Influence of Anthropometric Height on Oculo-Manual Coordinative Reaction Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants—First Phase
2.2. Experimental Protocol—First Phase
2.3. Participants—Second Phase
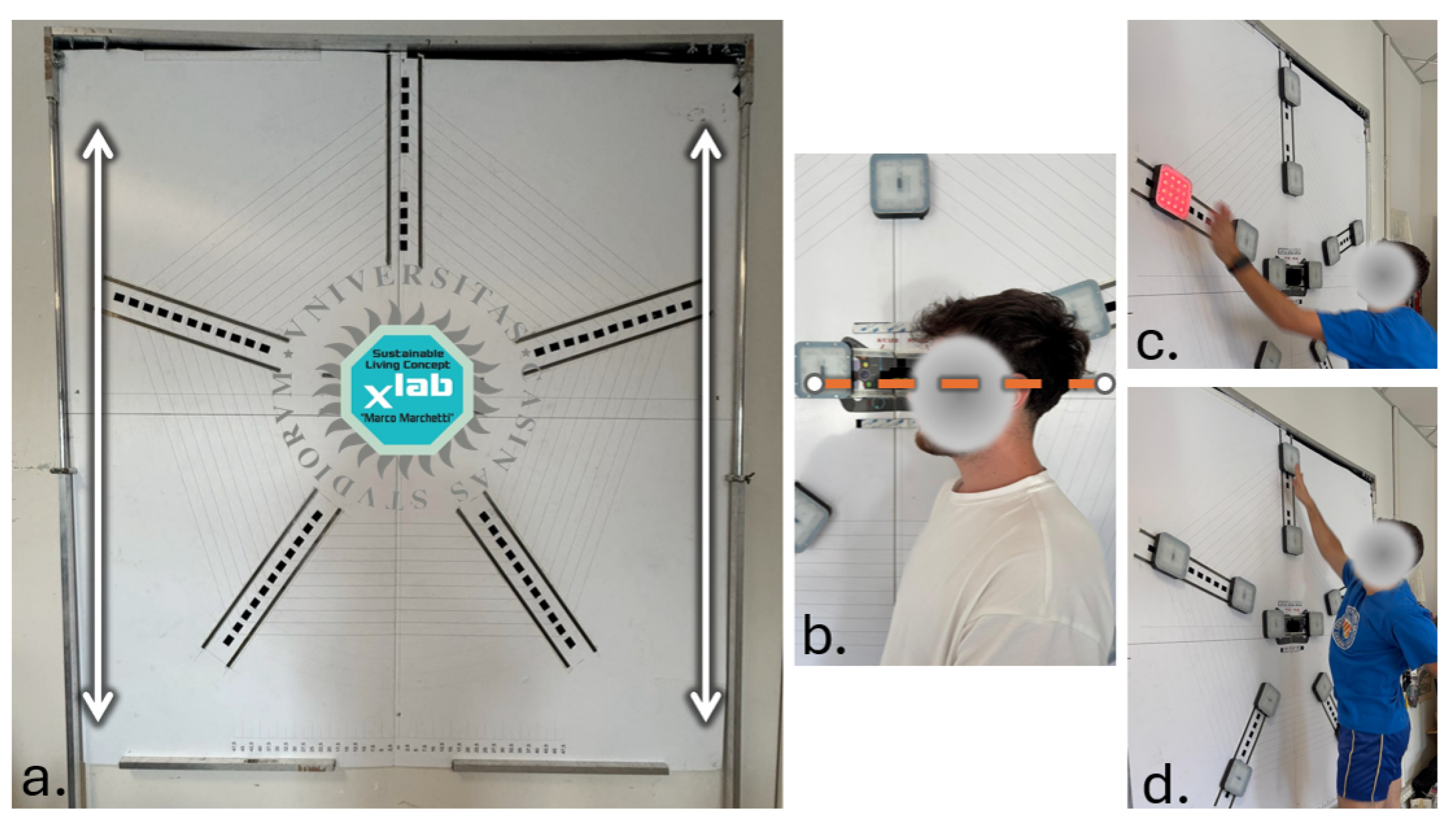
- FP: The panel and the lights were positioned to replicate the exact testing conditions used during the first phase assessment. An exception was made for the panel structure, which was designed to be horizontally translatable to accommodate the two testing configurations.
