Neuromuscular and Psychological Performance Monitoring During One Season in Spanish Marine Corps
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedures
2.3.1. Physical Performance Tests
2.3.2. Psychological Performance Tests
2.4. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1
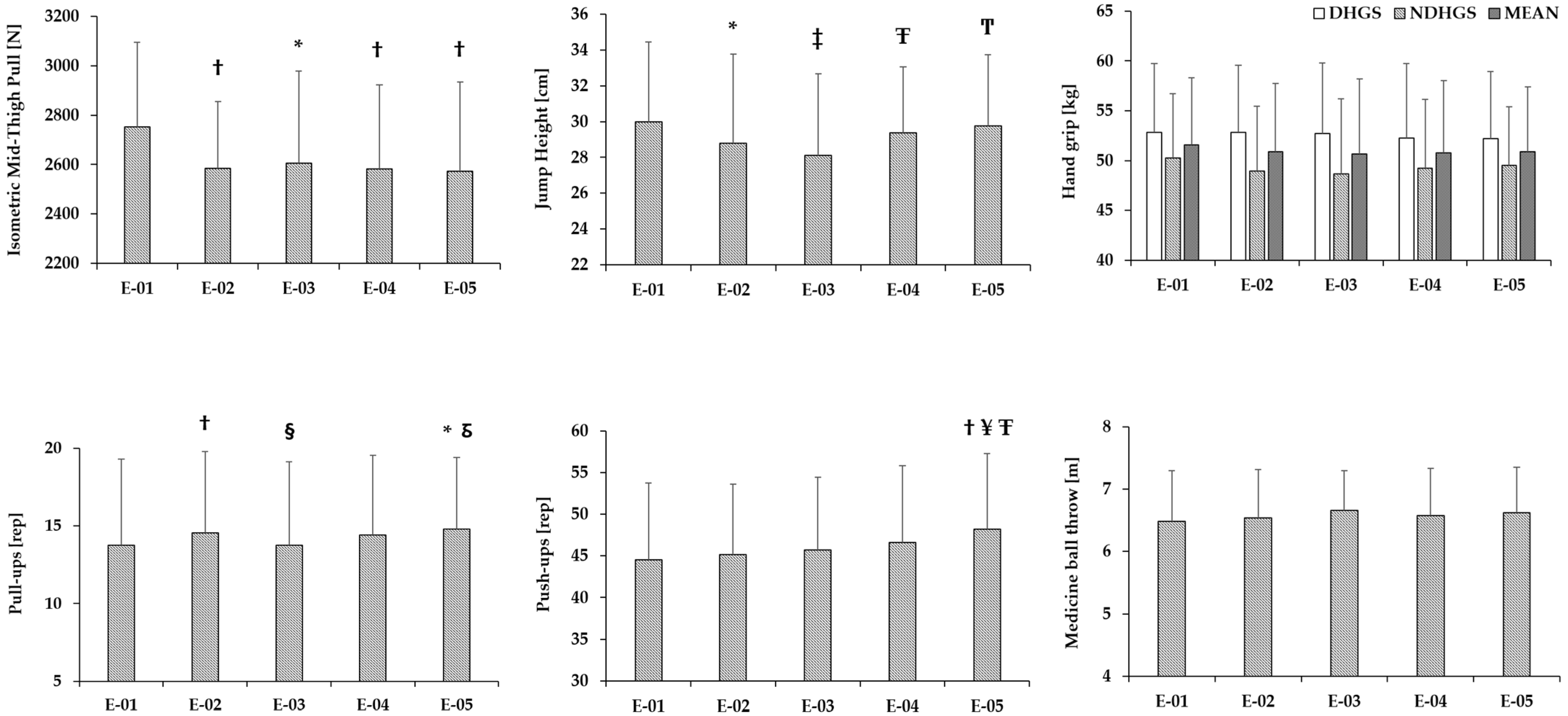
Appendix A.2
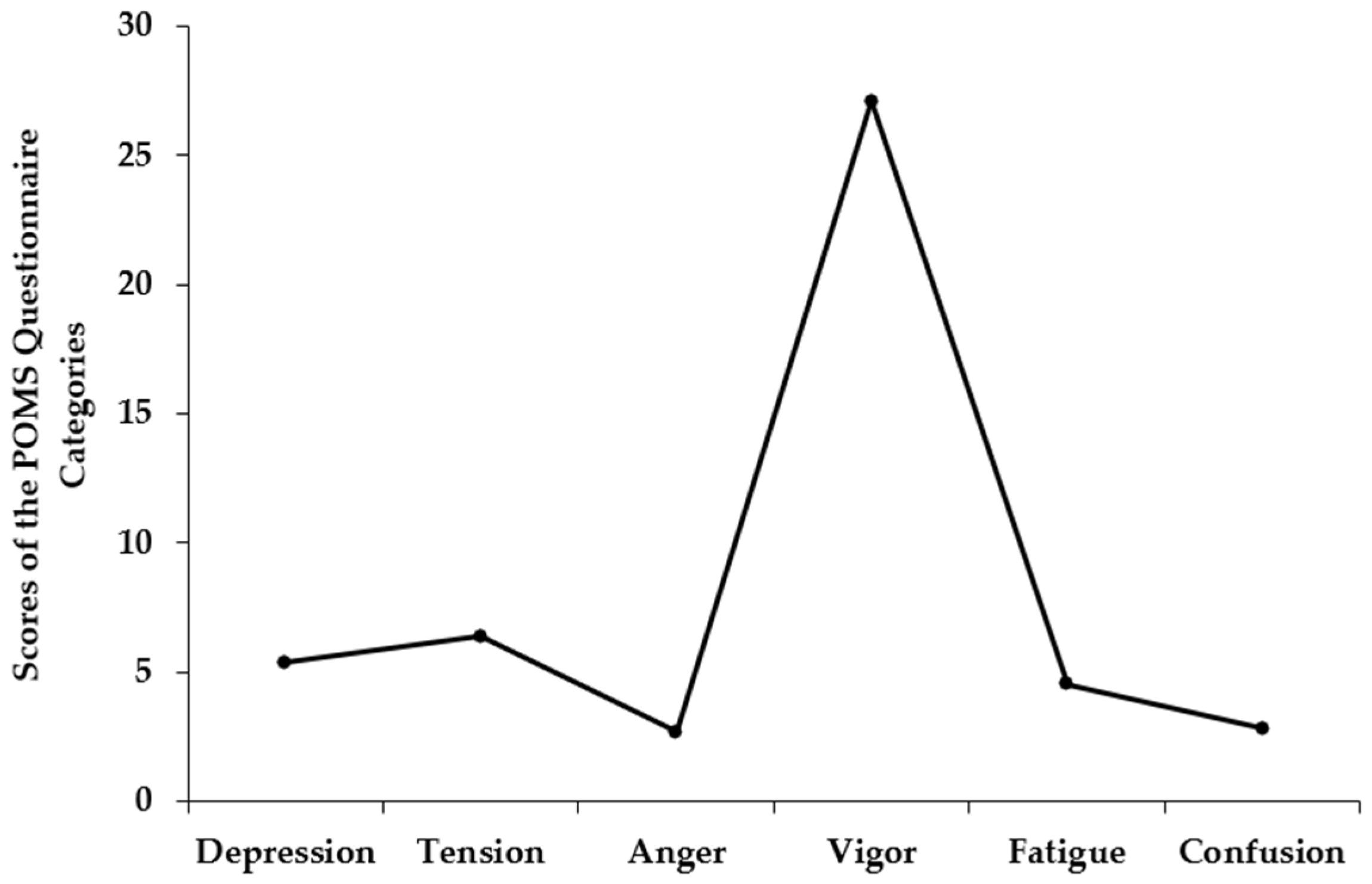

Appendix A.3
| Variables | E01 | CV1 | E02 | ∆%1 | ES1 | CV2 | E03 | ∆%2 | ES2 | CV3 | E04 | ∆%3 | ES3 | CV4 | E05 | ∆%4 | ES4 | CV5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BM [kg] | 79.01 ± 7.96 | 1.4 | 79.52 ± 7.99 * | 0.7 | −0.51 | 1.4 | 81.00 ± 7.99 ‡Ψ | 2.5 | −1.23 | 1.4 | 79.23 ± 7.61 Ͳ | 0.3 | −0.16 | 1.4 | 78.82 ± 7.53 ¥ͲƱ | −0.2 | 0.14 | 1.4 |
| PLUmax [reps] | 13.77 ± 5.52 | 9.1 | 14.54 ± 5.26 † | 5.6 | −0.62 | 8.6 | 13.77 ± 5.35 § | 0.0 | 0.00 | 9.1 | 14.42 ± 5.12 | 4.7 | −0.29 | 8.7 | 14.81 ± 4.58 *δ | 7.6 | −0.46 | 8.4 |
| PUmax [reps] | 44.50 ± 9.23 | 7.3 | 45.15 ± 8.43 | 1.5 | −0.17 | 7.1 | 45.73 ± 8.68 | 2.8 | −0.26 | 7.1 | 46.62 ± 9.23 | 4.8 | −0.32 | 6.9 | 48.23 ± 9.03 †¥ŦΦ | 8.4 | −0.61 | 6.7 |
| MBT [m] | 6.48 ± 0.81 | 4.4 | 6.54 ± 0.77 | 0.9 | −0.13 | 4.3 | 6.66 ± 0.64 | 2.8 | −0.42 | 4.2 | 6.58 ± 0.75 | 1.5 | −0.26 | 4.3 | 6.62 ± 0.73 | 2.2 | −0.27 | 4.3 |
| DHGS [kg] | 52.85 ± 6.90 | 6.0 | 52.81 ± 6.75 | -0.1 | 0.01 | 6.0 | 52.71 ± 7.08 | −0.3 | 0.03 | 6.0 | 52.28 ± 7.47 | −1.1 | 0.10 | 6.1 | 52.21 ± 6.72 | −1.2 | 0.10 | 6.1 |
| NDHGS [kg] | 50.28 ± 6.43 | 6.9 | 48.97 ± 6.47 | -6.3 | 0.26 | 7.0 | 48.67 ± 7.54 | −6.9 | 0.25 | 7.1 | 49.23 ± 6.93 | −5.8 | 0.24 | 7.0 | 49.52 ± 5.91 | −5.3 | 0.19 | 7.0 |
| IMTP [N] | 2752.46 ± 342.21 | 5.9 | 2584.23 ± 271.02 † | -6.1 | 0.64 | 6.3 | 2605.12 ± 372.69 † | −5.4 | 0.54 | 6.2 | 2581.96 ± 340.68 † | −6.2 | 0.66 | 6.3 | 2573.96 ± 360.31 † | −6.5 | 0.65 | 6.3 |
| VJ CMJ [cm] | 30.00 ± 4.45 | 6.0 | 28.78 ± 4.98 * | −4.1 | 0.44 | 6.3 | 28.12 ± 4.54 ‡ | −6.3 | 0.71 | 6.4 | 29.38 ± 3.67 Ŧ | −2.1 | 0.20 | 6.1 | 29.77 ± 3.95 T | −0.8 | 0.08 | 6.0 |
| PP CMJ [W] | 3602.35 ± 476.47 | 6.2 | 3609.31 ± 504.99 | 0.2 | -0.03 | 6.2 | 3449.02 ± 862.03 | −4.3 | 0.22 | 6.5 | 3583.69 ± 439.62 | −0.5 | 0.06 | 6.3 | 3625.50 ± 411.79 | 0.6 | −0.08 | 6.2 |
| RFDEcc CMJ [N/s] | 4772.62 ± 2064.86 | 26.3 | 5757.88 ± 2611.65 † | 20.6 | −0.69 | 21.8 | 5039.15 ± 2153.34 | 5.6 | −0.11 | 24.9 | 5016.73 ± 1312.61 | 5.1 | −0.14 | 25.1 | 5798.50 ± 1818.77 †Φ | 21.5 | −0.68 | 21.7 |
| RFDCon CMJ [N/s] | 1563.40 ± 1327.59 | 30.8 | 1439.46 ± 1167.51 | −7.9 | 0.08 | 33.5 | 1526.46 ± 996.93 | −2.4 | 0.03 | 31.6 | 1308.65 ± 926.29 | −16.3 | 0.15 | 36.8 | 1379.58 ± 927.84 | −11.8 | 0.15 | 34.9 |
| PEcc CMJ [N] | 1770.23 ± 291.65 | 8.5 | 1865.62 ± 353.98 | 5.4 | −0.62 | 8.1 | 1866.50 ± 474.11 | 5.4 | −0.23 | 8.1 | 1777.35 ± 239.55 | 0.4 | −0.04 | 8.5 | 1848.38 ± 265.44 | 4.4 | −0.47 | 8.2 |
| PCon CMJ [N] | 1858.15 ± 257.10 | 5.4 | 1932.92 ± 333.30 * | 4.0 | −0.45 | 5.2 | 1891.92 ± 249.29 | 1.8 | −0.26 | 5.3 | 1835.19 ± 209.59 ¥δ | −1.2 | 0.16 | 5.5 | 1900.77 ± 243.61 Ʊ | 2.3 | −0.29 | 5.3 |
| DSI (%) | 0.68 ± 0.13 | . | 0.75 ± 0.15 | . | . | . | 0.73 ± 0.14 | . | . | . | 0.71 ± 0.12 | . | . | . | 0.74 ± 0.14 | . | . | . |
Appendix A.4
| Weeks | Depression Dejection | ES | Tension Anxiety | ES | Anger Hostility | ES | Vigor Activity | ES | Fatigue Inertia | ES | Confusion Bewilderment | ES | TMD | Cronbach α |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 4.07 ± 4.47 | 0.91 | 6.41 ± 4.26 | 1.50 | 2.59 ± 2.90 | 0.89 | 24.93 ± 6.92 | 3.60 | 2.63 ± 3.19 | 0.83 | 3.89 ± 2.56 | 1.52 | 156 | 0.89 |
| 2 | 3.59 ± 4.94 | 0.73 | 6.11 ± 4.99 | 1.22 | 2.00 ± 3.06 | 0.65 | 26.48 ± 7.49 | 3.54 | 2.52 ± 3.06 | 0.82 | 2.37 ± 1.94 | 1.22 | 33 | 0.91 |
| 3 | 4.70 ± 6.95 | 0.68 | 7.04 ± 4.46 | 1.58 | 3.33 ± 5.00 | 0.67 | 26.22 ± 5.47 | 4.80 | 5.15 ± 5.34 | 0.96 | 3.52 ± 2.67 | 1.32 | 233 | 0.89 |
