Aquatic Therapy Versus Land-Based Therapy in Patients with Parkinson’s Disease: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Strategy
2.2. Selection Criteria
2.3. Data Extraction
2.4. Assessment of Methodological Quality and Risk of Bias
3. Results
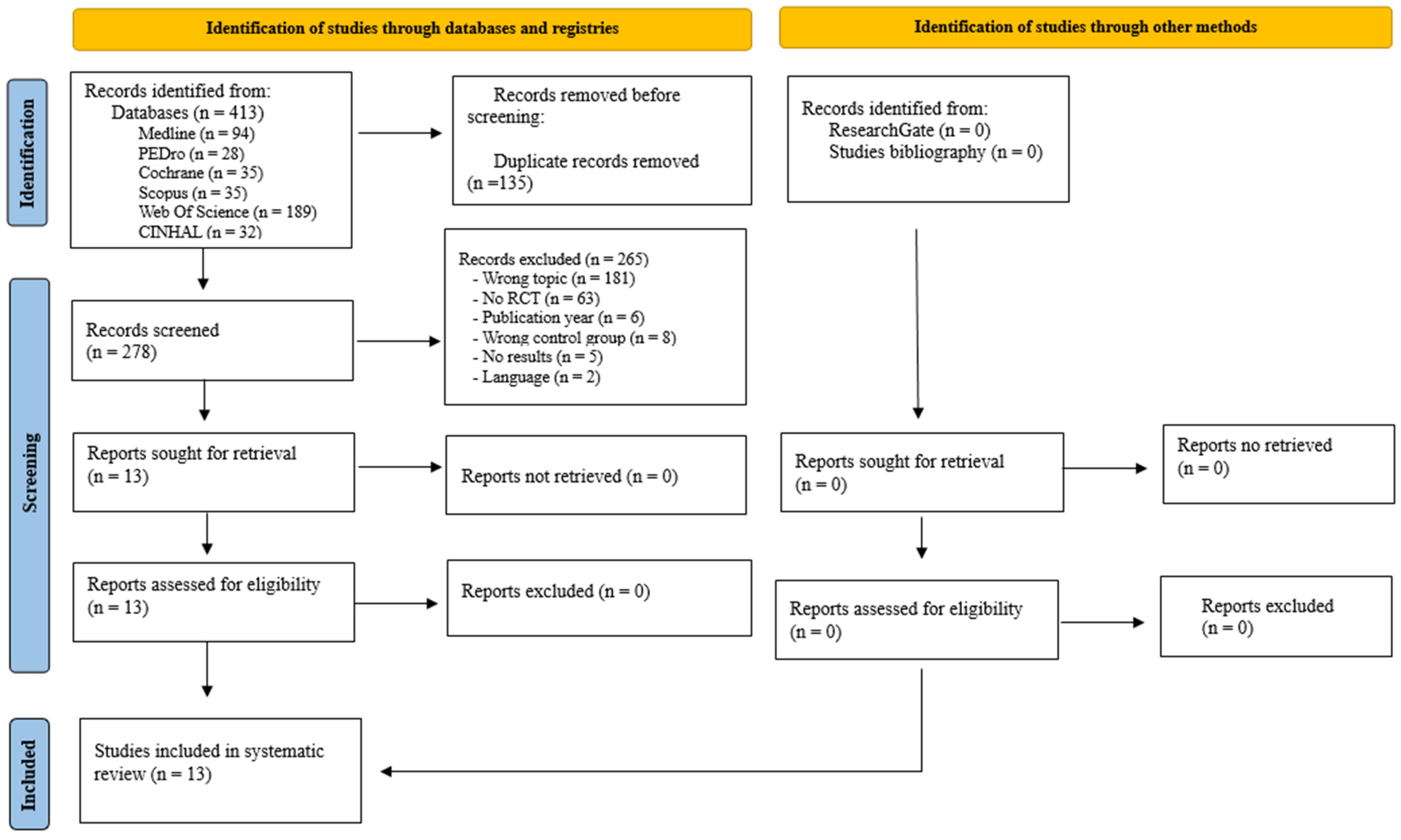
3.1. Study Selection
3.2. Assessment of Methodological Quality
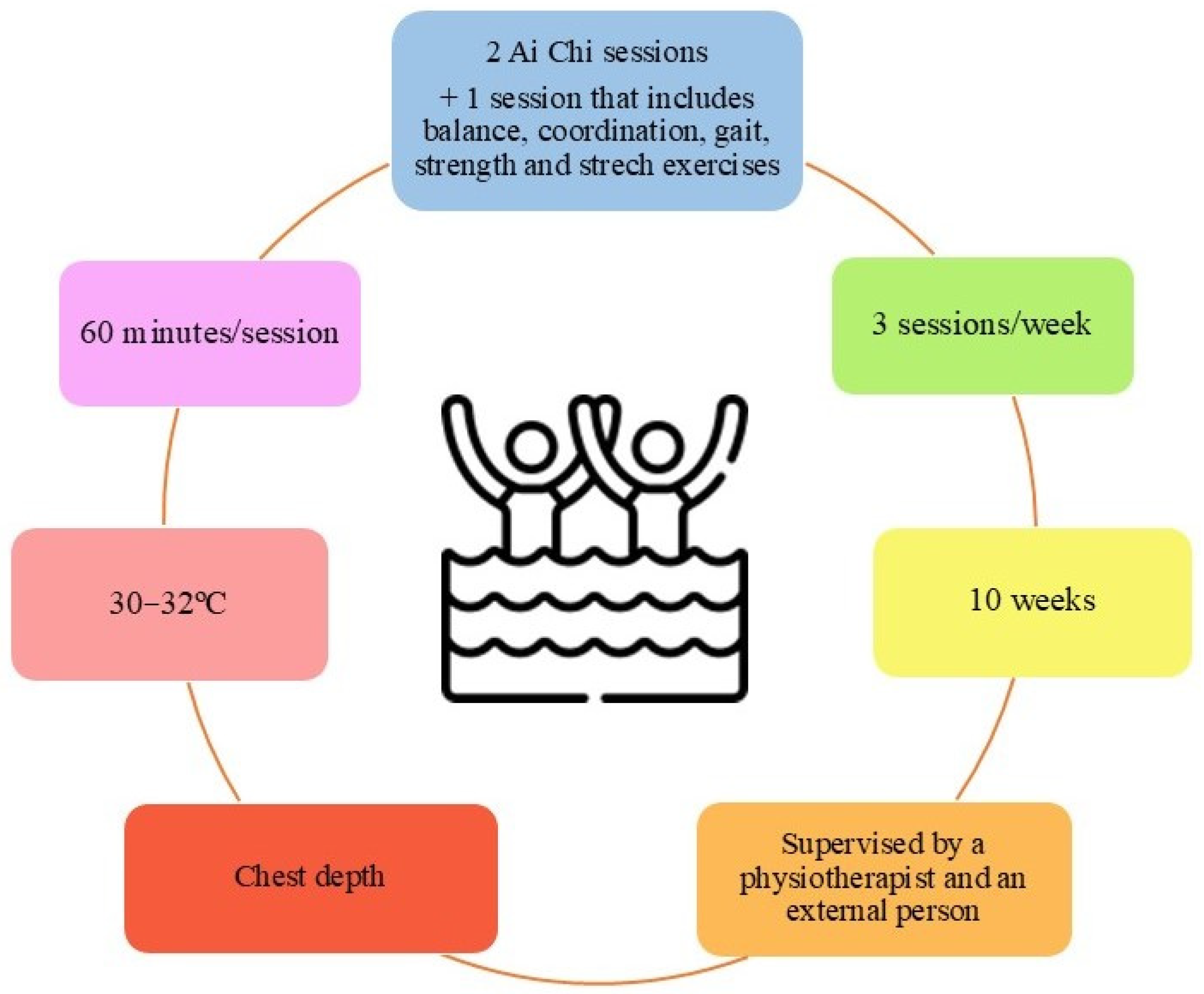
3.3. Characteristics of Participants and Interventions
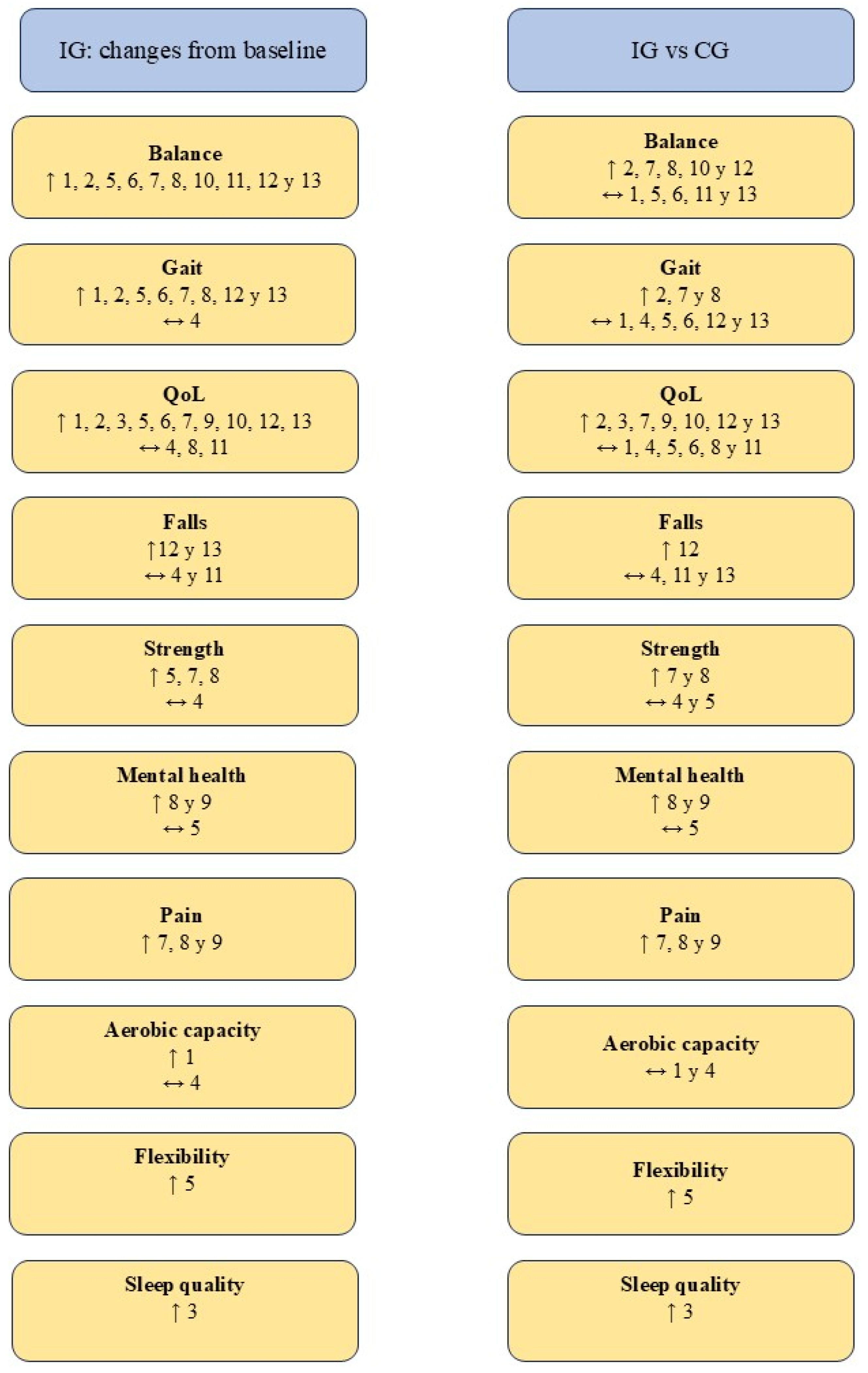
3.4. Evaluation of the Results
3.4.1. Balance
3.4.2. Gait
3.4.3. Quality of Life
3.4.4. Strength
3.4.5. Other Parameters Evaluated
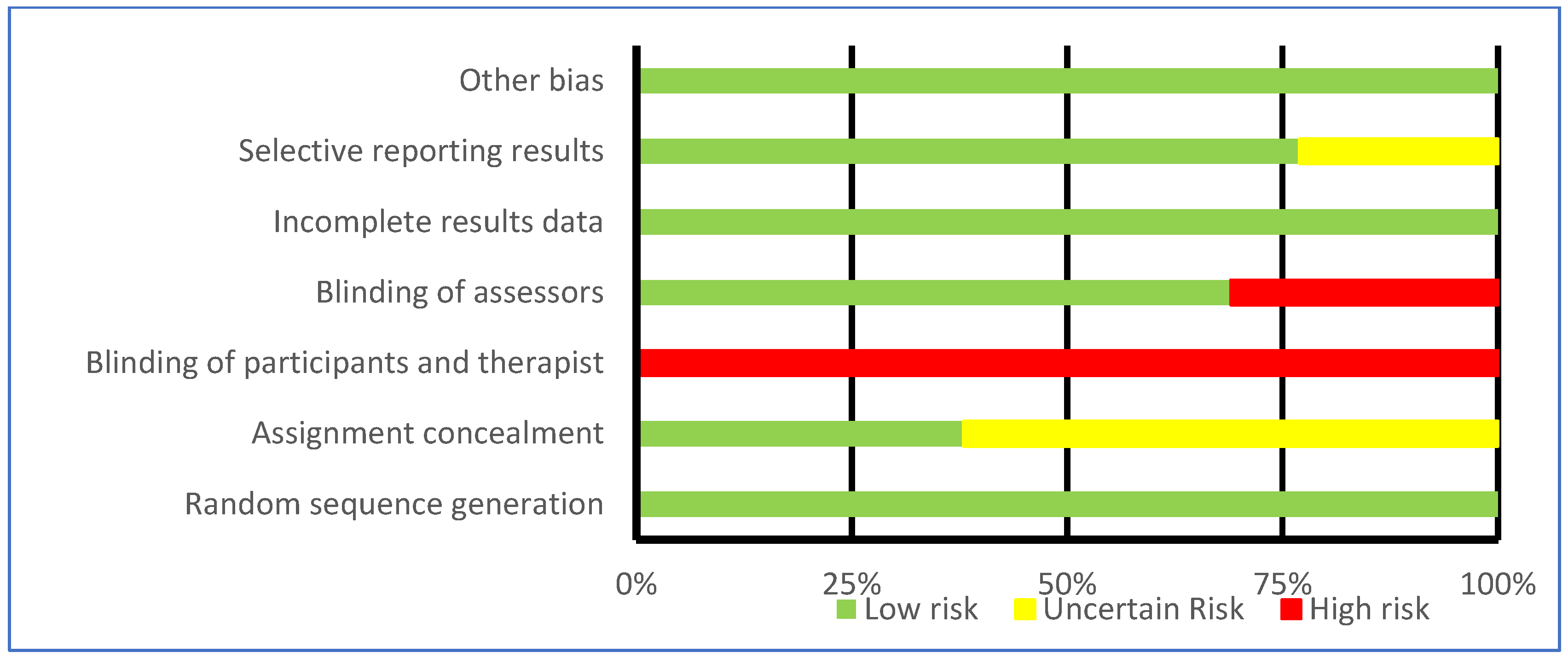
3.5. Bias Assessment
4. Discussion
4.1. Balance
4.2. Gait
4.3. Quality of Life
4.4. Strength and Flexibility
4.5. Practical Applications
4.6. Reflections on the Role of Aquatic and Land-Based Physical Exercise in Parkinson’s Disease
4.7. Future Lines
4.8. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMPK | AMP-activated protein kinase |
| AT | Aquatic-based therapeutic exercise |
| BDNF | Brain-derived neurotrophic factor |
| CASPe | Critical Appraisal Skills Program in Spanish |
| CG | Control group |
| FNDCS | Fibronectin type III domain-containing proteins |
| IG | Intervention group |
| IGF-1 | Insulin-like growth factor 1 |
| IL-10 | Interleukin 10 |
| LT | Land-based therapeutic exercise |
| MeSH | Medical Subject Headings |
| PD | Parkinson’s Disease |
| PEDro | Physiotherapy Evidence Database |
| QoL | Quality of life |
| TNFα | Tumor necrosis factor-alpha |
| PGC-1α | Peroxime proliferator-activated receptor gamma coactivator 1-alpha |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-analyses |
| Tpa | Tissue plasminogen activator |