- AP: The panel was individually adjusted to align with each participant’s natural visual focus, defined as the horizontal line of sight when looking straight ahead with the head in a neutral position (166 cm ± 0.07). Anatomically, this corresponds to the primary visual axis, which extends from the center of the fovea in the retina to the point of fixation in the environment, and is considered the most accurate and sensitive area for processing visual stimuli [24,38].
2.4. Experimental Protocol—Second Phase
- Reaction Test Total Time : total time required to deactivate all 54 lights targets.
- Reaction Test Intertime : average time interval between successive light deactivations out of a total of 54 lights.
3. Statistical Analysis
4. Results
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FP | Fixed Panel |
| AP | Adjustable Panel |
| RT | Reaction Time |
| Reaction Time Total Time | |
| Reaction Time Intertime |
References
- Müller, M.; Drašinac, G.; Jakšić, D. The impact of coordinative abilities on the development of self-regulated Learning. J. Phys. Educ. Sport 2025, 25, 92. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The Proprioceptive Senses: Their Roles in Signaling Body Shape, Body Position and Movement, and Muscle Force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Soltys, J.S.; Wilson, S.E. The Role of the Central Nervous System in the Integration of Proprioceptive Activity. In Proceedings of the ASME 2012 Summer Bioengineering Conference, Parts A and B, Fajardo, Puerto Rico, USA, 20–23 June 2012; SBC2012. American Society of Mechanical Engineers: New York, NY, USA, 2012; pp. 237–238. [Google Scholar] [CrossRef]
- Lin, J. The impact of team-based learning on students with different self-regulated learning abilities. J. Comput. Assist. Learn. 2019, 35, 758–768. [Google Scholar] [CrossRef]
- Carissimo, C.; Cerro, G.; Libero, T.D.; Ferrigno, L.; Marino, A.; Rodio, A. Objective Evaluation of Coordinative Abilities and Training Effectiveness in Sports Scenarios: An Automated Measurement Protocol. IEEE Access 2023, 11, 76996–77008. [Google Scholar] [CrossRef]
- Diotaiuti, P.; Corrado, S.; Tosti, B.; Spica, G.; Di Libero, T.; D’Oliveira, A.; Zanon, A.; Rodio, A.; Andrade, A.; Mancone, S. Evaluating the effectiveness of neurofeedback in chronic pain management: A narrative review. Front. Psychol. 2024, 15, 1369487. [Google Scholar] [CrossRef] [PubMed]
- van Schooten, K.S.; Duran, L.; Visschedijk, M.; Pijnappels, M.; Lord, S.R.; Richardson, J.; Delbaere, K. Catch the ruler: Concurrent validity and test–retest reliability of the ReacStick measures of reaction time and inhibitory executive function in older people. Aging Clin. Exp. Res. 2019, 31, 1147–1154. [Google Scholar] [CrossRef]
- Di Libero, T.; Carissimo, C.; Cerro, G.; Abbatecola, A.; Marino, A.; Miele, G.; Ferrigno, L.; Rodio, A. Motor abilities analysis using a standardized tapping test enhanced by a detailed processing stage: Gender and age comparison. In Proceedings of the 2023 IEEE International Symposium on Medical Measurements and Applications (MeMeA), Jeju, Republic of Korea, 14–16 June 2023; pp. 1–6. [Google Scholar] [CrossRef]