| 4 | 5.07 ± 8.37 | 0.61 | 6.41 ± 4.49 | 1.43 | 2.56 ± 4.52 | 0.57 | 27.44 ± 5.76 | 4.76 | 5.63 ± 4.65 | 1.21 | 3.48 ± 3.34 | 1.04 | 184 | 0.92 |
| 5 | 6.37 ± 9.96 | 0.64 | 7.26 ± 5.62 | 1.29 | 2.37 ± 4.51 | 0.53 | 27.81 ± 5.67 | 4.91 | 5.59 ± 4.94 | 1.13 | 2.78 ± 2.81 | 0.99 | 207 | 0.92 |
| 6 | 6.74 ± 10.60 | 0.64 | 7.15 ± 6.50 | 1.10 | 2.78 ± 5.73 | 0.48 | 27.85 ± 5.28 | 5.27 | 5.30 ± 5.26 | 1.01 | 2.37 ± 2.62 | 0.91 | 205 | 0.94 |
| 7 | 2.70 ± 3.89 | 0.70 | 5.59 ± 3.65 | 1.53 | 1.37 ± 2.72 | 0.50 | 27.85 ± 5.33 | 5.22 | 3.67 ± 4.58 | 0.80 | 2.30 ± 2.33 | 0.98 | -30 | 0.84 |
| 8 | 5.78 ± 9.86 | 0.59 | 6.19 ± 5.76 | 1.07 | 2.56 ± 5.02 | 0.51 | 27.89 ± 5.80 | 4.81 | 3.56 ± 4.25 | 0.84 | 2.48 ± 2.59 | 0.96 | 102 | 0.92 |
| 9 | 6.26 ± 10.01 | 0.63 | 6.11 ± 4.69 | 1.30 | 2.96 ± 4.93 | 0.60 | 26.81 ± 7.85 | 3.41 | 5.89 ± 6.00 | 0.98 | 2.70 ± 3.28 | 0.82 | 222 | 0.94 |
| 10 | 6.41 ± 10.86 | 0.59 | 6.11 ± 4.86 | 1.26 | 3.48 ± 6.42 | 0.54 | 27.48 ± 6.02 | 4.57 | 5.07 ± 6.06 | 0.84 | 3.11 ± 3.83 | 0.81 | 211 | 0.95 |
| 11 | 7.00 ± 13.90 | 0.50 | 6.33 ± 4.45 | 1.42 | 3.07 ± 6.32 | 0.49 | 27.48 ± 6.82 | 4.03 | 5.19 ± 6.11 | 0.85 | 2.96 ± 3.31 | 0.90 | 221 | 0.94 |
| 12 | 6.33 ± 13.55 | 0.47 | 5.70 ± 5.19 | 1.10 | 3.44 ± 6.83 | 0.50 | 26.93 ± 8.97 | 3.00 | 4.15 ± 5.07 | 0.82 | 2.07 ± 2.59 | 0.80 | 159 | 0.94 |
| Mean | 5.42 ± 9.42 | 0.77 | 6.37 ± 4.91 | 1.80 | 2.71 ± 4.96 | 0.70 | 27.10 ± 6.49 | 5.47 | 4.53 ± 5.02 | 1.15 | 2.84 ± 2.87 | 1.34 | 158.58 ± 83.06 | 0.92 |
Appendix A.5
| Scales | S/R | E-01 | E-02 | E-03 | E-04 | E-05 | E-06 | E-07 | E-08 | E-09 | E-10 | E-11 | E-12 | Mean | ES |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| TS | S | 5.28 ± 4.76 | 5.06 ± 4.77 | 5.68 ± 5.35 | 5.60 ± 5.48 | 5.90 ± 5.85 | 5.73 ± 5.59 | 5.04 ± 5.37 | 5.15 ± 5.37 | 6.22 ± 6.19 | 5.37 ± 5.84 | 5.45 ± 5.93 | 5.08 ± 6.07 | 5.46 ± 5.57 | 1.73 |
| TR | R | 18.26 ± 3.31 | 18.15 ± 3.89 | 17.59 ± 4.30 | 17.49 ± 4.72 | 17.62 ± 4.42 | 17.44 ± 4.55 | 17.99 ± 4.70 | 17.24 ± 5.13 | 17.40 ± 5.08 | 17.49 ± 4.95 | 16.84 ± 5.77 | 17.03 ± 6.36 | 17.54 ± 4.83 | 5.13 |
| NSSS | S | 4.83 ± 4.58 | 4.35 ± 4.43 | 5.02 ± 5.10 | 5.02 ± 5.25 | 5.38 ± 5.51 | 5.30 ± 5.39 | 4.48 ± 4.97 | 4.79 ± 5.11 | 5.57 ± 5.77 | 4.97 ± 5.49 | 4.85 ± 5.59 | 4.70 ± 5.84 | 4.94 ± 5.27 | 1.56 |
| 1. General stress | S | 1.81 ± 2.30 | 1.59 ± 2.04 | 2.37 ± 3.80 | 2.52 ± 3.46 | 3.07 ± 3.72 | 3.00 ± 3.91 | 2.00 ± 2.90 | 3.22 ± 4.39 | 3.00 ± 4.19 | 2.96 ± 4.06 | 3.04 ± 4.69 | 2.93 ± 5.38 | 2.63 ± 3.15 | 0.83 |
| 2. Emotional stress | S | 2.48 ± 2.82 | 2.11 ± 2.59 | 2.37 ± 3.56 | 2.41 ± 3.43 | 2.85 ± 3.30 | 2.63 ± 3.63 | 1.63 ± 2.51 | 3.11 ± 3.92 | 2.70 ± 4.06 | 2.89 ± 4.10 | 2.56 ± 3.97 | 2.74 ± 4.44 | 2.54 ± 2.94 | 0.86 |
| 3. Social stress | S | 2.85 ± 3.25 | 1.89 ± 2.55 | 2.56 ± 3.83 | 2.70 ± 3.53 | 2.26 ± 3.03 | 3.44 ± 4.60 | 2.37 ± 3.21 | 2.85 ± 3.74 | 2.74 ± 4.39 | 2.89 ± 3.88 | 2.41 ± 4.20 | 3.11 ± 5.55 | 2.67 ± 3.09 | 0.86 |
| 4. Conflicts/ Pressure | S | 8.37 ± 4.34 | 7.22 ± 4.70 | 7.56 ± 3.92 | 7.63 ± 4.04 | 8.22 ± 5.32 | 8.52 ± 5.54 | 7.19 ± 5.51 | 8.04 ± 4.82 | 7.33 ± 5.51 | 7.30 ± 5.21 | 7.67 ± 5.62 | 6.74 ± 5.41 | 7.65 ± 4.00 | 1.91 |
| 5. Fatigue | S | 9.11 ± 5.54 | 9.81 ± 5.09 | 11.26 ± 5.40 | 11.30 ± 6.60 | 11.00 ± 6.67 | 10.26 ± 6.28 | 10.33 ± 6.04 | 8.96 ± 6.47 | 11.63 ± 6.12 | 9.41 ± 6.59 | 9.93 ± 6.98 | 8.74 ± 6.57 | 10.15 ± 4.95 | 2.05 |
| 6. Lack of energy | S | 4.63 ± 3.86 | 3.59 ± 2.53 | 4.07 ± 3.95 | 3.67 ± 4.08 | 4.48 ± 4.81 | 4.07 ± 4.15 | 3.93 ± 3.71 | 3.48 ± 4.35 | 4.67 ± 4.71 | 4.30 ± 5.57 | 4.00 ± 4.44 | 4.11 ± 5.13 | 4.08 ± 3.51 | 1.16 |
| 7. Physical complaints | S | 4.56 ± 3.45 | 4.22 ± 3.21 | 4.93 ± 4.19 | 4.89 ± 4.20 | 5.74 ± 5.03 | 5.19 ± 4.32 | 3.93 ± 3.12 | 3.85 ± 3.90 | 6.89 ± 5.47 | 5.07 ± 5.31 | 4.33 ± 4.42 | 4.56 ± 6.08 | 4.85 ± 3.36 | 1.44 |
