Myoelectric Activity of the Peroneal Muscles Following Lateral Ankle Sprain: A Cross-Sectional Analysis
Abstract
1. Introduction
2. Materials and Methods
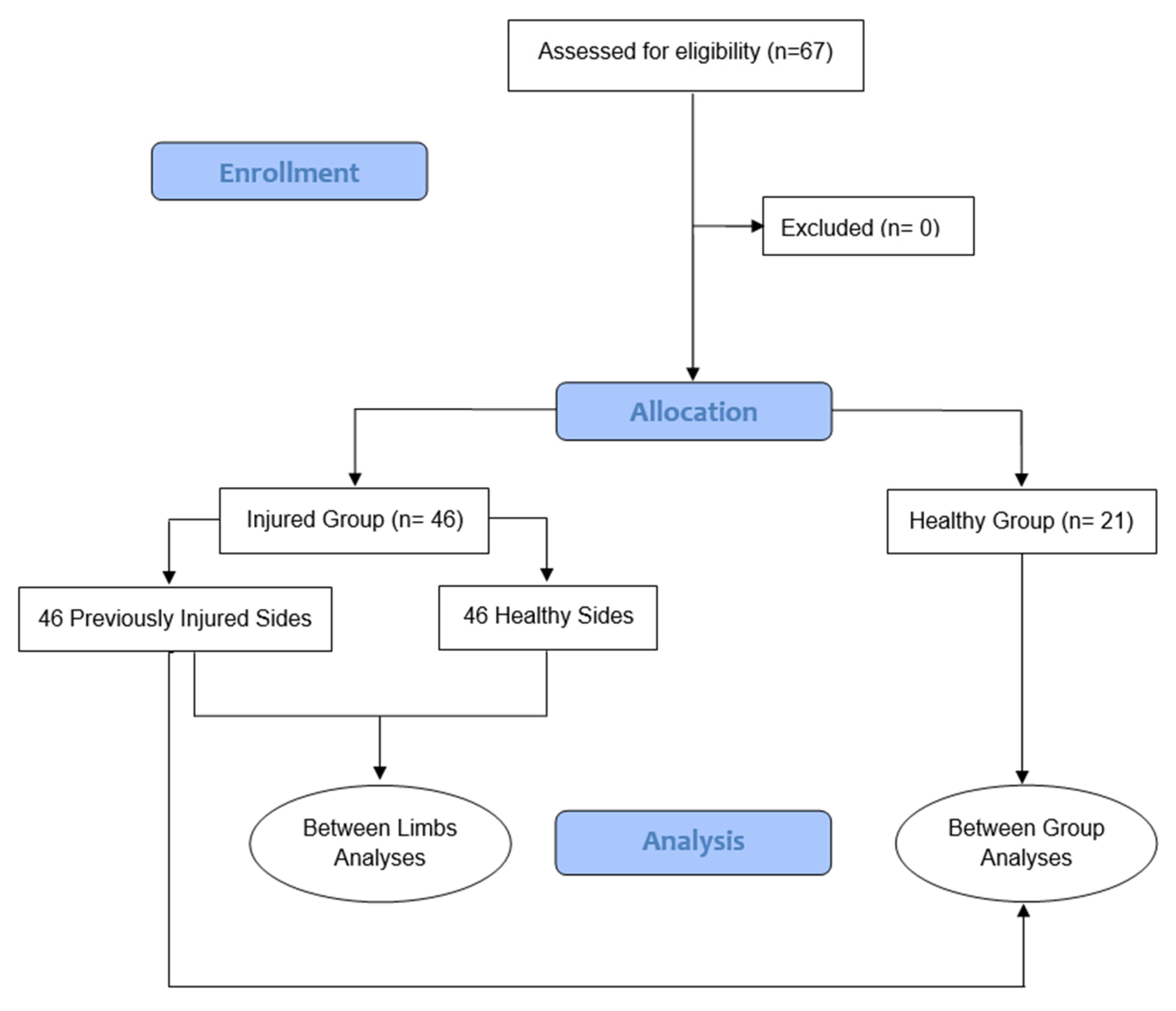
2.1. Design and Participants
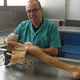
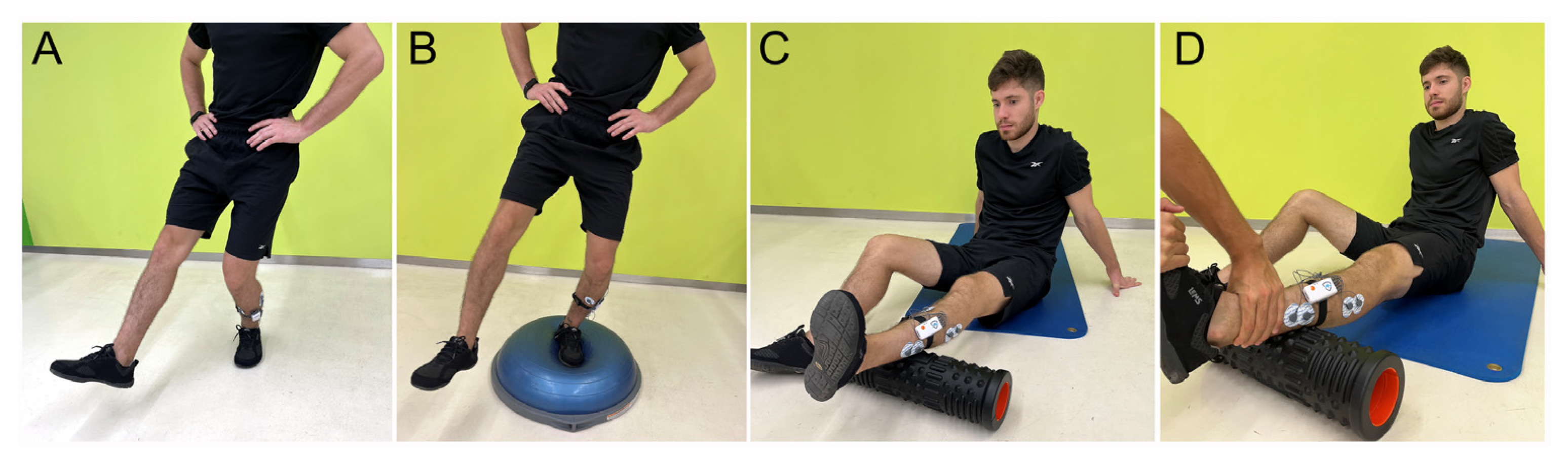
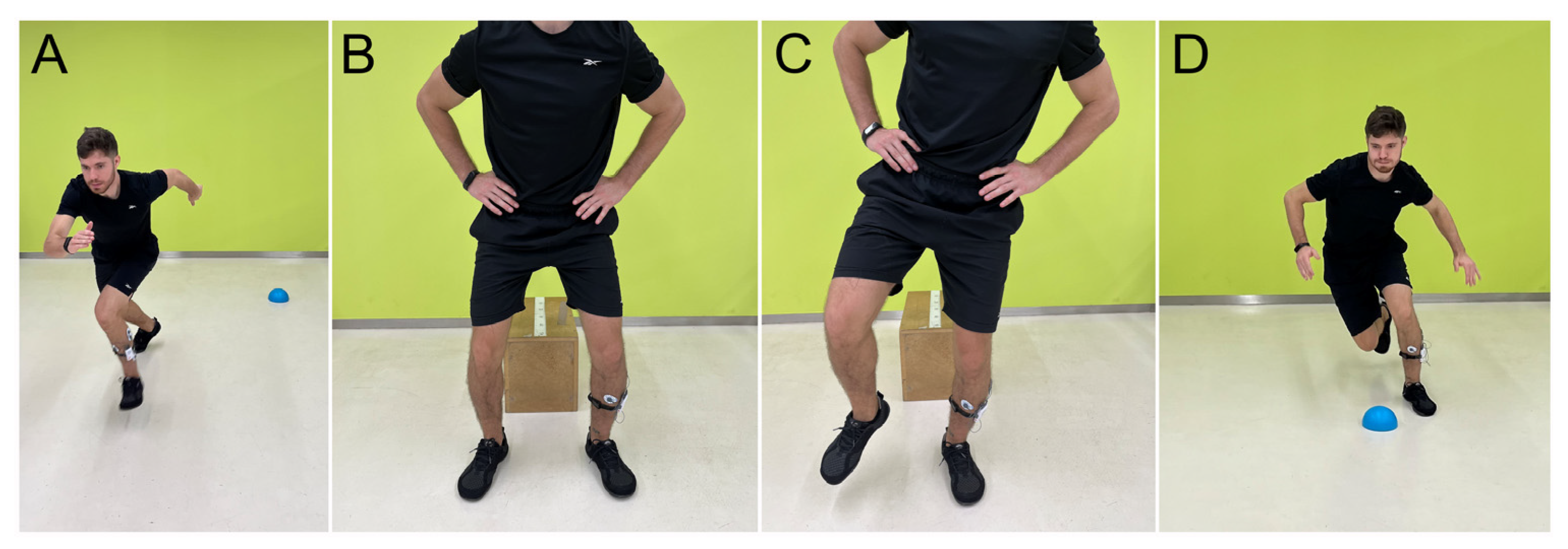
2.2. Testing Procedure
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gribble, P.A.; Bleakley, C.M.; Caulfield, B.M.; Docherty, C.L.; Fourchet, F.; Fong, D.T.-P.; Hertel, J.; Hiller, C.E.; Kaminski, T.W.; McKeon, P.O.; et al. 2016 consensus statement of the International Ankle Consortium: Prevalence, impact and long-term consequences of lateral ankle sprains. Br. J. Sports Med. 2016, 50, 1493–1495. [Google Scholar] [CrossRef] [PubMed]
- Rosa, B.B.; Asperti, A.M.; Helito, C.P.; Demange, M.K.; Fernandes, T.L.; Hernandez, A.J. Epidemiology of sports injuries on collegiate athletes at a single center. Acta Ortop. Bras. 2014, 22, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Doherty, C.; Delahunt, E.; Caulfield, B.; Hertel, J.; Ryan, J.; Bleakley, C. The Incidence and Prevalence of Ankle Sprain Injury: A Systematic Review and Meta-Analysis of Prospective Epidemiological Studies. Sports Med. 2014, 44, 123–140. [Google Scholar] [CrossRef]
- Herzog, M.M.; Kerr, Z.Y.; Marshall, S.W.; Wikstrom, E.A. Epidemiology of ankle sprains and chronic ankle instability. J. Athl. Train. 2019, 54, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Mauntel, T.C.; Wikstrom, E.A.; Roos, K.G.; Djoko, A.; Dompier, T.P.; Kerr, Z.Y. The Epidemiology of High Ankle Sprains in National Collegiate Athletic Association Sports. Am. J. Sports Med. 2017, 45, 2156–2163. [Google Scholar] [CrossRef]
- Hunt, K.J.; Hurwit, D.; Robell, K.; Gatewood, C.; Botser, I.B.; Matheson, G. Incidence and Epidemiology of Foot and Ankle Injuries in Elite Collegiate Athletes. Am. J. Sports Med. 2017, 45, 426–433. [Google Scholar] [CrossRef]
- Herzog, M.M.; Mack, C.D.; Dreyer, N.A.; Wikstrom, E.A.; Padua, D.A.; Kocher, M.S.; DiFiori, J.P.; Marshall, S.W. Ankle Sprains in the National Basketball Association, 2013–2014 Through 2016–2017. Am. J. Sports Med. 2019, 47, 2651–2658. [Google Scholar] [CrossRef]