- Di Libero, T.; D’Ermo, A.; Tosti, B.; Corrado, S.; Diotaiuti, P.; Rodio, A. The 100-Days: Physical Exercise and Challenges to Assess, Maintain and Improve Physical Fitness During Lockdown. Sports 2024, 12, 337. [Google Scholar] [CrossRef]
- Aqil, M.; Knapen, T.; Dumoulin, S.O. Computational model links normalization to chemoarchitecture in the human visual system. Sci. Adv. 2024, 10, eadj6102. [Google Scholar] [CrossRef]
- Smithson, H.E.; Young, L.K.; Hauperich, A.K.; Hexley, A.C.; Regan, S.E. Are fixational eye movements adaptive? Two tests of the interaction between photoreceptor sampling, eye movements and psychophysical performance. J. Vis. 2019, 19, 31. [Google Scholar] [CrossRef]
- Gegenfurtner, K.R. The Interaction Between Vision and Eye Movements. Perception 2016, 45, 1333–1357. [Google Scholar] [CrossRef]
- Rangaswamy, N.V.; Patel, H.M.; Locke, K.G.; Hood, D.C.; Birch, D.G. A Comparison of Visual Field Sensitivity to Photoreceptor Thickness in Retinitis Pigmentosa. Investig. Opthalmol. Vis. Sci. 2010, 51, 4213. [Google Scholar] [CrossRef] [PubMed]
- Hanakawa, T.; Immisch, I.; Toma, K.; Dimyan, M.A.; Van Gelderen, P.; Hallett, M. Functional Properties of Brain Areas Associated With Motor Execution and Imagery. J. Neurophysiol. 2003, 89, 989–1002. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Harvey, B.M.; Jorge, L.; Canário, N.; Machado, F.; Soares, M.; d’Almeida, O.C.; Castelo-Branco, M. Simultaneous changes in visual acuity, cortical population receptive field size, visual field map size, and retinal thickness in healthy human aging. Brain Struct. Funct. 2021, 226, 2839–2853. [Google Scholar] [CrossRef] [PubMed]
- Manakhov, P.; Sidenmark, L.; Pfeuffer, K.; Gellersen, H. Gaze on the Go: Effect of Spatial Reference Frame on Visual Target Acquisition During Physical Locomotion in Extended Reality. In Proceedings of the CHI Conference on Human Factors in Computing Systems, CHI ’24, Honolulu, HI, USA, 11–16 May 2024; ACM: New York, NY, USA, 2024; pp. 1–16. [Google Scholar] [CrossRef]
- Arede, J.; Carvalho, M.; Esteves, P.; de las Heras, B.; Leite, N. Exploring the Effects of LED Lighting Training Program on Motor Performance among Young Athletes. Creat. Res. J. 2020, 33, 63–73. [Google Scholar] [CrossRef]
- Yu, L.H.; Lee, S.N. The Effects of a Reaction Time and Eye-hand Coordination Training in Youth Players. J. Coach. Dev. 2023, 25, 204–215. [Google Scholar] [CrossRef]
- Deore, D.N. A Cross Sectional Study on the Relationship Between the Body Mass Index (BMI) and the Audiovisual Reaction Time (ART). J. Clin. Diagn. Res. 2012, 6, 1466. [Google Scholar] [CrossRef]
- Śliż, M.; Paśko, W.; Dziadek, B.; Godek, Ł.; Bliźniak, K.; Gouveia, É.R.; Przednowek, K. The influence of selected anthropometric parameters on psychomotor abilities among professional Rugby Union players. BMC Sport. Sci. Med. Rehabil. 2023, 15, 125. [Google Scholar] [CrossRef]
- Khan, A.D.; Ashwini, A.; Malipatil, B. Effect of Body Mass Index and Gender on Visual and Auditory Reaction Times in Young Adults. J. US-China Med. Sci. 2015, 12, 64–69. [Google Scholar] [CrossRef]
- Kwon, M.; Liu, R. Linkage between retinal ganglion cell density and the nonuniform spatial integration across the visual field. Proc. Natl. Acad. Sci. USA 2019, 116, 3827–3836. [Google Scholar] [CrossRef]