| NSSR | R | 18.27 ± 3.66 | 17.66 ± 4.23 | 17.30 ± 4.45 | 17.16 ± 5.05 | 17.31 ± 4.55 | 17.01 ± 4.69 | 17.61 ± 4.79 | 17.21 ± 4.93 | 17.18 ± 4.90 | 17.26 ± 5.02 | 16.60 ± 5.57 | 16.78 ± 6.36 | 17.28 ± 4.89 | 5.14 |
| 8. Success | R | 17.78 ± 2.95 | 16.96 ± 4.00 | 17.41 ± 4.26 | 17.04 ± 4.71 | 17.15 ± 4.04 | 16.78 ± 4.87 | 18.11 ± 4.40 | 16.63 ± 5.64 | 17.44 ± 4.81 | 16.63 ± 5.00 | 17.07 ± 5.45 | 16.74 ± 6.20 | 17.15 ± 3.98 | 4.31 |
| 9. Social recovery | R | 19.93 ± 3.26 | 19.11 ± 3.86 | 17.22 ± 4.44 | 18.33 ± 5.20 | 18.44 ± 5.03 | 17.74 ± 4.92 | 18.89 ± 4.36 | 18.26 ± 4.55 | 18.33 ± 5.26 | 18.85 ± 4.58 | 17.41 ± 5.58 | 18.22 ± 6.34 | 18.39 ± 4.13 | 4.45 |
| 10. Physical recovery | R | 18.30 ± 3.01 | 17.67 ± 3.59 | 17.11 ± 3.97 | 16.74 ± 5.07 | 17.30 ± 4.47 | 17.26 ± 4.25 | 17.78 ± 4.41 | 17.44 ± 4.92 | 16.26 ± 4.75 | 16.85 ± 4.66 | 16.19 ± 5.19 | 16.22 ± 6.83 | 17.09 ± 3.74 | 4.57 |
| 11. General well-being | R | 20.78 ± 2.38 | 20.37 ± 3.52 | 20.63 ± 3.98 | 20.11 ± 4.03 | 20.04 ± 3.50 | 19.67 ± 3.86 | 19.85 ± 4.36 | 19.85 ± 4.12 | 19.81 ± 4.50 | 19.85 ± 5.04 | 19.41 ± 5.32 | 19.41 ± 6.13 | 19.98 ± 3.58 | 5.58 |
| 12. Sleep quality | R | 14.59 ± 3.39 | 14.19 ± 3.61 | 14.11 ± 3.24 | 13.59 ± 4.06 | 13.63 ± 3.12 | 13.59 ± 3.49 | 13.44 ± 4.03 | 13.89 ± 3.36 | 14.04 ± 3.20 | 14.11 ± 4.06 | 12.93 ± 4.55 | 13.30 ± 4.85 | 13.78 ± 2.84 | 4.86 |
| SSS | S | 6.32 ± 5.04 | 6.72 ± 5.14 | 7.22 ± 4.63 | 6.95 ± 5.78 | 7.11 ± 6.45 | 6.77 ± 5.95 | 6.33 ± 6.03 | 5.99 ± 5.88 | 7.74 ± 6.87 | 6.30 ± 6.54 | 6.85 ± 6.47 | 5.95 ± 6.53 | 6.69 ± 6.04 | 1.84 |
| 13. Disturbed breaks | S | 6.00 ± 3.86 | 6.48 ± 3.84 | 7.19 ± 4.88 | 7.07 ± 5.07 | 6.15 ± 5.27 | 5.30 ± 4.37 | 5.67 ± 4.84 | 4.48 ± 4.65 | 6.93 ± 6.00 | 5.15 ± 5.25 | 6.74 ± 5.76 | 5.19 ± 5.66 | 6.03 ± 3.98 | 1.52 |
| 14. Emotional exhaustion | S | 3.19 ± 3.36 | 2.74 ± 2.88 | 4.19 ± 4.58 | 3.15 ± 3.48 | 4.22 ± 4.97 | 3.04 ± 3.95 | 2.74 ± 4.07 | 3.93 ± 5.55 | 3.67 ± 4.82 | 3.44 ± 5.12 | 2.85 ± 4.34 | 3.00 ± 4.56 | 3.35 ± 3.53 | 0.95 |
| 15. Injury | S | 9.78 ± 5.40 | 10.93 ± 4.85 | 10.30 ± 5.76 | 10.63 ± 5.99 | 10.96 ± 7.11 | 11.96 ± 5.42 | 10.59 ± 6.25 | 9.56 ± 5.81 | 12.63 ± 6.58 | 10.30 ± 7.16 | 10.96 ± 6.53 | 9.67 ± 7.37 | 10.69 ± 5.17 | 2.07 |
| SSR | R | 18.24 ± 2.82 | 18.76 ± 3.34 | 17.95 ± 4.10 | 17.90 ± 4.26 | 18.00 ± 4.24 | 17.97 ± 4.34 | 18.45 ± 4.56 | 17.28 ± 5.39 | 17.68 ± 5.31 | 17.79 ± 4.86 | 17.13 ± 6.02 | 17.34 ± 6.37 | 17.87 ± 4.74 | 4.96 |
| 16. Being in shape | R | 18.52 ± 2.95 | 18.22 ± 3.21 | 17.41 ± 4.02 | 17.74 ± 4.17 | 17.11 ± 4.06 | 17.37 ± 3.90 | 18.67 ± 4.36 | 16.85 ± 5.63 | 17.26 ± 5.04 | 17.15 ± 4.87 | 16.67 ± 5.20 | 17.19 ± 6.47 | 17.51 ± 3.60 | 4.86 |
| 17. Personal accomplishment | R | 16.81 ± 2.95 | 17.70 ± 3.44 | 17.15 ± 4.40 | 16.93 ± 4.68 | 17.22 ± 4.35 | 17.22 ± 4.79 | 16.96 ± 4.92 | 16.74 ± 5.08 | 15.78 ± 5.80 | 16.74 ± 4.87 | 16.59 ± 6.90 | 16.22 ± 6.65 | 16.84 ± 4.08 | 4.13 |
| 18. Self-efficacy | R | 19.00 ± 2.51 | 19.19 ± 3.37 | 18.85 ± 3.56 | 18.33 ± 3.96 | 18.59 ± 3.92 | 18.37 ± 4.16 | 19.07 ± 4.31 | 17.85 ± 5.52 | 18.41 ± 4.98 | 18.59 ± 4.50 | 17.78 ± 5.04 | 17.81 ± 6.45 | 18.49 ± 3.48 | 5.31 |
| 19. Self-regulation | R | 18.63 ± 2.47 | 19.93 ± 3.06 | 18.41 ± 4.37 | 18.59 ± 4.23 | 19.07 ± 4.51 | 18.93 ± 4.45 | 19.11 ± 4.55 | 17.67 ± 5.55 | 19.26 ± 4.99 | 18.67 ± 5.12 | 17.48 ± 6.95 | 18.15 ± 6.09 | 18.66 ± 3.99 | 4.68 |
| Cronbach α | 0.87 | 0.82 | 0.85 | 0.84 | 0.79 | 0.81 | 0.83 | 0.82 | 0.87 | 0.81 | 0.87 | 0.93 | 0.84 | . |
Appendix A.6
| RESTQ-Sport Scales | S/R | POMS Categories | |||||
|---|---|---|---|---|---|---|---|
| Depression Dejection | Tension Anxiety | Anger Hostility | Vigor Activity | Fatigue Inertia | Confusion Bewilderment | ||
| TS | S | 0.79 ‡ | 0.83 ‡ | 0.74 ‡ | −0.60 ‡ | 0.89 ‡ | 0.42 δ |
| TR | R | −0.80 ‡ | −0.55 † | −0.73 ‡ | 0.90 ‡ | −0.56 † | −0.56 † |
| NSSS | S | 0.84 ‡ | 0.84 ‡ | 0.80 ‡ | −0.65 ‡ | 0.84 ‡ | 0.44 δ |