- Vuurberg, G.; Hoorntje, A.; Wink, L.M.; van der Doelen, B.F.W.; van den Bekerom, M.P.; Dekker, R.; van Dijk, C.N.; Krips, R.; Loogman, M.C.M.; Ridderikhof, M.L.; et al. Diagnosis, treatment and prevention of ankle sprains: Update of an evidence-based clinical guideline. Br. J. Sports Med. 2018, 52, 956. [Google Scholar] [CrossRef]
- Doherty, C.; Bleakley, C.; Hertel, J.; Caulfield, B.; Ryan, J.; Delahunt, E. Recovery From a First-Time Lateral Ankle Sprain and the Predictors of Chronic Ankle Instability. Am. J. Sports Med. 2016, 44, 995–1003. [Google Scholar] [CrossRef]
- Miklovic, T.M.; Donovan, L.; Protzuk, O.A.; Kang, M.S.; Feger, M.A. Acute lateral ankle sprain to chronic ankle instability: A pathway of dysfunction. Phys. Sportsmed. 2018, 46, 116–122. [Google Scholar] [CrossRef]
- Delahunt, E.; Bleakley, C.M.; Bossard, D.S.; Caulfield, B.M.; Docherty, C.L.; Doherty, C.; Fourchet, F.; Fong, D.T.; Hertel, J.; Hiller, C.E.; et al. Clinical assessment of acute lateral ankle sprain injuries (ROAST): 2019 consensus statement and recommendations of the International Ankle Consortium. Br. J. Sports Med. 2018, 52, 1304–1310. [Google Scholar] [CrossRef] [PubMed]
- Hoch, M.C.; McKeon, P.O. Peroneal reaction time after ankle sprain: A systematic review and meta-analysis. Med. Sci. Sports Exerc. 2014, 46, 546–556. [Google Scholar] [CrossRef] [PubMed]
- Sierra-Guzmán, R.; Jiménez, F.; Abián-Vicén, J. Predictors of chronic ankle instability: Analysis of peroneal reaction time, dynamic balance and isokinetic strength. Clin. Biomech. 2018, 54, 28–33. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.; Thomas, R. Ankle instability: Presentation and management. Orthop. Trauma. 2015, 29, 145–151. [Google Scholar] [CrossRef]
- Sánchez-Ureña, B.; Rojas-Valverde, D.; Gutiérrez-Vargas, R. Effectiveness of Two Cold Water Immersion Protocols on Neuromuscular Function Recovery: A Tensiomyography Study. Front. Physiol. 2018, 9, 766. [Google Scholar] [CrossRef]
