A Transcutaneous Randomized Pulsed Radiofrequency Application for Spine Pain Conditions: A Case Series
Abstract
1. Introduction
- Systemically, by placing the electrodes over the axillary artery and the forearm, and all the immune cells in the blood pass through the PRF electric field and are exposed, having an effect in the whole body.
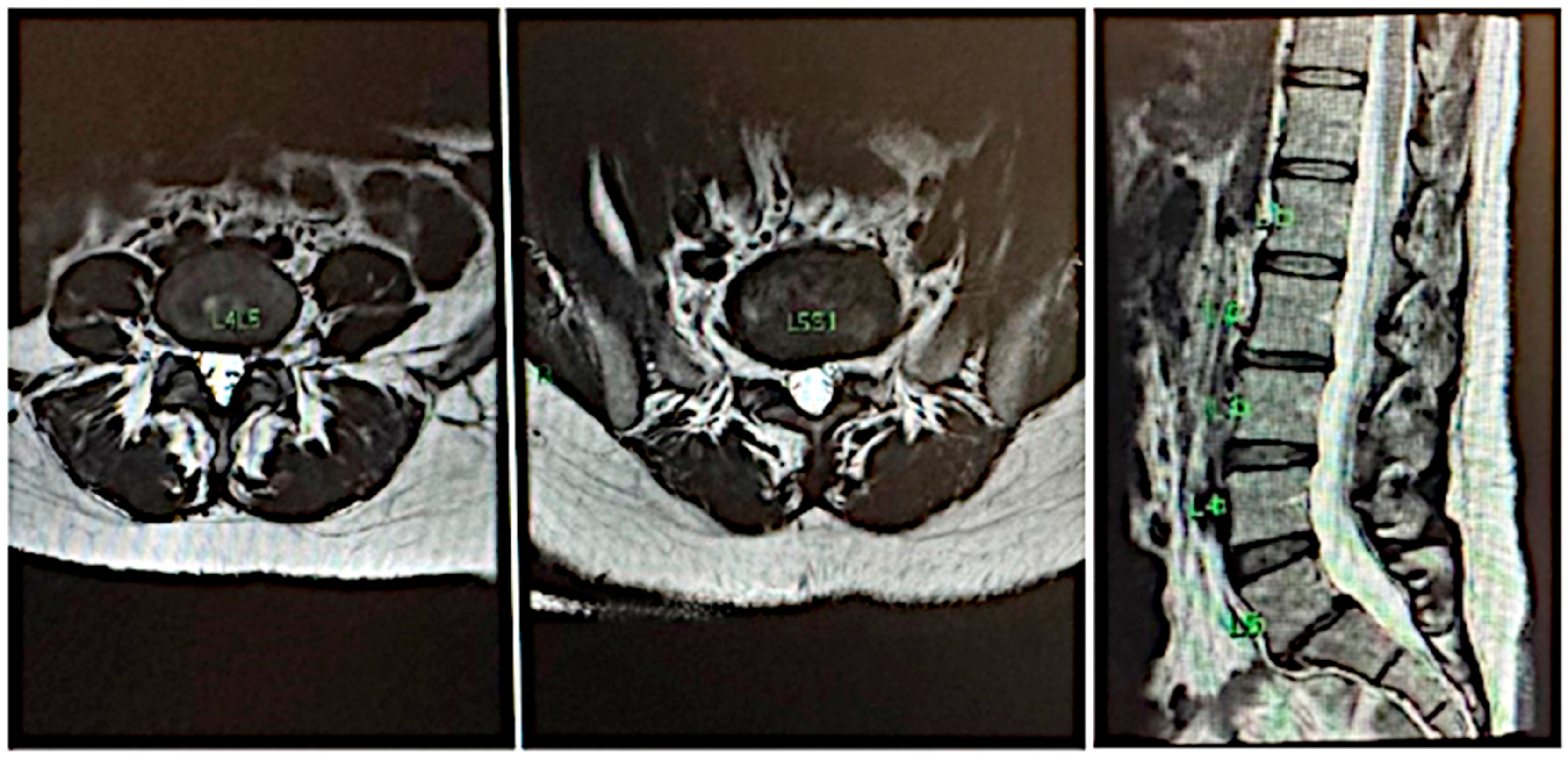
2. CASE 1: Lumbar Root Pain
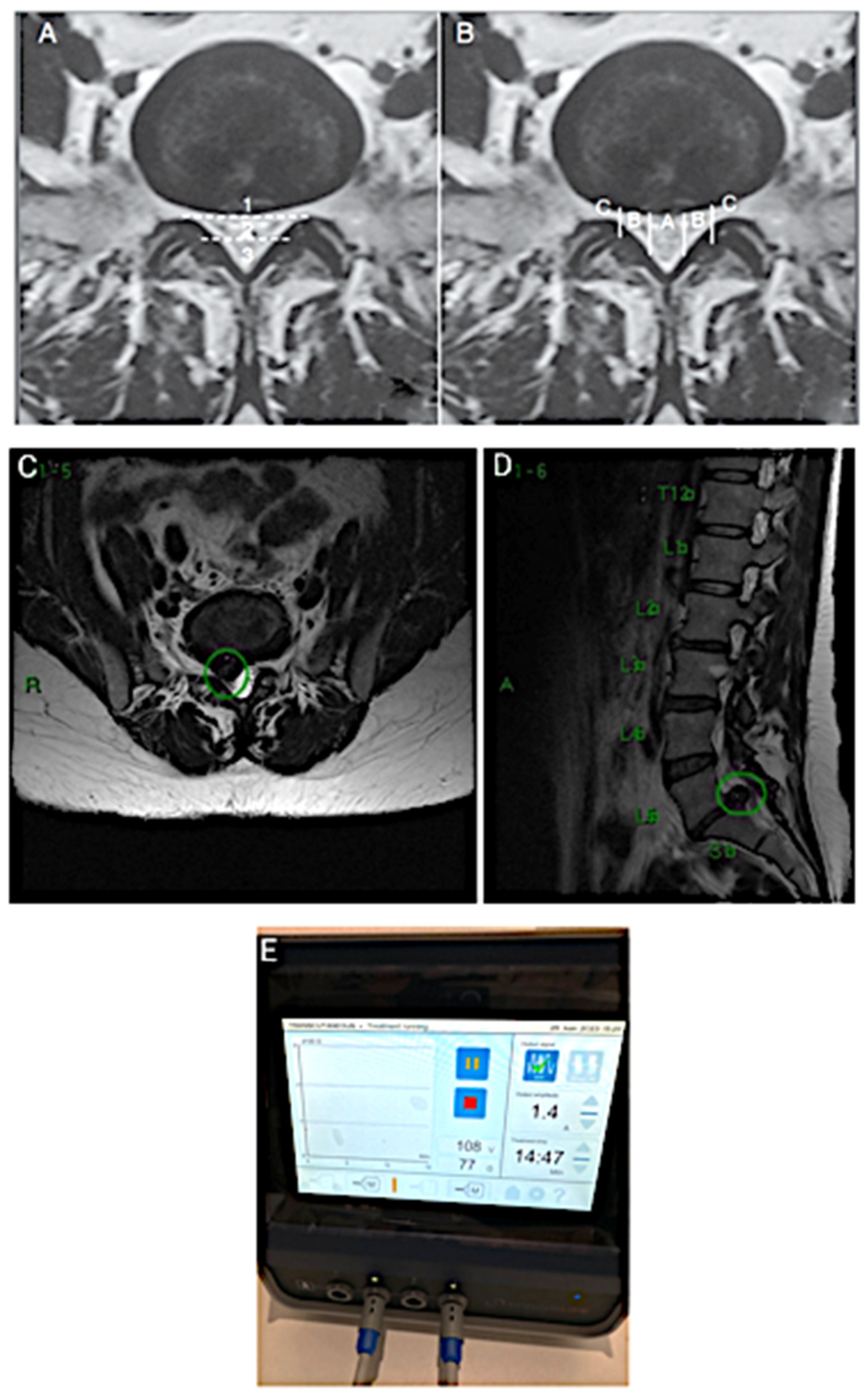
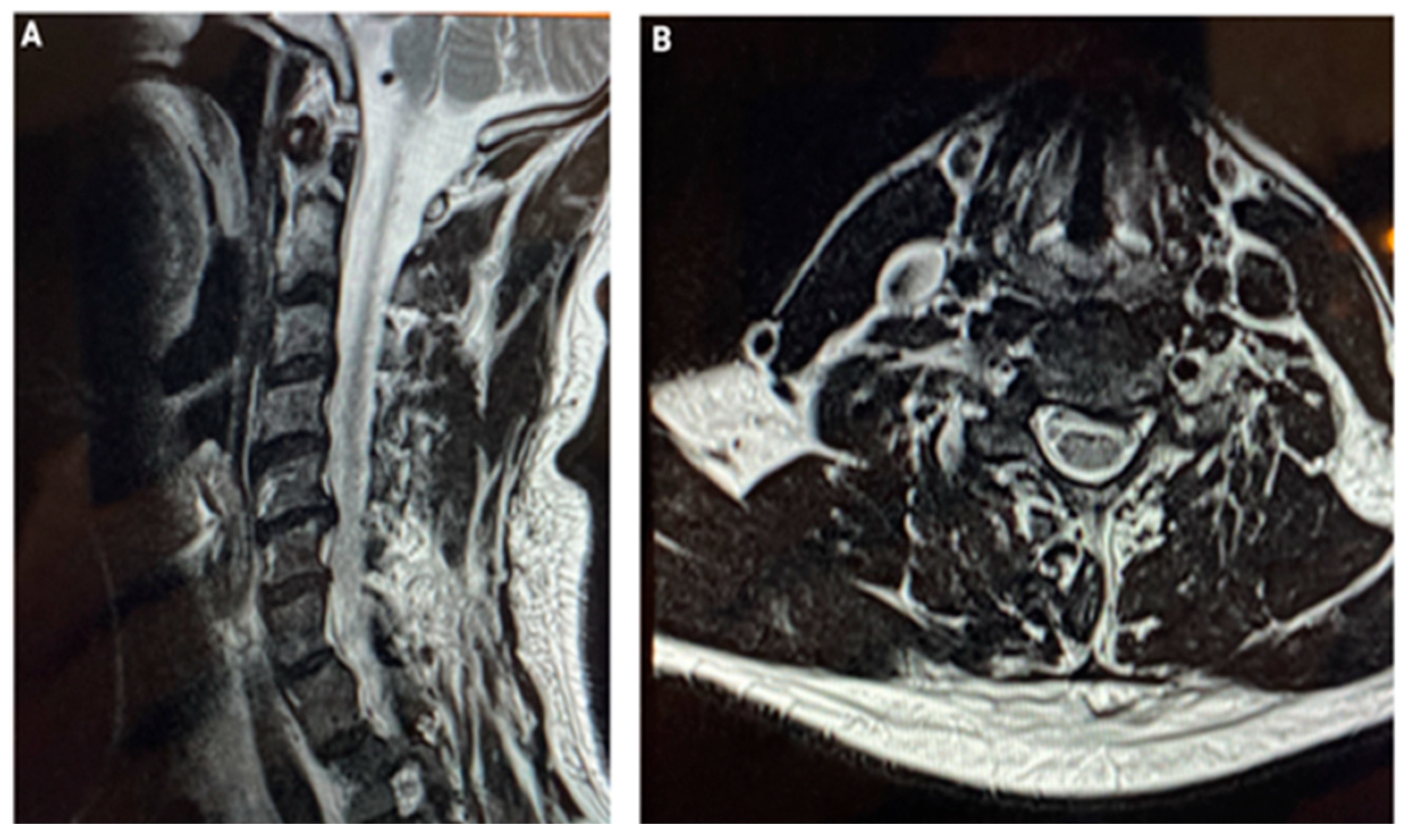
3. CASE 2: Cervical Spine Degenerative Disease
4. CASE 3: Lumbar Disc with Compression and Central Stenosis
5. Results
6. Discussion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huang, R.Y.; Liao, C.C.; Tsai, S.Y.; Yen, C.T.; Lin, C.W.; Chen, T.C.; Lin, W.T.; Chang, C.H.; Wen, Y.R. Rapid and Delayed Effects of Pulsed Radiofrequency on Neuropathic Pain: Electrophysiological, Molecular, and Behavioral Evidence Supporting Long-Term Depression. Pain Physician 2017, 20, E269–E283. [Google Scholar] [PubMed]
- Vanneste, T.; Van Lantschoot, A.; Van Boxem, K.; Van Zundert, J. Pulsed radiofrequency in chronic pain. Curr. Opin. Anaesthesiol. 2017, 30, 577–582. [Google Scholar] [CrossRef] [PubMed]
- Chakour, M.C.; Gibson, S.J.; Bradbeer, M.; Helme, R.D. The effect of age on A delta- and C-fibre thermal pain perception. Pain 1996, 64, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, M.E.; van Kleef, M. Characteristics and mode of action of radiofrequency lesions. Curr. Rev. Pain 1998, 2, 143–150. [Google Scholar] [CrossRef]
- Cui, Y.; Shen, H.; Chen, Y.; Zhang, W.; Zhu, J.; Duan, Z.; Liao, Z.; Weiqiang, L. Study on the process of intervertebral disc disease by the theory of continuum damage mechanics. Clin. Biomech. 2022, 98, 105738. [Google Scholar] [CrossRef] [PubMed]
- Me, S. The effect of pulsed radiofrequency fields applied to the dorsal root ganglion: A preliminary report. Pain Clin. 1998, 11, 109–117. [Google Scholar]