Appendix A. Checklist PRISMA 2020 [27]
| Section and Topic | Item | Checklist Item | Location Where Item Is Reported |
| Title | |||
| Title | 1 | Identify the report as a systematic review. | 1 |
| Abstract | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | 1–2 |
| Introduction | |||
| Rationale | 3 | Describe the rationale for this review in the context of existing knowledge. | 2 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | 2 |
| Methods | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for this review and how studies were grouped for the syntheses. | 3 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | 3 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | 3 and 29 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of this review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and, if applicable, details of automation tools used in the process. | 3 |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | 3 |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g., for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | 3 |
| 10b | List and define all other variables for which data were sought (e.g., participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | 3 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study, whether they worked independently, and, if applicable, details of automation tools used in this process. | 3 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g., risk ratio, mean difference) used in the synthesis or presentation of results. | - |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g., tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | - |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling missing summary statistics or data conversions. | - | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | - | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s) and method(s) to identify the presence and extent of statistical heterogeneity and software package(s) used. | - | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g., subgroup analysis, meta-regression). | - | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | - | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | - |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | - |
| Results | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in this review, ideally using a flow diagram. | 3–5 |
| 16b | Cite studies that might appear to meet the inclusion criteria but which were excluded, and explain why they were excluded. | 3–5 | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | 8 and 10–18 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | 6–8 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g., confidence/credible interval), ideally using structured tables or plots. | 8–16 and 19 |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | - |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was performed, present for each the summary estimate and its precision (e.g., confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | - | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | - | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | - | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | - |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | - |
| Discussion | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | 19–23 |
| 23b | Discuss any limitations of the evidence included in this review. | 23 | |