| 1. General stress | S | 0.89 ‡ | 0.82 ‡ | 0.82 ‡ | −0.72 ‡ | 0.80 ‡ | 0.46 δ |
| 2. Emotional stress | S | 0.93 ‡ | 0.83 ‡ | 0.93 ‡ | −0.73 ‡ | 0.70 ‡ | 0.54 † |
| 3. Social stress | S | 0.90 ‡ | 0.73 ‡ | 0.92 ‡ | −0.73 ‡ | 0.64 ‡ | 0.52 † |
| 4. Conflicts/ Pressure | S | 0.56 † | 0.64 ‡ | 0.48 δ | −0.41 δ | 0.51 † | 0.09 |
| 5. Fatigue | S | 0.51 † | 0.64 ‡ | 0.48 δ | −0.34 | 0.82 ‡ | 0.27 |
| 6. Lack of energy | S | 0.85 ‡ | 0.84 ‡ | 0.79 ‡ | −0.71 ‡ | 0.80 ‡ | 0.48 δ |
| 7. Physical complaints | S | 0.75 ‡ | 0.75 ‡ | 0.71 ‡ | −0.57 † | 0.91 ‡ | 0.48 δ |
| NSSR | R | −0.81 ‡ | −0.55 † | −0.75 ‡ | 0.89 ‡ | −0.53 † | −0.50 † |
| 8. Success | R | −0.72 ‡ | −0.50 † | −0.62 ‡ | 0.86 ‡ | −0.50 † | −0.55 † |
| 9. Social recovery | R | −0.77 ‡ | −0.46 δ | −0.75 ‡ | 0.85 ‡ | −0.36 | −0.41 δ |
| 10. Physical recovery | R | −0.78 ‡ | −0.60 ‡ | −0.71 ‡ | 0.84 ‡ | −0.68 ‡ | −0.47 δ |
| 11. General well-being | R | −0.85 ‡ | −0.54 † | −0.83 ‡ | 0.89 ‡ | −0.46 δ | −0.54 † |
| 12. Sleep quality | R | −0.55 † | −0.39 δ | −0.47 δ | 0.61 ‡ | −0.43 δ | −0.31 |
| SSS | S | 0.72 ‡ | 0.80 ‡ | 0.68 ‡ | -0.54 † | 0.91 ‡ | 0.40 δ |
| 13. Disturbed breaks | S | 0.74 ‡ | 0.72 ‡ | 0.74 ‡ | −0.59 ‡ | 0.86 ‡ | 0.48 δ |
| 14. Emotional exhaustion | S | 0.89 ‡ | 0.84 ‡ | 0.81 ‡ | −0.74 ‡ | 0.77 ‡ | 0.51 † |
| 15. Injury | S | 0.36 | 0.56 † | 0.31 | −0.17 | 0.73 ‡ | 0.14 |
| SSR | R | −0.78 ‡ | −0.53 † | −0.69 ‡ | 0.88 ‡ | −0.57 † | −0.60 ‡ |
| 16. Being in shape | R | −0.77 ‡ | −0.61 ‡ | −0.67 ‡ | 0.81 ‡ | −0.72 ‡ | −0.53 † |
| 17. Personal accomplishment | R | −0.68 ‡ | −0.39 δ | −0.63 ‡ | 0.84 ‡ | −0.44 δ | −0.55 † |
| 18. Self-efficacy | R | −0.78 ‡ | −0.55 † | −0.71 ‡ | 0.86 ‡ | −0.54 † | −0.65 ‡ |
| 19. Self-regulation | R | −0.74 ‡ | −0.49 † | −0.63 ‡ | 0.86 ‡ | −0.50 † | −0.54 † |
References
- Kyröläinen, H.; Pihlainen, K.; Vaara, J.P.; Ojanen, T.; Santtila, M. Optimising training adaptations and performance in military environment. J. Sci. Med. Sport 2018, 21, 1131–1138. [Google Scholar] [CrossRef]
- de Visser, E.J.; Dorfman, A.; Chartrand, D.; Lamon, J.; Freedy, E.; Weltman, G. Building resilience with the Stress Resilience Training System: Design validation and applications. Work 2016, 54, 351–366. [Google Scholar] [CrossRef]
- Szivak, T.K.; Kraemer, W.J. Physiological Readiness and Resilience: Pillars of Military Preparedness. J. Strength Cond. Res. 2015, 29, S34–S39. [Google Scholar] [CrossRef]
- Nindl, B.C.; Alvar, B.A.; R Dudley, J.; Favre, M.W.; Martin, G.J.; Sharp, M.A.; Warr, B.J.; Stephenson, M.D.; Kraemer, W.J. Executive Summary From the National Strength and Conditioning Association’s Second Blue Ribbon Panel on Military Physical Readiness: Military Physical Performance Testing. J. Strength Cond. Res. 2015, 29, S216–S220. [Google Scholar] [CrossRef]
- Cáceres-Diego, B.; Alcaraz, P.E.; Marín-Pagán, C. Neuromuscular Responses to 5 K Time Trial Load Carried by Spanish Army Marines. Sports 2025, 13, 129. [Google Scholar] [CrossRef]
- Cáceres-Diego, B.; Marín-Pagán, C.; Martínez de Baños, P.; Alcaraz, P.E. Effects of a 30 K Military Loaded Carriage on the Neuromuscular System in Spanish Army Marines. Sports 2025, 13, 76. [Google Scholar] [CrossRef] [PubMed]
- Tornero-Aguilera, J.F.; Robles-Pérez, J.J.; Clemente-Suárez, V.J. Effect of Combat Stress in the Psychophysiological Response of Elite and Non-Elite Soldiers. J. Med. Syst. 2017, 41, 100. [Google Scholar] [CrossRef]
- Tait, J.L.; Drain, J.R.; Corrigan, S.L.; Drake, J.M.; Main, L.C. Impact of military training stress on hormone response and recovery. PLoS ONE 2022, 17, e0265121. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.E.; Bernards, J.R.; Jameson, J.T.; Johnson, D.C.; Kelly, K.R. The Benefit of Mental Skills Training on Performance and Stress Response in Military Personnel. Front. Psychol. 2019, 10, 2964. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.L.; Russo, M.B. Neurocognitive monitors: Toward the prevention of cognitive performance decrements and catastrophic failures in the operational environment. Aviat. Space Environ. Med. 2007, 78, B144–B152. [Google Scholar]
