Effects of Clenching Strength on Step Reaction Time
Abstract
1. Introduction
2. Materials and Methods
2.1. Subject
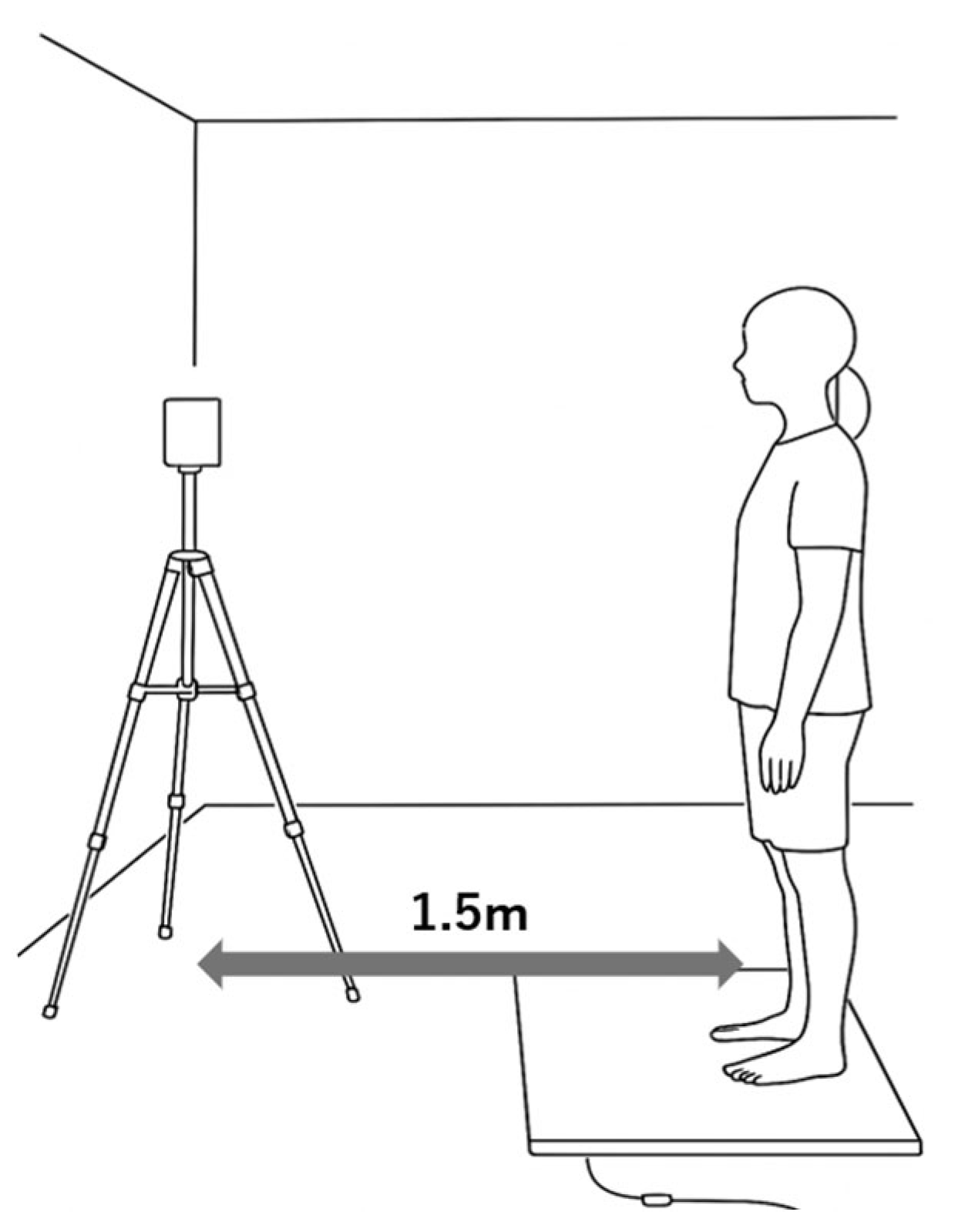
2.2. Electromyography Measurement (EMG)
2.3. Clenching Conditions
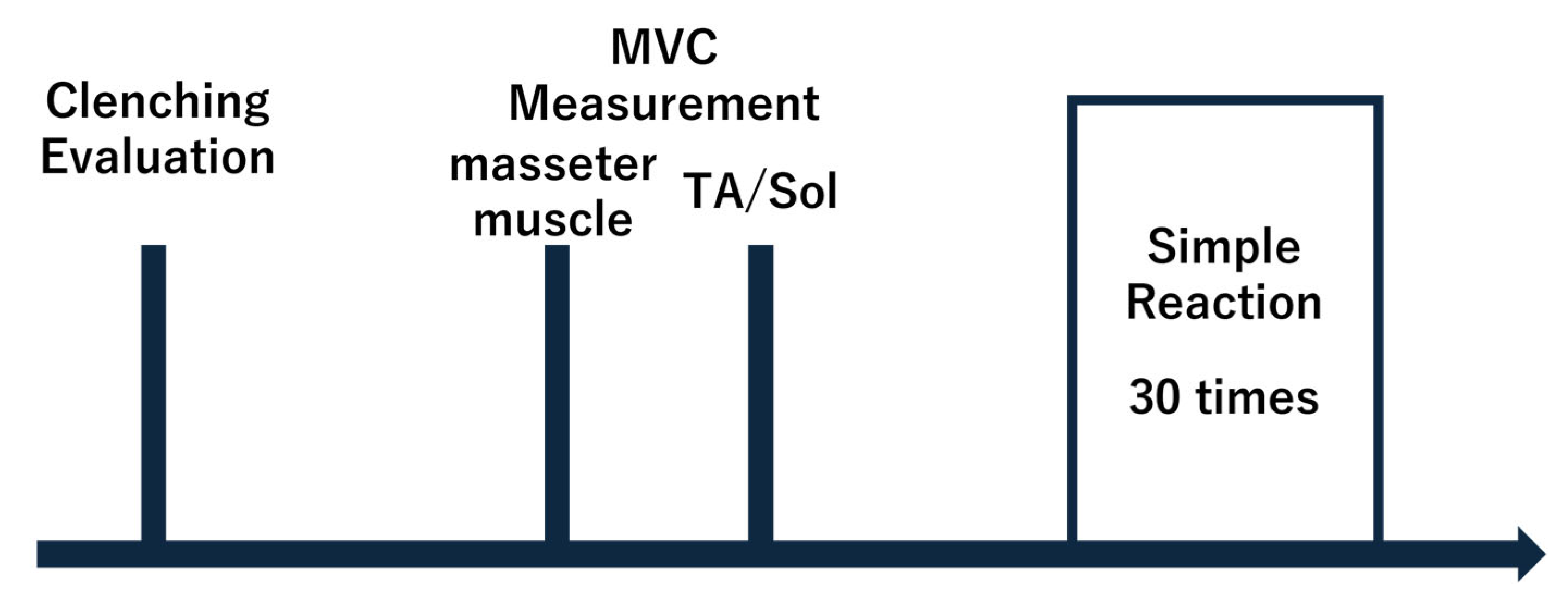
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
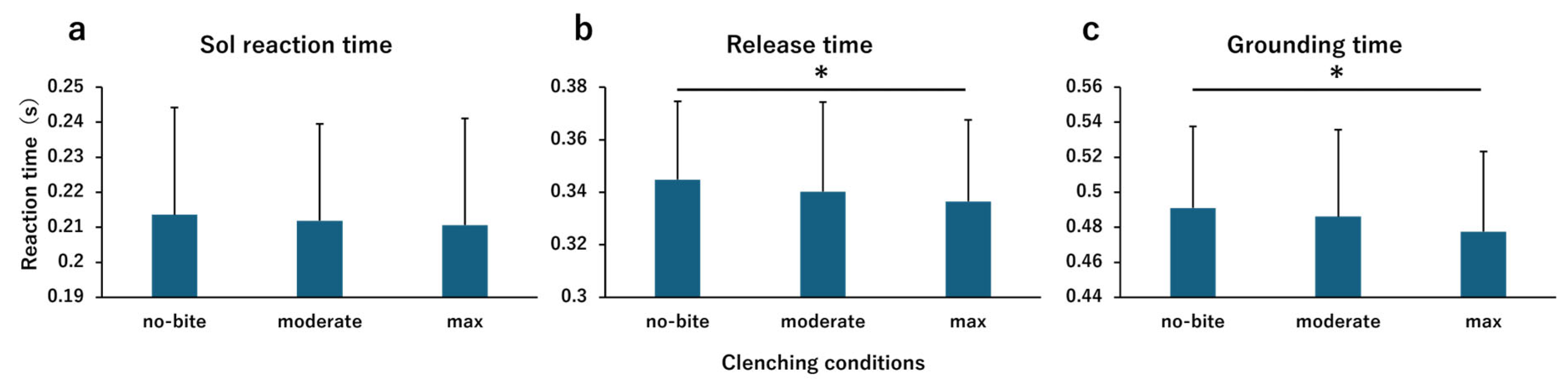
Comparison Between the Clenching Conditions
4. Discussion
4.1. Comparison Between the Clenching Conditions
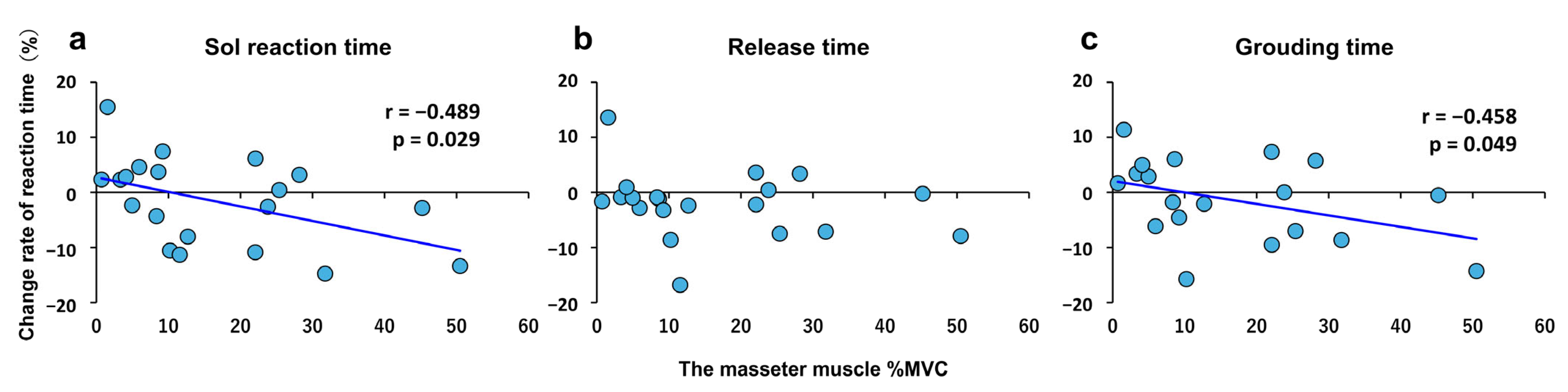
4.2. Relationship Between Clenching Strength and Rate of Change in Reaction Time Under the Moderate Condition
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Paul, D.J.; Gabbett, T.J.; Nassis, G.P. Agility in Team Sports: Testing, Training and Factors Affecting Performance. Sports Med. 2016, 46, 421–442. [Google Scholar] [CrossRef] [PubMed]
- Nuri, L.; Shadmehr, A.; Ghotbi, N.; Attarbashi Moghadam, B. Reaction time and anticipatory skill of athletes in open and closed skill-dominated sport. Eur. J. Sport. Sci. 2013, 13, 431–436. [Google Scholar] [CrossRef]
- Żak, M.; Mikrut, G.; Sobota, G. Measurement of Simple Reaction Time of the Cyclist in the Laboratory and Natural Environment Condition. Sensors 2023, 23, 3898. [Google Scholar] [CrossRef] [PubMed]
- Kennefick, M.; Maslovat, D.; Carlsen, A.N. The time course of corticospinal excitability during a simple reaction time task. PLoS ONE 2014, 9, e113563. [Google Scholar] [CrossRef] [PubMed]
- Tønnessen, E.; Haugen, T.; Shalfawi, S.A. Reaction time aspects of elite sprinters in athletic world championships. J. Strength Cond. Res. 2013, 27, 885–892. [Google Scholar] [CrossRef]