| 23c | Discuss any limitations of the review processes used. | 23 | |
| 23d | Discuss implications of the results for practice, policy, and future research. | 21–23 | |
| Other information | |||
| Registration and protocol | 24a | Provide registration information for this review, including register name and registration number, or state that this review was not registered. | 2 |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | 2 | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | 2 | |
| Support | 25 | Describe sources of financial or non-financial support for this review and the role of the funders or sponsors in this review. | 24 |
| Competing interests | 26 | Declare any competing interests of review authors. | 24 |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in this review. | - |
Appendix B. Search Strategy Employed in Databases
| Database | Search Strategy |
| Medline/Pubmed | (Parkinson Disease (MeSH) OR Parkinson OR Parkinson’s Disease) AND (Aquatic Therapy (MeSH) OR Aquatic Exercise Therapy OR Water Exercise Therapy OR Ai Chi Therapy) |
| Scopus | (TITLE-ABS-KEY (“parkinson disease” OR Parkinson OR “Parkinson’s Disease”) AND TITLE-ABS-KEY (“Aquatic Therapy” OR “Aquatic Exercise Therapy” OR “Water Exercise Therapy” OR “ai chi therapy”)) |
| Web of Science | Parkinson Disease (topic) AND Aquatic Therapy (Topic) |
| PEDro | Parkinson disease AND hydrotherapy, balneotherapy |
| CINAHL | (Parkinson disease OR Parkinson OR Parkinson’s Disease) AND (Aquatic therapy OR Aquatic Exercise Therapy OR Water Exercise Therapy OR ai chi therapy) |
| Cochrane | Parkinson Disease AND Aquatic Therapy |
References