- Taylor, M.K.; Sausen, K.P.; Mujica-Parodi, L.R.; Potterat, E.G.; Yanagi, M.A.; Kim, H. Neurophysiologic methods to measure stress during survival, evasion, resistance, and escape training. Aviat. Space Environ. Med. 2007, 78, B224–B230. [Google Scholar]
- Bustamante-Sánchez, Á.; Tornero-Aguilera, J.F.; Fernández-Elías, V.E.; Hormeño-Holgado, A.J.; Dalamitros, A.A.; Clemente-Suárez, V.J. Effect of Stress on Autonomic and Cardiovascular Systems in Military Population: A Systematic Review. Cardiol. Res. Pract. 2020, 2020, 7986249. [Google Scholar] [CrossRef]
- Passi, T.; Lukander, K.; Laarni, J.; Närväinen, J.; Rissanen, J.; Vaara, J.P.; Pihlainen, K.; Kallinen, K.; Ojanen, T.; Mauno, S.; et al. Effects of overnight military training and acute battle stress on the cognitive performance of soldiers in simulated urban combat. Front. Psychol. 2022, 13, 925157. [Google Scholar] [CrossRef]
- Kettunen, O.; Kyröläinen, H.; Santtila, M.; Vuorimaa, T.; Vasankari, T.J. Greater levels of cardiorespiratory and muscular fitness are associated with low stress and high mental resources in normal but not overweight men. BMC Public Health 2016, 16, 788. [Google Scholar] [CrossRef]
- Conkright, W.R.; Beckner, M.E.; Sinnott, A.M.; Eagle, S.R.; Martin, B.J.; Lagoy, A.D.; Proessl, F.; Lovalekar, M.; Doyle, T.L.; Agostinelli, P.; et al. Neuromuscular Performance and Hormonal Responses to Military Operational Stress in Men and Women. J. Strength Cond. Res. 2021, 35, 1296–1305. [Google Scholar] [CrossRef]
- Beckner, M.E.; Conkright, W.R.; Eagle, S.R.; Martin, B.J.; Sinnott, A.M.; LaGoy, A.D.; Proessl, F.; Lovalekar, M.; Jabloner, L.R.; Roma, P.G.; et al. Impact of simulated military operational stress on executive function relative to trait resilience, aerobic fitness, and neuroendocrine biomarkers. Physiol. Behav. 2021, 236, 113413. [Google Scholar] [CrossRef]
- Szivak, T.K.; Lee, E.C.; Saenz, C.; Flanagan, S.D.; Focht, B.C.; Volek, J.S.; Maresh, C.M.; Kraemer, W.J. Adrenal Stress and Physical Performance During Military Survival Training. Aerosp. Med. Hum. Perform. 2018, 89, 99–107. [Google Scholar] [CrossRef]
- Taylor, M.K.; Markham, A.E.; Reis, J.P.; Padilla, G.A.; Potterat, E.G.; Drummond, S.P.A.; Mujica-Parodi, L.R. Physical fitness influences stress reactions to extreme military training. Mil. Med. 2008, 173, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Szivak, T.K. Strength training for the warfighter. J. Strength Cond. Res. 2012, 26, S107–S118. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, W.J.; Ratamess, N.A.; Flanagan, S.D.; Shurley, J.P.; Todd, J.S.; Todd, T.C. Understanding the Science of Resistance Training: An Evolutionary Perspective. Sports Med. 2017, 47, 2415–2435. [Google Scholar] [CrossRef] [PubMed]
- Friedl, K.E.; Knapik, J.J.; Häkkinen, K.; Baumgartner, N.; Groeller, H.; Taylor, N.A.S.; Duarte, A.F.; Kyröläinen, H.; Jones, B.H.; Kraemer, W.J.; et al. Perspectives on Aerobic and Strength Influences on Military Physical Readiness: Report of an International Military Physiology Roundtable. J. Strength Cond. Res. 2015, 29, S10–S23. [Google Scholar] [CrossRef] [PubMed]
- Abt, J.P.; Oliver, J.M.; Nagai, T.; Sell, T.C.; Lovalekar, M.T.; Beals, K.; Wood, D.E.; Lephart, S.M. Block-Periodized Training Improves Physiological and Tactically Relevant Performance in Naval Special Warfare Operators. J. Strength Cond. Res. 2016, 30, 39–52. [Google Scholar] [CrossRef] [PubMed]
- Vantarakis, A.; Chatzinikolaou, A.; Avloniti, A.; Vezos, N.; Douroudos, I.I.; Draganidis, D.; Jamurtas, A.Z.; Kambas, A.; Kalligeros, S.; Fatouros, I.G. A 2-Month Linear Periodized Resistance Exercise Training Improved Musculoskeletal Fitness and Specific Conditioning of Navy Cadets. J. Strength Cond. Res. 2017, 31, 1362–1370. [Google Scholar] [CrossRef]