- Neto, E.M.; da Silva, E.A.; Nunes, H.R.C.; Bazan, R.; de Souza, L.; Luvizutto, G.J. Effect of transcranial direct current stimulation in addition to visuomotor training on choice reaction time and cognition function in amateur soccer players (FAST trial): A randomized control trial. Neurosci. Lett. 2022, 766, 136346. [Google Scholar] [CrossRef]
- Collet, C. Strategic aspects of reaction time in world-class sprinters. Percept. Mot. Skills 1999, 88, 65–75. [Google Scholar] [CrossRef]
- Akintunde, A.; Buxton, D.F. Differential sites of origin and collateralization of corticospinal neurons in the rat: A multiple fluorescent retrograde tracer study. Brain Res. 1992, 575, 86–92. [Google Scholar] [CrossRef]
- Kamiyama, T.; Kameda, H.; Murabe, N.; Fukuda, S.; Yoshioka, N.; Mizukami, H.; Ozawa, K.; Sakurai, M. Corticospinal tract development and spinal cord innervation differ between cervical and lumbar targets. J. Neurosci. 2015, 35, 1181–1191. [Google Scholar] [CrossRef]
- Maier, M.A.; Armand, J.; Kirkwood, P.A.; Yang, H.W.; Davis, J.N.; Lemon, R.N. Differences in the corticospinal projection from primary motor cortex and supplementary motor area to macaque upper limb motoneurons: An anatomical and electrophysiological study. Cereb. Cortex 2002, 12, 281–296. [Google Scholar] [CrossRef]
- Kutukcu, Y.; Marks, W.J., Jr.; Goodin, D.S.; Aminoff, M.J. Simple and choice reaction time in Parkinson’s disease. Brain Res. 1999, 815, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Zangrandi, A.; Mioli, A.; D’Alonzo, M.; Formica, D.; Pellegrino, G.; Di Pino, G. Conditioning transcranial magnetic stimulation of ventral premotor cortex shortens simple reaction time. Cortex 2019, 121, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Devanathan, D.; Madhavan, S. Effects of anodal tDCS of the lower limb M1 on ankle reaction time in young adults. Exp. Brain Res. 2016, 234, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Holtermann, A.; Roeleveld, K.; Engstrøm, M.; Sand, T. Enhanced H-reflex with resistance training is related to increased rate of force development. Eur. J. Appl. Physiol. 2007, 101, 301–312. [Google Scholar] [CrossRef]