- Cho, H.K.; Cho, Y.W.; Kim, E.H.; Sluijter, M.E.; Hwang, S.J.; Ahn, S.H. Changes in pain behavior and glial activation in the spinal dorsal horn after pulsed radiofrequency current administration to the dorsal root ganglion in a rat model of lumbar disc herniation: Laboratory investigation. J. Neurosurg. Spine 2013, 19, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Sluijter, M.E.; Teixeira, A.; Serra, V.; Balogh, S.; Schianchi, P. Intra-articular application of pulsed radiofrequency for arthrogenic pain--report of six cases. Pain Pract. 2008, 8, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Erdine, S.; Bilir, A.; Cosman, E.R.; Cosman, E.R., Jr. Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract. 2009, 9, 407–417, Erratum in Pain Pract. 2010, 10, 264. [Google Scholar] [CrossRef] [PubMed]
- Filippiadis, D.; Tsochatzis, A.; Petsatodis, E.; Galanis, S.; Velonakis, G.; Giankoulof, C.; Kelekis, A. Intra-Articular Application of Sluijter-Teixera Poisson Pulsed Radiofrequency in Symptomatic Patients with Knee Osteoarthritis: Focus upon Clinical Efficacy and Safety. Pain Res. Manag. 2021, 2021, 5554631. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schianchi, P.M.; Sluijter, M.E.; Balogh, S.E. The Treatment of Joint Pain with Intra-articular Pulsed Radiofrequency. Anesth. Pain Med. 2013, 3, 250–255. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brasil, L.J.; Marroni, N.; Schemitt, E.; Colares, J. Effects of Pulsed Radiofrequency on a Standard Model of Muscle Injury in Rats. Anesth. Pain Med. 2020, 10, e97372. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sluijter, M.E.; Teixeira, A.; Vissers, K.; Brasil, L.J.; van Duijn, B. The Anti-Inflammatory Action of Pulsed Radiofrequency-A Hypothesis and Potential Applications. Med. Sci. 2023, 11, 58. