.; Epstein, S.E. Dynamic limitation of coronary vasodilator reserve in patients with dilated cardiomyopathy and chest pain. J. Am. Coll. Cardiol. 1987, 10, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Treasure, C.B.; AVita, J.; ACox, D.; Fish, R.D.; Gordon, J.B.; Mudge, G.H.; Colucci, W.S.; Sutton, M.G.; Selwyn, A.P.; Alexander, R.W. Endothelium-dependent dilation of the coronary microvasculature is impaired in dilated cardiomyopathy. Circulation 1990, 81, 772–779. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Sakai, Y.; Morooka, S.; Hayashi, T.; Takayanagi, K.; Yamanaka, T.; Kakoi, H.; Takabatake, Y. Coronary flow reserve in patients with dilated cardiomyopathy. Am. Heart J. 1993, 125, 93–98. [Google Scholar] [CrossRef]
- Inoue, T.; Sakai, Y.; Morooka, S.; Hayashi, T.; Takayanagi, K.; Yamaguchi, H.; Kakoi, H.; Takabatake, Y. Vasodilatory capacity of coronary resistance vessels in dilated cardiomyopathy. Am. Heart J. 1994, 127, 376–381. [Google Scholar] [CrossRef]
- Arena, R.; Guazzi, M.; Myers, J.; Peberdy, M.A. Prognostic value of heart rate recovery in patients with heart failure. Am. Heart J. 2006, 151, 851-e7–851-e13. [Google Scholar] [CrossRef]
- Fortin, M.; Turgeon, P.-Y.; Nadreau, É.; Grégoire, P.; Maltais, L.-G.; Sénéchal, M.; Provencher, S.; Maltais, F. Prognostic value of oxygen kinetics during recovery from cardiopulmonary exercise testing in patients with chronic heart failure. Can. J. Cardiol. 2015, 31, 1259–1265. [Google Scholar] [CrossRef] [PubMed]
- Cohen-Solal, A.; Laperche, T.; Morvan, D.; Geneves, M.; Caviezel, B.; Gourgon, R. Prolonged kinetics of recovery of oxygen consumption after maximal graded exercise in patients with chronic heart failure: Analysis with gas exchange measurements and NMR spectroscopy. Circulation 1995, 91, 2924–2932. [Google Scholar] [CrossRef]
- Nanas, S.; Nanas, J.; Kassiotis, C.; Nikolaou, C.; Tsagalou, E.; Sakellariou, D.; Terovitis, I.; Papazachou, O.; Drakos, S.; Papamichalopoulos, A.; et al. Early recovery of oxygen kinetics after submaximal exercise test predicts functional capacity in patients with chronic heart failure. Eur. J. Heart Fail. 2001, 3, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Karagodin, I.; Aba-Omer, O.; Sparapani, R.; Strande, J.L. Aortic stiffening precedes onset of heart failure with preserved ejection fraction in patients with asymptomatic diastolic dysfunction. BMC Cardiovasc. Disord. 2017, 17, 62. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.D.; Levy, D.; Dannenberg, A.L.; Garrison, R.J.; Castelli, W.P. Association of echocardiographic left ventricular mass with body size, blood pressure and physical activity (the Framingham Study). Am. J. Cardiol. 1990, 65, 371–376. [Google Scholar] [CrossRef]
- Gulsin, G.S.; Swarbrick, D.J.; Hunt, W.H.; Levelt, E.; Graham-Brown, M.P.; Parke, K.S.; Wormleighton, J.V.; Lai, F.Y.; Yates, T.; Wilmot, E.G.; et al. Relation of aortic stiffness to left ventricular remodeling in younger adults with type 2 diabetes. Diabetes 2018, 67, 1395–1400. [Google Scholar] [CrossRef]
- Wasmund, S.L.; Owan, T.; Yanowitz, F.G.; Adams, T.D.; Hunt, S.C.; Hamdan, M.H.; Litwin, S.E. Improved heart rate recovery after marked weight loss induced by gastric bypass surgery: Two-year follow up in the Utah Obesity Study. Heart Rhythm 2011, 8, 84–90. [Google Scholar] [CrossRef]
- Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Snader, C.E.; Lauer, M.S. Heart-rate recovery immediately after exercise as a predictor of mortality. N. Engl. J. Med. 1999, 341, 1351–1357. [Google Scholar] [CrossRef]