- Molero-Chamizo, A.; Alameda Bailén, J.R.; Garrido Béjar, T.; García López, M.; Jaén Rodríguez, I.; Gutiérrez Lérida, C.; Pérez Panal, S.; González Ángel, G.; Lemus Corchero, L.; Ruiz Vega, M.J.; et al. Poststimulation time interval-dependent effects of motor cortex anodal tDCS on reaction-time task performance. Cogn. Affect. Behav. Neurosci. 2018, 18, 167–175. [Google Scholar] [CrossRef]
- Hirabayashi, R.; Edama, M.; Saito, A.; Yamada, Y.; Nawa, R.; Onishi, H. Effects of Clenching Strength on Exercise Performance: Verification Using Spinal Function Assessments. Sports Health 2022, 14, 404–414. [Google Scholar] [CrossRef]
- Buscà, B.; Moreno-Doutres, D.; Peña, J.; Morales, J.; Solana-Tramunt, M.; Aguilera-Castells, J. Effects of jaw clenching wearing customized mouthguards on agility, power and vertical jump in male high-standard basketball players. J. Exerc. Sci. Fit. 2018, 16, 5–11. [Google Scholar] [CrossRef]
- Ebben, W.P.; Flanagan, E.P.; Jensen, R.L. Jaw clenching results in concurrent activation potentiation during the countermovement jump. J. Strength Cond. Res. 2008, 22, 1850–1854. [Google Scholar] [CrossRef]
- Nakamura, T.; Yoshida, Y.; Churei, H.; Aizawa, J.; Hirohata, K.; Ohmi, T.; Ohji, S.; Takahashi, T.; Enomoto, M.; Ueno, T.; et al. The Effect of Teeth Clenching on Dynamic Balance at Jump-Landing: A Pilot Study. J. Appl. Biomech. 2017, 33, 211–215. [Google Scholar] [CrossRef]
- Nukaga, H.; Takeda, T.; Nakajima, K.; Narimatsu, K.; Ozawa, T.; Ishigami, K.; Funato, K. Masseter Muscle Activity in Track and Field Athletes: A Pilot Study. Open Dent. J. 2016, 10, 474–485. [Google Scholar] [CrossRef][Green Version]