- Lai, Z.; Wang, X.; Lee, S.; Hou, X.; Wang, L. Effects of whole body vibration exercise on neuromuscular function for individuals with knee osteoarthritis: Study protocol for a randomized controlled trial. Trials 2017, 18, 437. [Google Scholar] [CrossRef]
- Pérez-Bellmunt, A.; Llurda-Almuzara, L.; Simon, M.; Navarro, R.; Casasayas, O.; López-de-Celis, C. Review Article Neuromuscular Response What is it and How to Measure it ? Phys. Med. Rehabil. J. 2019, 2, 118. [Google Scholar]
- Lynn, S.K.; Watkins, C.M.; Wong, M.A.; Balfany, K.; Feeney, D.F. Validity and reliability of surface electromyography measurements from a wearable athlete performance system. J. Sport. Sci. Med. 2018, 17, 205–215. [Google Scholar]
- Areia, C.; Barreira, P.; Montanha, T.; Oliveira, J.; Ribeiro, F. Neuromuscular changes in football players with a previous hamstring injury. Physiotherapy 2019, 105, e120. [Google Scholar] [CrossRef]
- Kazemi, K.; Arab, A.M.; Abdollahi, I.; López-López, D.; Calvo-Lobo, C. Electromiography comparison of distal and proximal lower limb muscle activity patterns during external perturbation in subjects with and without functional ankle instability. Hum. Mov. Sci. 2017, 55, 211–220. [Google Scholar] [CrossRef]
- Lin, J.Z.; Lin, Y.A.; Lee, H.J. Are landing biomechanics altered in elite athletes with chronic ankle instability. J. Sport. Sci. Med. 2019, 18, 653–662. [Google Scholar]
- Allet, L.; Zumstein, F.; Eichelberger, P.; Armand, S.; Punt, I.M. Neuromuscular Control Mechanisms During Single-Leg Jump Landing in Subacute Ankle Sprain Patients: A Case Control Study. PM R 2017, 9, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, L.; Donovan, L.; Hart, J.M.; Hertel, J. Eversion Strength and Surface Electromyography Measures With and Without Chronic Ankle Instability Measured in 2 Positions. Foot Ankle Int. 2017, 38, 769–778. [Google Scholar] [CrossRef]
- Rodrigues, K.A.; Soares, R.J.; Tomazini, J.E. The influence of fatigue in evertor muscles during lateral ankle sprain. Foot 2019, 40, 98–104. [Google Scholar] [CrossRef]
- Sharif, B.; Welck, M.; Saifuddin, A. MRI of the distal tibiofibular joint. Skelet. Radiol. 2020, 49, 1–17. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Int. J. Surg. 2014, 12, 1495–1499. [Google Scholar] [CrossRef]