- Heilbronn, B.E.; Doma, K.; Gormann, D.; Schumann, M.; Sinclair, W.H. Effects of Periodized vs. Nonperiodized Resistance Training on Army-Specific Fitness and Skills Performance. J. Strength Cond. Res. 2020, 34, 738–753. [Google Scholar] [CrossRef] [PubMed]
- Vrijkotte, S.; Roelands, B.; Pattyn, N.; Meeusen, R. The Overtraining Syndrome in Soldiers: Insights from the Sports Domain. Mil. Med. 2019, 184, e192–e200. [Google Scholar] [CrossRef]
- Rosendal, L.; Langberg, H.; Skov-Jensen, A.; Kjaer, M. Incidence of injury and physical performance adaptations during military training. Clin. J. Sport. Med. 2003, 13, 157–163. [Google Scholar] [CrossRef]
- Nikolaidis, P.T.; Papaioannou, K.G.; Rosemann, T.; Knechtle, B. Exercise Testing of Muscle Strength in Military. Mil. Med. 2019, 184, e426–e430. [Google Scholar] [CrossRef]
- Merrigan, J.J.; Stone, J.D.; Thompson, A.G.; Hornsby, W.G.; Hagen, J.A. Monitoring Neuromuscular Performance in Military Personnel. Int. J. Environ. Res. Public Health 2020, 17, 9147. [Google Scholar] [CrossRef]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1971. [Google Scholar]
- Kellmann, M.; Kallus, K.W. Der Erholungs-Belastungsfragebogen für Sportler (EBF-Sport)—Manual [Recovery-Stress Questionnaire for Athletes. Manual]; Swets Test Service: Frankfurt, Germany, 2000. [Google Scholar]
- McDonald, D.G.; Norton, J.P.; Hodgdon, J.A. Training success in U.S. Navy special forces. Aviat. Space Environ. Med. 1990, 61, 548–554. [Google Scholar]
- Knapik, J.; Staab, J.; Bahrke, M.; Reynolds, K.; Vogel, J.; O’Connor, J. Soldier performance and mood states following a strenuous road march. Mil. Med. 1991, 156, 197–200. [Google Scholar] [CrossRef]
- McNair, D.M.; Lorr, M.; Droppleman, L.F. Revised Manual for the Profile of Mood States; Educational and Industrial Testing Services: San Diego, CA, USA, 1992. [Google Scholar]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Markovic, G.; Dizdar, D.; Jukic, I.; Cardinale, M. Reliability and factorial validity of squat and countermovement jump tests. J. Strength Cond. Res. 2004, 18, 551–555. [Google Scholar]
- Hébert-Losier, K.; Beaven, C.M. The MARS for squat, countermovement, and standing long jump performance analyses: Are measures reproducible? J. Strength Cond. Res. 2014, 28, 1849–1857. [Google Scholar] [CrossRef]
- Grgic, J.; Scapec, B.; Mikulic, P.; Pedisic, Z. Test-retest reliability of isometric mid-thigh pull maximum strength assessment: A systematic review. Biol. Sport 2022, 39, 407–414. [Google Scholar] [CrossRef]
- Sheppard, J.M.; Chapman, D.; Taylor, K.L. An Evaluation of a Strength Qualities Assessment Method for the Lower Body. JASC 2011, 19, 4–10. [Google Scholar]
- Leyk, D.; Gorges, W.; Ridder, D.; Wunderlich, M.; Rüther, T.; Sievert, A.; Essfeld, D. Hand-grip strength of young men, women and highly trained female athletes. Eur. J. Appl. Physiol. 2007, 99, 415–421. [Google Scholar] [CrossRef]
- Canino, M.C.; Foulis, S.A.; Zambraski, E.J.; Cohen, B.S.; Redmond, J.E.; Hauret, K.G.; Frykman, P.N.; Sharp, M.A. U.S. Army Physical Demands Study: Differences in Physical Fitness and Occupational Task Performance Between Trainees and Active Duty Soldiers. J. Strength Cond. Res. 2019, 33, 1864–1870. [Google Scholar] [CrossRef] [PubMed]
- Hauschild, V.D.; DeGroot, D.W.; Hall, S.M.; Grier, T.L.; Deaver, K.D.; Hauret, K.G.; Hauret, K.G.; Jones, B.H. Fitness tests and occupational tasks of military interest: A systematic review of correlations. Occup. Environ. Med. 2017, 74, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Moreno, M.; Pareja-Blanco, F.; Diaz-Cueli, D.; González-Badillo, J.J. Determinant factors of pull-up performance in trained athletes. J. Sports Med. Phys. Fit. 2016, 56, 825–833. [Google Scholar]