- Ebben, W.P. A brief review of concurrent activation potentiation: Theoretical and practical constructs. J. Strength Cond. Res. 2006, 20, 985–991. [Google Scholar] [CrossRef]
- De Cicco, V.; Tramonti Fantozzi, M.P.; Cataldo, E.; Barresi, M.; Bruschini, L.; Faraguna, U.; Manzoni, D. Trigeminal, Visceral and Vestibular Inputs May Improve Cognitive Functions by Acting through the Locus Coeruleus and the Ascending Reticular Activating System: A New Hypothesis. Front. Neuroanat. 2018, 11, 130. [Google Scholar] [CrossRef] [PubMed]
- Ertuglu, L.A.; Karacan, I.; Yilmaz, G.; Türker, K.S. Standardization of the Jendrassik maneuver in Achilles tendon tap reflex. Clin. Neurophysiol. Pract. 2017, 3, 1–5. [Google Scholar] [CrossRef]
- Yamada, Y.; Hirabayashi, R.; Okada, Y.; Yokota, H.; Sekine, C.; Edama, M. Effects of remote facilitation on ankle joint movement: Focusing on occlusal strength and balance. Health Sci. Rep. 2023, 6, e1098. [Google Scholar] [CrossRef]
- Aagaard, P.; Simonsen, E.B.; Andersen, J.L.; Magnusson, P.; Dyhre-Poulsen, P. Neural adaptation to resistance training: Changes in evoked V-wave and H-reflex responses. J. Appl. Physiol. (1985) 2002, 92, 2309–2318. [Google Scholar] [CrossRef] [PubMed]
- Vangsgaard, S.; Taylor, J.L.; Hansen, E.A.; Madeleine, P. Changes in H reflex and neuromechanical properties of the trapezius muscle after 5 weeks of eccentric training: A randomized controlled trial. J. Appl. Physiol. (1985) 2014, 116, 1623–1631. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Arima, T.; Takeuchi, T.; Honda, K.; Tomonaga, A.; Tanosoto, T.; Ohata, N.; Svensson, P. Effects of interocclusal distance on bite force and masseter EMG in healthy participants. J. Oral Rehabil. 2013, 40, 900–908. [Google Scholar] [CrossRef]