- McKinney, J.; Velghe, J.; Fee, J.; Isserow, S.; Drezner, J.A. Defining Athletes and Exercisers. Am. J. Cardiol. 2019, 123, 532–535. [Google Scholar] [CrossRef]
- Wikstrom, E.A.; Cain, M.S.; Chandran, A.; Song, K.; Regan, T.; Migel, K.; Kerr, Z.Y. Lateral ankle sprain and subsequent ankle sprain risk: A systematic review. J. Athl. Train. 2021, 56, 578–585. [Google Scholar] [CrossRef]
- De Noronha, M.; França, L.C.; Haupenthal, A.; Nunes, G.S. Intrinsic predictive factors for ankle sprain in active university students: A prospective study. Scand. J. Med. Sci. Sport. 2013, 23, 541–547. [Google Scholar] [CrossRef]
- Stegeman, D.; Hermens, H. Standards for surface electromyography: The European project Surface EMG for non-invasive assessment of muscles (SENIAM). Enschede Roessingh Res. Dev. 2007, 10, 108–112. [Google Scholar]
- Kim, H.-Y. Statistical notes for clinical researchers: Effect size. Restor. Dent. Endod. 2015, 40, 328. [Google Scholar] [CrossRef] [PubMed]
- Koldenhoven, R.M.; Feger, M.A.; Fraser, J.J.; Saliba, S.; Hertel, J. Surface electromyography and plantar pressure during walking in young adults with chronic ankle instability. Knee Surg. Sports Traumatol. Arthrosc. 2016, 24, 1060–1070. [Google Scholar] [CrossRef] [PubMed]
- Deodato, I.; Cortes-Rodriguez, A.E.; Aracil-Marco, A.; Martinez-Garcia, D.; Lozano-Berges, G. Inertial sensors-based assessment to detect hallmarks of chronic ankle instability during single-leg standing: Is the healthy limb “healthy”? Clin. Biomech. 2023, 109, 106036. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Anson, J.; Waddington, G.; Adams, R.; Liu, Y. Chronic ankle instability is associated with proprioception deficits: A systematic review and meta-analysis. J. Sport Health Sci. 2021, 10, 182–195. [Google Scholar] [CrossRef]
- Sole, G.; Milosavljevic, S.; Nicholson, H.; Sullivan, S.J. Selective strength loss and decreased muscle activity in hamstring injury. J. Orthop. Sports Phys. Ther. 2011, 41, 354–363. [Google Scholar] [CrossRef]