- Watson, A.B. A formula for human retinal ganglion cell receptive field density as a function of visual field location. J. Vis. 2014, 14, 15. [Google Scholar] [CrossRef]
- Kupers, E.R.; Benson, N.C.; Carrasco, M.; Winawer, J. Asymmetries around the visual field: From retina to cortex to behavior. PLoS Comput. Biol. 2022, 18, e1009771. [Google Scholar] [CrossRef] [PubMed]
- Goodale, M.A.; Danckert, J. Superior performance for visually guided pointing in the lower visual field. Exp. Brain Res. 2001, 137, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Lawrence, G.P. Differences in visuomotor control between the upper and lower visual fields. Exp. Brain Res. 2005, 164, 395–398. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.A.; Elias, L.J. Upper and lower visual field differences in perceptual asymmetries. Brain Res. 2011, 1387, 108–115. [Google Scholar] [CrossRef]
- Ellison, P.; Kearney, P.; Sparks, S.; Murphy, P.; Marchant, D. Further evidence against eye–hand coordination as a general ability. Int. J. Sport. Sci. Coach. 2017, 13, 687–693. [Google Scholar] [CrossRef]
- Shaw, B.S.; Breukelman, G.; Millard, L.; Moran, J.; Sandercock, G.; Shaw, I. Maximal aerobic exercise and acute visual performance in females: Implications for concussion side-line testing. J. Optom. 2024, 17, 100515. [Google Scholar] [CrossRef]
- Bujak, Z.; Gierczuk, D. Changes in Response Time in Elite Taekwon-Do Athletes and Wrestlers Resulting From Led Lighting Training. Pol. J. Sport Tour. 2024, 31, 17–23. [Google Scholar] [CrossRef]
- Gierczuk, D.; Bujak, Z.; Cieśliński, I. Effects of Led Lighting Training on Response Time in Greco-Roman Wrestlers. Pol. J. Sport Tour. 2023, 30, 11–16. [Google Scholar] [CrossRef]
- Gierczuk, D.; Bujak, Z. Reliability and Accuracy of Batak Lite Tests Used for Assessing Coordination Motor Abilities in Wrestlers. Pol. J. Sport Tour. 2014, 21, 72–76. [Google Scholar] [CrossRef]
- Di Libero, T.; Carissimo, C.; Zagaglia, A.; Cerro, G.; Ferrigno, L.; Rodio, A. Assessment of coordinative abilities through upper extremity wearable device technology. In Proceedings of the 2022 IEEE International Workshop on Sport, Technology and Research (STAR), Trento-Cavalese, Italy, 6–8 July 2022; pp. 175–179. [Google Scholar] [CrossRef]
- Di Libero, T.; Carissimo, C.; Cerro, G.; Abbatecola, A.M.; Marino, A.; Miele, G.; Ferrigno, L.; Rodio, A. An Overall Automated Architecture Based on the Tapping Test Measurement Protocol: Hand Dexterity Assessment through an Innovative Objective Method. Sensors 2024, 24, 4133. [Google Scholar] [CrossRef]
- Rodio, A.; Di Libero, T.; Biffi, A.; Fernando, F.; Fattorini, L. Automotive workers: The role of coordinative and conditional abilities as effectiveness wellness indicator. Front. Public Health 2024, 12, 1447358. [Google Scholar] [CrossRef]
- Kuriyan, R. Body composition techniques. Indian J. Med. Res. 2018, 148, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Weale, R.A. Ishihara Tables. BMJ 1953, 1, 1148. [Google Scholar] [CrossRef] [PubMed]
- Previc, F.H. Functional specialization in the lower and upper visual fields in humans: Its ecological origins and neurophysiological implications. Behav. Brain Sci. 1990, 13, 519–542. [Google Scholar] [CrossRef]
- jamovi, version 2.4.5; Computer Software; The jamovi Project: Sydney, Australia, 2023.