- Kumagai, H.; Suzuki, T.; Hamada, T.; Sondang, P.; Fujitani, M.; Nikawa, H. Occlusal force distribution on the dental arch during various levels of clenching. J. Oral Rehabil. 1999, 26, 932–935. [Google Scholar] [CrossRef]
- Lord, S.R.; Fitzpatrick, R.C. Choice stepping reaction time: A composite measure of falls risk in older people. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M627–M632. [Google Scholar] [CrossRef]
- Tisserand, R.; Robert, T.; Chabaud, P.; Bonnefoy, M.; Chèze, L. Elderly Fallers Enhance Dynamic Stability Through Anticipatory Postural Adjustments during a Choice Stepping Reaction Time. Front. Hum. Neurosci. 2016, 10, 613. [Google Scholar] [CrossRef]
- Björkquist, S.E. An EMG study of simple reaction time. Scand. J. Rehabil. Med. Suppl. 1974, 3, 109–114. [Google Scholar]
- Meijers, L.M.; Eijkman, E.G. The motor system in simple reaction time experiments. Acta Psychol. 1974, 38, 367–377. [Google Scholar] [CrossRef]
- Smith, A.M.; Hepp-Reymond, M.C.; Wyss, U.R. Relation of activity in precentral cortical neurons to force and rate of force change during isometric contractions of finger muscles. Exp. Brain Res. 1975, 23, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Evarts, E.V. Relation of pyramidal tract activity to force exerted during voluntary movement. J. Neurophysiol. 1968, 31, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Ebben, W.P.; Kaufmann, C.E.; Fauth, M.L.; Petushek, E.J. Kinetic analysis of concurrent activation potentiation during back squats and jump squats. J. Strength Cond. Res. 2010, 24, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Peters, R.M.; Dalton, B.H.; Blouin, J.S.; Inglis, J.T. Precise coding of ankle angle and velocity by human calf muscle spindles. Neuroscience 2017, 349, 98–105. [Google Scholar] [CrossRef]