- Vanderburgh, P.M.; Edmonds, T. The Effect of Experimental Alterations in Excess Mass on Pull-up Performance in Fit Young Men. J. Strength Cond. Res. 1997, 11, 230–233. [Google Scholar]
- Mayhew, J.L.; Ball, T.E.; Arnold, M.D.; Bowen, J.C. Push-ups As a Measure of Upper Body Strength. J. Strength Cond. Res. 1991, 5, 16. [Google Scholar]
- Knapik, J.J.; Sharp, M.A.; Darakjy, S.; Jones, S.B.; Hauret, K.G.; Jones, B.H. Temporal changes in the physical fitness of US Army recruits. Sports Med. 2006, 36, 613–634. [Google Scholar] [CrossRef]
- Aandstad, A. Association Between Performance in Muscle Fitness Field Tests and Skeletal Muscle Mass in Soldiers. Mil. Med. 2020, 185, e839–e846. [Google Scholar] [CrossRef]
- Aandstad, A. Temporal changes in physical fitness in Norwegian male and female military conscripts between 2006 and 2020. Scand. J. Med. Sci. Sports 2023, 33, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Andrade Fernández, E.; Arce Fernández, C.; Seoane Pesqueira, G. Adaptación al español del cuestionario «perfil de los estados de ánimo» en una muestra de deportistas. Psicothema 2002, 14, 708–713. [Google Scholar]
- Arce, C.; Andrade, E.M.; Seoane, G. Problemas semánticos en la adaptación del POMS al castellano. Psicothema 2000, 12, S47–S51. [Google Scholar]
- Balaguer Solá, I.; Fuentes, I.; Meliá Navarro, J.L.; García Merita, M.L.; Pérez Recio, G. El perfil de los estados de ánimo (POMS): Baremo para estudiantes valencianos y su aplicación en el contexto deportivo. Revista de Psicología del Deporte 1993, 2, 39–52. [Google Scholar]
- González-Boto, R.; Salguero, A.; Tuero, C.; Márquez, S.; Kellmann, M. Spanish adaptation and analysis by structural equation modeling of an instrument for monitoring overtraining: The recovery-stress questionnaire (RESTQ-sport). SBP J. 2008, 36, 635–649. [Google Scholar] [CrossRef]
- Kellmann, M.; Kallus, K.W. “The Recovery-Stress Questionnaire for Athletes,” in The Recovery-Stress Questionnaires. In User Manual; Kallus, K.W., Kellmann, M., Eds.; Pearson: Frankfurt, Germany, 2016; pp. 86–131. [Google Scholar]
- Cronbach, L.J. Coefficient alpha and the internal structure of tests. Psychometrika 1951, 16, 297–334. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, W.G. Measures of reliability in sports medicine and science. Sports Med. 2000, 30, 1–15. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum: Hillsdale, NJ, USA, 1988; p. 567. [Google Scholar]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef]
- Bergeron, M.F.; Nindl, B.C.; Deuster, P.A.; Baumgartner, N.; Kane, S.F.; Kraemer, W.J.; Sexauer, L.R.; Thompson, W.R.; O’COnnor, F.G. Consortium for Health and Military Performance and American College of Sports Medicine consensus paper on extreme conditioning programs in military personnel. Curr. Sports Med. Rep. 2011, 10, 383–389. [Google Scholar] [CrossRef]
- Santtila, M.; Kyröläinen, H.; Häkkinen, K. Changes in maximal and explosive strength, electromyography, and muscle thickness of lower and upper extremities induced by combined strength and endurance training in soldiers. J. Strength Cond. Res. 2009, 23, 1300–1308. [Google Scholar] [CrossRef]
- Knapik, J.J.; Rieger, W.; Palkoska, F.; Van Camp, S.; Darakjy, S. United States Army physical readiness training: Rationale and evaluation of the physical training doctrine. J. Strength Cond. Res. 2009, 23, 1353–1362. [Google Scholar] [CrossRef]
- Armstrong, L.E.; Bergeron, M.F.; Lee, E.C.; Mershon, J.E.; Armstrong, E.M. Overtraining Syndrome as a Complex Systems Phenomenon. Front. Netw. Physiol. 2022, 1, 794392. [Google Scholar] [CrossRef]
- Corrigan, S.L.; Bulmer, S.; Roberts, S.S.H.; Warmington, S.; Drain, J.; Main, L.C. Monitoring Responses to Basic Military Training with Heart Rate Variability. Med. Sci. Sports Exerc. 2022, 54, 1506–1514. [Google Scholar] [CrossRef]