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Papa, A.; Di Dato, M.T.; Lo Bianco, G.; Gazzerro, G.; Salzano, A.M.; Di Costanzo, E.; Tammaro, D.; Schatman, M.E.; Varrassi, G. Intraarticular STP Radiofrequency for Painful Osteoarthritis in the Knee: A Retrospective Single Center Analysis. J. Pain Res. 2021, 14, 2441–2447. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jorge, D.M.F.; Huber, S.C.; Rodrigues, B.L.; Da Fonseca, L.F.; Azzini, G.O.M.; Parada, C.A.; Paulus-Romero, C.; Lana, J.F.S.D. The Mechanism of Action between Pulsed Radiofrequency and Orthobiologics: Is There a Synergistic Effect? Int. J. Mol. Sci. 2022, 23, 11726. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sam, J.; Catapano, M.; Sahni, S.; Ma, F.; Abd-Elsayed, A.; Visnjevac, O. Pulsed Radiofrequency in Interventional Pain Management: Cellular and Molecular Mechanisms of Action—An Update and Review. Pain Physician 2021, 24, 525–532. [Google Scholar] [PubMed]
- Huygen, F.; Patijn, J.; Rohof, O.; Lataster, A.; Mekhail, N.; van Kleef, M.; Van Zundert, J. 9. Painful shoulder complaints. Pain Pract. 2010, 10, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Cahana, A.; Vutskits, L.; Muller, D. Acute differential modulation of synaptic transmission and cell survival during exposure to pulsed and continuous radiofrequency energy. J. Pain 2003, 4, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Rohof, O.J. Radiofrequency treatment of peripheral nerves. Pain Pract. 2002, 2, 257–260. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.; Sluijter, M.E. Intradiscal high-voltage, long-duration pulsed radiofrequency for discogenic pain: A preliminary report. Pain Med. 2006, 7, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Migliore, M.; Lansky, P. Computational model of the effects of stochastic conditioning on the induction of long-term potentiation and depression. Biol. Cybern. 