- Wisthoff, B.; Matheny, S.; Struminger, A.; Gustavsen, G.; Glutting, J.; Swanik, C.; Kaminski, T.W. Ankle Strength Deficits in a Cohort of College Athletes with Chronic Ankle Instability. J. Sport Rehabil. 2019, 28, 752–757. [Google Scholar] [CrossRef]
- Fraser, J.J.; Koldenhoven, R.M.; Jaffri, A.H.; Park, J.S.; Saliba, S.F.; Hart, J.M.; Hertel, J. Foot impairments contribute to functional limitation in individuals with ankle sprain and chronic ankle instability. Knee Surg. Sport. Traumatol. Arthrosc. 2018, 28, 1600–1610. [Google Scholar] [CrossRef]
- Cho, B.K.; Park, J.K.; Choi, S.M.; Kang, S.W.; SooHoo, N.F. The peroneal strength deficits in patients with chronic ankle instability compared to ankle sprain copers and normal individuals. Foot Ankle Surg. 2019, 25, 231–236. [Google Scholar] [CrossRef]
- Sakai, S.; Urabe, Y.; Sasadai, J.; Morikawa, M.; Maeda, N. Quantity and quality of the peroneal nerve muscles in leg with chronic ankle instability assessed by ultrasonography. Gait Posture 2018, 65, 413–414. [Google Scholar] [CrossRef]
- Perron, M.; Moffet, H.; Nadeau, S.; Hébert, L.J.; Belzile, S. Persistence of long term isokinetic strength deficits in subjects with lateral ankle sprain as measured with a protocol including maximal preloading. Clin. Biomech. 2014, 29, 1151–1157. [Google Scholar] [CrossRef]
| Control Group (n = 21) | Ankle Sprain Group (n = 46) | ||
|---|---|---|---|
| Mean ± SD or n (%) | Mean ± SD or n (%) | p-Value | |
| Gender | |||
| Female | 10 (47.6%) | 26 (56.5%) | 0.498 Ŧ |
| Male | 11 (52.4%) | 20 (43.5%) | |
| Age | 20.81 ± 3.74 | 21.28 ± 4.31 | 0.795 * |
| Height | 173.14 ± 6.88 | 172.59 ± 9.48 | 0.850 * |
| Weight | 64.57 ± 8.92 | 66.33 ± 12.73 | 0.556 * |
| BMI | 21.46 ± 1.81 | 22.17 ± 3.09 | 0.503 * |
| Dominance | |||
| Left | 4 (19%) | 10 (21.7%) | 0.802 Ŧ |
| Right | 17 (81%) | 36 (78.3%) | |
| Sport | |||
| Basketball | 1 (5.6%) | 6 (11.4%) | |
| Fitness | 6 (33.3%) | 6 (11.4%) | |
| Soccer | 5 (22.2%) | 22 (50%) | 0.013 Ŧ Ŧ |
| Hockey | 4 (16.7%) | 11 (25%) | |
| Others | 5 (22.2%) | 1 (2.3%) |
| Previously Injured Side Mean ± SD (n = 46) | Control Side Mean ± SD (n = 46) | ES | p-Value | |
|---|---|---|---|---|