- Bustos, D.; Guedes, J.C.; Vaz, M.P.; Pombo, E.; Fernandes, R.J.; Costa, J.T.; Baptista, J.S. Non-Invasive Physiological Monitoring for Physical Exertion and Fatigue Assessment in Military Personnel: A Systematic Review. Int. J. Environ. Res. Public. Health 2021, 18, 8815. [Google Scholar] [CrossRef]
- González Boto, R.; Salguero del Valle, A.; Tuero del Prado, C.; Márquez, S. Validez concurrente de la versión española del cuestionario de recuperación-estrés para deportistas (RESTQ-Sport). Revista de Psicología del Deporte 2009, 18, 53–72. [Google Scholar]
- Reynoso-Sánchez, L.F.; Pérez-Verduzco, G.; Celestino-Sánchez, M.Á.; López-Walle, J.M.; Zamarripa, J.; Rangel-Colmenero, B.R.; Muñoz-Helú, H.; Hernández-Cruz, G. Competitive Recovery-Stress and Mood States in Mexican Youth Athletes. Front. Psychol. 2020, 11, 627828. [Google Scholar] [CrossRef] [PubMed]
- Vaara, J.P.; Groeller, H.; Drain, J.; Kyröläinen, H.; Pihlainen, K.; Ojanen, T.; Connaboy, C.; Santtila, M.; Agostinelli, P.; Nindl, B.C. Physical training considerations for optimizing performance in essential military tasks. Eur. J. Sport. Sci. 2022, 22, 43–57. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.N.; Young, M.D.; Crum, A.J. Stress, Mindsets, and Success in Navy SEALs Special Warfare Training. Front. Psychol. 2019, 10, 2962. [Google Scholar] [CrossRef]
- Gibson, N.; Drain, J.R.; Larsen, P.; Michael, S.; Groeller, H.; Sampson, J.A. A Comprehensive Analysis of Injuries During Army Basic Military Training. Mil. Med. 2024, 189, 652–660. [Google Scholar] [CrossRef]
- de Lira, C.A.B.; Morais, N.S.; Menezes, V.A.; Andrade, M.S.; Vancini, R.L.; Braz, A.G.; Malysz, T. Identifying mood disorders and health-related quality of life of individuals submitted to mandatory military service. Acta Neuropsychiatr. 2021, 33, 9–14. [Google Scholar] [CrossRef]
- Knapik, J.J.; Johnson, R.F.; Ang, P.; Meiselman, H.L.; Bensel, C.K.; Johnson, W.; Flynn, B.; Hanlon, W.; Kirk, J.; Harman, E.; et al. Road March Performance of Special Operations Soldiers Carrying Various Loads and Load Distributions; Technical Report: T14–93; US Army Research Institute of Environmental Medicine: Natick, MA, USA, 1993. [Google Scholar]
- Gnacinski, S.L.; Meyer, B.B.; Wahl, C.A. Psychometric Properties of the RESTQ-Sport-36 in a Collegiate Student-Athlete Population. Front. Psychol. 2021, 12, 671919. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.C.; Kotwal, R.S.; Forsten, R.D. Preparing soldiers for the stress of combat. J. Spec. Oper. Med. 2012, 12, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Suarez, V.J.; Palomera, P.R.; Robles-Pérez, J.J. Psychophysiological response to acute-high-stress combat situations in professional soldiers. Stress Health 2018, 34, 247–252. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cáceres-Diego, B.; Alcaraz, P.E.; Marín-Pagán, C. Neuromuscular and Psychological Performance Monitoring During One Season in Spanish Marine Corps. J. Funct. Morphol. Kinesiol. 2025, 10, 324. https://doi.org/10.3390/jfmk10030324
Cáceres-Diego B, Alcaraz PE, Marín-Pagán C. Neuromuscular and Psychological Performance Monitoring During One Season in Spanish Marine Corps. Journal of Functional Morphology and Kinesiology. 2025; 10(3):324. https://doi.org/10.3390/jfmk10030324
Chicago/Turabian StyleCáceres-Diego, Beltrán, Pedro E. Alcaraz, and Cristian Marín-Pagán. 2025. "Neuromuscular and Psychological Performance Monitoring During One Season in Spanish Marine Corps" Journal of Functional Morphology and Kinesiology 10, no. 3: 324. https://doi.org/10.3390/jfmk10030324
APA StyleCáceres-Diego, B., Alcaraz, P. E., & Marín-Pagán, C. (2025). Neuromuscular and Psychological Performance Monitoring During One Season in Spanish Marine Corps. Journal of Functional Morphology and Kinesiology, 10(3), 324. https://doi.org/10.3390/jfmk10030324