- Héroux, M.E.; Dakin, C.J.; Luu, B.L.; Inglis, J.T.; Blouin, J.S. Absence of lateral gastrocnemius activity and differential motor unit behavior in soleus and medial gastrocnemius during standing balance. J. Appl. Physiol. (1985) 2014, 116, 140–148. [Google Scholar] [CrossRef]
- Kunugi, S.; Holobar, A.; Kodera, T.; Toyoda, H.; Watanabe, K. Motor unit firing patterns of lower leg muscles during isometric plantar flexion with flexed knee joint position. J. Electromyogr. Kinesiol. 2022, 67, 102720. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sugai, N.; Hirabayashi, R.; Okada, Y.; Yoshida, Y.; Okouchi, T.; Yokota, H.; Ishigaki, T.; Komiya, M.; Edama, M. Effects of Clenching Strength on Step Reaction Time. J. Funct. Morphol. Kinesiol. 2025, 10, 264. https://doi.org/10.3390/jfmk10030264
Sugai N, Hirabayashi R, Okada Y, Yoshida Y, Okouchi T, Yokota H, Ishigaki T, Komiya M, Edama M. Effects of Clenching Strength on Step Reaction Time. Journal of Functional Morphology and Kinesiology. 2025; 10(3):264. https://doi.org/10.3390/jfmk10030264
Chicago/Turabian StyleSugai, Nao, Ryo Hirabayashi, Yoshiyuki Okada, Yuriko Yoshida, Takeru Okouchi, Hirotake Yokota, Tomonobu Ishigaki, Makoto Komiya, and Mutsuaki Edama. 2025. "Effects of Clenching Strength on Step Reaction Time" Journal of Functional Morphology and Kinesiology 10, no. 3: 264. https://doi.org/10.3390/jfmk10030264
APA StyleSugai, N., Hirabayashi, R., Okada, Y., Yoshida, Y., Okouchi, T., Yokota, H., Ishigaki, T., Komiya, M., & Edama, M. (2025). Effects of Clenching Strength on Step Reaction Time. Journal of Functional Morphology and Kinesiology, 10(3), 264. https://doi.org/10.3390/jfmk10030264