| Maximal Voluntary Isometric Contraction PB RMS | 271.01 ± 147.59 | 269.09 ± 192.90 | 0.01 | 0.746 * |
| Maximal Voluntary Isometric Contraction PL RMS | 204.10 ± 107.57 | 192.91 ± 92.56 | 0.11 | 0.522 ** |
| Dynamic Eversions PB RMS | 19.09 ± 12.08 | 23.49 ± 16.72 | 0.30 | 0.192 * |
| Dynamic Eversions PL RMS | 26.70 ± 34.27 | 27.91 ± 21.52 | 0.04 | 0.220 * |
| Single Leg Squat PB RMS | 31.99 ± 22.07 | 37.86 ± 33.18 | 0.21 | 0.912 * |
| Single Leg Squat PL RMS | 61.57 ± 97.43 | 55.82 ± 42.47 | 0.08 | 0.975 * |
| Drop Jump PB RMS | 12.61 ± 7.70 | 17.16 ± 19.69 | 0.30 | 0.667 * |
| Drop Jump PL RMS | 20.11 ± 19.65 | 19.40 ± 16.26 | 0.04 | 0.868 * |
| Unilateral Drop Jump PB RMS | 24.90 ± 14.76 | 36.96 ± 49.65 | 0.33 | 0.294 * |
| Unilateral Drop Jump PL RMS | 39.26 ± 34.19 | 37.39 ± 20.55 | 0.07 | 0.792 * |
| Bosu PB RMS | 48.74 ± 22.19 | 60.48 ± 42.38 | 0.35 | 0.103 * |
| Bosu PL RMS | 71.28 ± 66.07 | 73.17 ± 53.27 | 0.03 | 0.935 * |
| Sprint PB RMS | 78.08 ± 39.03 | 94.90 ± 72.00 | 0.29 | 0.411 * |
| Sprint PL RMS | 128.54 ± 112.30 | 119.71 ± 107.64 | 0.08 | 0.483 * |
| Change of Direction PB RMS | 76.68 ± 44.38 | 100.82 ± 79.22 | 0.38 | 0.115 * |
| Change of Direction PL RMS | 146.29 ± 173.23 | 122.34 ± 90.58 | 0.17 | 0.769 * |
| Injured Group Mean ± SD (n = 46) | Healthy Group Mean ± SD (n = 21) | ES | p-Value | |
|---|---|---|---|---|
| Maximal Voluntary Isometric Contraction PB RMS | 249.13 ± 108.23 | 227.35 ± 93.09 | 0.22 | 0.434 * |
| Maximal Voluntary Isometric Contraction PL RMS | 191.05 ± 60.04 | 186.93 ± 106.05 | 0.05 | 0.875 * |
| Dynamic Eversions PB RMS | 25.64 ± 18.63 | 21.78 ± 16.28 | 0.22 | 0.298 ** |
| Dynamic Eversions PL RMS | 28.64 ± 16.62 | 29.69 ± 34.64 | 0.04 | 0.226 ** |
| Single Leg Squat PB RMS | 36.65 ± 23.49 | 36.44 ± 33.46 | 0.01 | 0.844 ** |
| Single Leg Squat PL RMS | 47.82 ± 30.54 | 50.81 ± 38.47 | 0.09 | 0.933 ** |
| Drop Jump PB RMS | 13.87 ± 13.86 | 12.42 ± 7.17 | 0.13 | 0.508 ** |
| Drop Jump PL RMS | 16.90 ± 17.24 | 19.78 ± 17.93 | 0.16 | 0.234 ** |
| Unilateral Drop Jump PB RMS | 27.14 ± 24.95 | 26.90 ± 16.76 | 0.01 | 0.525 ** |
| Unilateral Drop Jump PL RMS | 35.85 ± 35.62 | 39.27 ± 32.38 | 0.10 | 0.579 ** |