1999, 81, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Ristmägi, A.; Jauhiainen, O.P.; Suominen, S.; Heikkilä, H.V. The efficacy of pulsed radiofrequency stimulation of the glenohumeral joint and suprascapular nerve for chronic shoulder pain and function compared to physiotherapy and exercise program without pain management. Arch. Clin. Trials. 2021, 1, 1–6. [Google Scholar] [CrossRef]
- Taverner, M.G.; Ward, T.L.; Loughnan, T.E. Transcutaneous Pulsed Radiofrequency Treatment in Patients with Painful Knee Awaiting Total Knee Joint Replacement. Clin. J. Pain 2010, 26, 429–432. [Google Scholar] [CrossRef]
- Teixeira, A.; Sluijter, M.E. Intravenous application of pulsed radiofrequency-4 case reports. Anesth. Pain Med. 2013, 3, 219–222. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cosman, E.R., Jr.; Cosman, E.R., Sr. Electric and thermal field effects in tissue around radiofrequency electrodes. Pain Med. 2005, 6, 405–424. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M. Electrical fields in wound healing-An overriding signal that directs cell migration. Semin. Cell Dev. Biol. 2009, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Taverner, M.; Loughnan, T. Transcutaneous pulsed radiofrequency treatment for patients with shoulder pain booked for surgery: A double-blind, randomized controlled trial. Pain Pract. 2014, 14, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Dwornik, M.; Puszczalowska-Lizis, E.; Wojcik, M.; Szajkowski, S.; Graczykowski, M.; Szymanski, D.; Marszalek, S. Efficacy of osteopathic manipulative treatment (93.6. ICD–9)—Systematic review. Med. Stud. 2024, 40, 289–307. [Google Scholar] [CrossRef]