- Cui, Q.N.; Razavi, B.; O’Neill, W.E.; Paige, G.D. Perception of Auditory, Visual, and Egocentric Spatial Alignment Adapts Differently to Changes in Eye Position. J. Neurophysiol. 2010, 103, 1020–1035. [Google Scholar] [CrossRef]
- Bragança, S.; Arezes, P.; Carvalho, M.; Ashdown, S.P.; Leão, C. Assessment of the intraday variability of anthropometric measurements in the work environment: A pilot study. Int. J. Occup. Saf. Ergon. 2017, 24, 516–526. [Google Scholar] [CrossRef]
- Rodrigues, L.P.; Luz, C.; Cordovil, R.; Bezerra, P.; Silva, B.; Camões, M.; Lima, R. Normative values of the motor competence assessment (MCA) from 3 to 23 years of age. J. Sci. Med. Sport 2019, 22, 1038–1043. [Google Scholar] [CrossRef]
- Di Libero, T.; Falese, L.; D’Ermo, A.; Tosti, B.; Corrado, S.; Iannaccone, A.; Diotaiuti, P.; Rodio, A. Physiological Profile Assessment and Self-Measurement of Healthy Students through Remote Protocol during COVID-19 Lockdown. J. Funct. Morphol. Kinesiol. 2024, 9, 170. [Google Scholar] [CrossRef]
| Descriptives | Shapiro–Wilk | ||
|---|---|---|---|
| Mean | SD | p | |
| Age (y) | 30.1 | 2.72 | <0.001 |
| Height (cm) | 177.1 | 7.42 | 0.037 |
| Weight (kg) | 75.7 | 10.99 | <0.001 |
| BMI () | 24.1 | 3.15 | <0.001 |
| Descriptives | Shapiro-Wilk | ||
|---|---|---|---|
| Mean | SD | p | |
| Age (y) | 24.0 | 4.35 | <0.001 |
| Height (m) | 1.80 | 0.08 | 0.907 |
| Weight (kg) | 72.8 | 11.43 | 0.375 |
| BMI () | 23.7 | 2.79 | 0.576 |
| Correlation Matrix (Height) | ||
|---|---|---|
| Mean ± SD | 49.30 ± 4.31 | 0.70 ± 0.10 |
| Spearman’s rho | −0.482 *** | −0.475 *** |
| Standardized β | −0.54 | −0.51 |
| 0.232 | 0.226 |
| Correlation Matrix (Weight) | ||
|---|---|---|
| Mean ± SD | 49.30 ± 4.31 | 0.70 ± 0.10 |
| Spearman’s rho | −0.222 *** | −0.217 *** |
| Standardized β | −0.24 | −0.22 |
| 0.049 | 0.047 |
| Correlation Matrix (BMI) | ||
|---|---|---|
| Mean ± SD | 49.30 ± 4.31 | 0.70 ± 0.10 |
| Spearman’s rho | 0.052 | 0.055 |
| Standardized β | 0.08 | 0.09 |
| 0.003 | 0.003 |
| Comparison | ||
|---|---|---|
| FP | AP | |
| (s) | 32.1 ± 3.26 | 30.0 ± 2.98 ** |
| (s) | 0.36 ± 0.057 | 0.30 ± 0.066 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodio, A.; Fattorini, L.; Falese, L.; D’Ermo, A.; Biffi, A.; Fernando, F.; Di Libero, T. Influence of Anthropometric Height on Oculo-Manual Coordinative Reaction Time. J. Funct. Morphol. Kinesiol. 2025, 10, 334. https://doi.org/10.3390/jfmk10030334
Rodio A, Fattorini L, Falese L, D’Ermo A, Biffi A, Fernando F, Di Libero T. Influence of Anthropometric Height on Oculo-Manual Coordinative Reaction Time. Journal of Functional Morphology and Kinesiology. 2025; 10(3):334. https://doi.org/10.3390/jfmk10030334
Chicago/Turabian StyleRodio, Angelo, Luigi Fattorini, Lavinia Falese, Annalisa D’Ermo, Alessandro Biffi, Fredrick Fernando, and Tommaso Di Libero. 2025. "Influence of Anthropometric Height on Oculo-Manual Coordinative Reaction Time" Journal of Functional Morphology and Kinesiology 10, no. 3: 334. https://doi.org/10.3390/jfmk10030334
APA StyleRodio, A., Fattorini, L., Falese, L., D’Ermo, A., Biffi, A., Fernando, F., & Di Libero, T. (2025). Influence of Anthropometric Height on Oculo-Manual Coordinative Reaction Time. Journal of Functional Morphology and Kinesiology, 10(3), 334. https://doi.org/10.3390/jfmk10030334