| Bossu PB RMS | 46.57 ± 23.18 | 51.90 ± 28.83 | 0.20 | 0.582 ** |
| Bossu PL RMS | 51.92 ± 19.97 | 73.40 ± 66.42 | 0.44 | 0.436 ** |
| Sprint PB RMS | 76.36 ± 60.66 | 74.31 ± 38.59 | 0.04 | 0.480 ** |
| Sprint PL RMS | 81.82 ± 44.36 | 106.13 ± 69.48 | 0.42 | 0.477 ** |
| Change of Direction PB RMS | 82.87 ± 61.08 | 74.37 ± 49.50 | 0.15 | 0.749 ** |
| Change of Direction PL RMS | 92.04 ± 55.85 | 98.39 ± 75.59 | 0.10 | 0.848 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casasayas-Cos, O.; Labata-Lezaun, N.; Pérez-Bellmunt, A.; López-de-Celis, C.; Smit, J.; Marimon-Serra, X.; Aiguadé-Aiguadé, R.; Sanahuja-Diez-Caballero, J.; Canet-Vintró, M.; Llurda-Almuzara, L. Myoelectric Activity of the Peroneal Muscles Following Lateral Ankle Sprain: A Cross-Sectional Analysis. J. Funct. Morphol. Kinesiol. 2025, 10, 179. https://doi.org/10.3390/jfmk10020179
Casasayas-Cos O, Labata-Lezaun N, Pérez-Bellmunt A, López-de-Celis C, Smit J, Marimon-Serra X, Aiguadé-Aiguadé R, Sanahuja-Diez-Caballero J, Canet-Vintró M, Llurda-Almuzara L. Myoelectric Activity of the Peroneal Muscles Following Lateral Ankle Sprain: A Cross-Sectional Analysis. Journal of Functional Morphology and Kinesiology. 2025; 10(2):179. https://doi.org/10.3390/jfmk10020179
Chicago/Turabian StyleCasasayas-Cos, Oriol, Noé Labata-Lezaun, Albert Pérez-Bellmunt, Carlos López-de-Celis, Johke Smit, Xavier Marimon-Serra, Ramón Aiguadé-Aiguadé, Joaquín Sanahuja-Diez-Caballero, Max Canet-Vintró, and Luis Llurda-Almuzara. 2025. "Myoelectric Activity of the Peroneal Muscles Following Lateral Ankle Sprain: A Cross-Sectional Analysis" Journal of Functional Morphology and Kinesiology 10, no. 2: 179. https://doi.org/10.3390/jfmk10020179
APA StyleCasasayas-Cos, O., Labata-Lezaun, N., Pérez-Bellmunt, A., López-de-Celis, C., Smit, J., Marimon-Serra, X., Aiguadé-Aiguadé, R., Sanahuja-Diez-Caballero, J., Canet-Vintró, M., & Llurda-Almuzara, L. (2025). Myoelectric Activity of the Peroneal Muscles Following Lateral Ankle Sprain: A Cross-Sectional Analysis. Journal of Functional Morphology and Kinesiology, 10(2), 179. https://doi.org/10.3390/jfmk10020179