- Nishime, E.O.; Cole, C.R.; Blackstone, E.H.; Pashkow, F.J.; Lauer, M.S. Heart rate recovery and treadmill exercise score as predictors of mortality in patients referred for exercise ECG. JAMA 2000, 284, 1392–1398. [Google Scholar] [CrossRef]
- Cole, C.R.; Foody, J.M.; Blackstone, E.H.; Lauer, M.S. Heart rate recovery after submaximal exercise testing as a predictor of mortality in a cardiovascularly healthy cohort. Ann. Intern. Med. 2000, 132, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Morshedi-Meibodi, A.; Larson, M.G.; Levy, D.; O’Donnell, C.J.; Vasan, R.S. Heart rate recovery after treadmill exercise testing and risk of cardiovascular disease events (The Framingham Heart Study). Am. J. Cardiol. 2002, 90, 848–852. [Google Scholar] [CrossRef]
- Adabag, A.S.; Grandits, G.A.; Prineas, R.J.; Crow, R.S.; Bloomfield, H.E.; Neaton, J.D.; MRFIT Research Group. Relation of heart rate parameters during exercise test to sudden death and all-cause mortality in asymptomatic men. Am. J. Cardiol. 2008, 101, 1437–1443. [Google Scholar] [CrossRef]
- Lipinski, M.J.; Vetrovec, G.W.; Gorelik, D.; Froelicher, V.F. The importance of heart rate recovery in patients with heart failure or left ventricular systolic dysfunction. J. Card. Fail. 2005, 11, 624–630. [Google Scholar] [CrossRef]
- Ben Driss, A.; Tabet, J.-Y.; Meurin, P.; Weber, H.; Dumaine, R.; Renaud, N.; Grosdemouge, A.; Beauvais, F.; Solal, A.C. Heart rate recovery identifies high risk heart failure patients with intermediate peak oxygen consumption values. Int. J. Cardiol. 2011, 149, 284–285. [Google Scholar] [CrossRef]
- Arena, R.; Myers, J.; Abella, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Guazzi, M. The prognostic value of the heart rate response during exercise and recovery in patients with heart failure: Influence of beta-blockade. Int. J. Cardiol. 2010, 138, 166–173. [Google Scholar] [CrossRef]
- Guazzi, M.; Myers, J.; Peberdy, M.A.; Bensimhon, D.; Chase, P.; Arena, R. Heart rate recovery predicts sudden cardiac death in heart failure. Int. J. Cardiol. 2010, 144, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Curtis, J.M.; Horton, E.S.; Bahnson, J.; Gregg, E.W.; Jakicic, J.M.; Regensteiner, J.G.; Ribisl, P.M.; Soberman, J.E.; Stewart, K.J.; Espeland, M.A.; et al. Prevalence and predictors of abnormal cardiovascular responses to exercise testing among individuals with type 2 diabetes: The Look AHEAD (Action for Health in Diabetes) study. Diabetes Care 2010, 33, 901–907. [Google Scholar] [CrossRef]
- Zafrir, B.; Azencot, M.; Dobrecky-Mery, I.; Lewis, B.S.; Flugelman, M.Y.; Halon, D.A. Resting heart rate and measures of effort-related cardiac autonomic dysfunction predict cardiovascular events in asymptomatic type 2 diabetes. Eur. J. Prev. Cardiol. 2016, 23, 1298–1306. [Google Scholar] [CrossRef]
- Cheng, Y.J.; Lauer, M.S.; Earnest, C.P.; Church, T.S.; Kampert, J.B.; Gibbons, L.W.; Blair, S.N. Heart rate recovery following maximal exercise testing as a predictor of cardiovascular disease and all-cause mortality in men with diabetes. Diabetes Care 2003, 26, 2052–2057. [Google Scholar] [CrossRef] [PubMed]
- Nagueh, S.F.; Appleton, C.P.; Gillebert, T.C.; Marino, P.N.; Oh, J.K.; Smiseth, O.A.; Waggoner, A.D.; Flachskampf, F.A.; Pellikka, P.A.; Evangelisa, A. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur. J. Echocardiogr. 2009, 10, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Mitter, S.S.; Shah, S.J.; Thomas, J.D. A test in context: E/A and E/e′ to assess diastolic dysfunction and LV filling pressure. J. Am. Coll. Cardiol. 2017, 69, 1451–1464. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.M. Ventricular remodeling in heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2013, 10, 341–349. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).