- Mikołajczyk-Kocięcka, A.; Kocięcki, M.; Cyryłowski, L.; Szylińska, A.; Rynio, P.; Gębska, M.; Szuba, E.; Kaźmierczak, J. Assessment of the Effectiveness of Fascial Manipulation in Patients with Degenerative Disc Disease of the Lumbosacral Spine. Life 2024, 15, 33. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, J.H.; Jones, J.C.; Lee, D.S.; Joseph, J.R. Transcutaneous electrical nerve stimulation for the treatment of acute postoperative pain following spine surgery: A scoping review. J. Neurosurg. Spine 2024, 41, 97–104. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jorge, D.d.M.F.; Rohof, O.; Jorge, M.B.F.; Teixeira, A.; de Oliveira, C.A.; Sobreiro, P.; Dos Santos, D.F.; Huber, S.C.; Lana, J.F.S.D. A Transcutaneous Randomized Pulsed Radiofrequency Application for Spine Pain Conditions: A Case Series. J. Funct. Morphol. Kinesiol. 2025, 10, 242. https://doi.org/10.3390/jfmk10030242
Jorge DdMF, Rohof O, Jorge MBF, Teixeira A, de Oliveira CA, Sobreiro P, Dos Santos DF, Huber SC, Lana JFSD. A Transcutaneous Randomized Pulsed Radiofrequency Application for Spine Pain Conditions: A Case Series. Journal of Functional Morphology and Kinesiology. 2025; 10(3):242. https://doi.org/10.3390/jfmk10030242
Chicago/Turabian StyleJorge, Daniel de Moraes Ferreira, Olav Rohof, Melina Brigato Ferreira Jorge, Alexandre Teixeira, Cezar Augusto de Oliveira, Pablo Sobreiro, Douglas Freitas Dos Santos, Stephany Cares Huber, and Jose Fabio Santos Duarte Lana. 2025. "A Transcutaneous Randomized Pulsed Radiofrequency Application for Spine Pain Conditions: A Case Series" Journal of Functional Morphology and Kinesiology 10, no. 3: 242. https://doi.org/10.3390/jfmk10030242
APA StyleJorge, D. d. M. F., Rohof, O., Jorge, M. B. F., Teixeira, A., de Oliveira, C. A., Sobreiro, P., Dos Santos, D. F., Huber, S. C., & Lana, J. F. S. D. (2025). A Transcutaneous Randomized Pulsed Radiofrequency Application for Spine Pain Conditions: A Case Series. Journal of Functional Morphology and Kinesiology, 10(3), 242. https://doi.org/10.3390/jfmk10030242