- Cabreira, V.; Massano, J. Parkinson’s Disease: Clinical Review and Update. Acta Med. Port. 2019, 32, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.T. Parkinson’s Disease and Parkinsonism. Am. J. Med. 2019, 132, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Warnecke, T.; Lummer, C.; Wilhelm, R.; Claus, I.; Lüttje, D. Parkinson’s disease. Inn. Med. 2023, 64, 131–138. [Google Scholar] [CrossRef]
- Reich, S.; Savitt, J. Parkinson’s Disease. Med. Clin. N. Am. 2019, 103, 337–350. [Google Scholar] [CrossRef]
- Cerri, S.; Mus, L.; Blandini, F. Parkinson’s Disease in Women and Men: What’s the Difference? J. Park. Dis. 2019, 9, 501–515. [Google Scholar] [CrossRef]
- Ascherio, A.; Schwarzschild, M. The epidemiology of Parkinson’s disease: Risk factors and prevention. Lancet Neurol. 2016, 15, 1257–1272. [Google Scholar] [CrossRef]
- Dorsey, E.; Sherer, T.; Okun, M.; Bloem, B. The Emerging Evidence of the Parkinson Pandemic. J. Park. Dis. 2018, 8, S3–S8. [Google Scholar] [CrossRef]
- Allen, L. Are we facing a noncommunicable disease pandemic? J. Epidemiol. Glob. Heal. 2017, 7, 5–9. [Google Scholar] [CrossRef]
- Bellou, V.; Belbasis, L.; Tzoulaki, I.; Evangelou, E.; Ioannidis, J. Environmental risk factors and Parkinson’s disease: An umbrella review of meta-analyses. Park. Relat. Disord. 2016, 23, 1–9. [Google Scholar] [CrossRef]
- Hvingelby, V.; Glud, A.; Sorensen, J.; Tai, Y.; Andersen, A.; Johnsen, E.; Moro, E.; Pavese, N. Interventions to improve gait in Parkinson’s disease: A systematic review of randomized controlled trials and network meta-analysis. J. Neurol. 2022, 269, 4068–4079. [Google Scholar] [CrossRef]
- Hao, Z.; Zhang, X.; Chen, P. Effects of Ten Different Exercise Intervention on Motor Function in Parkinson’s Disease Patients: A Network Meta-Analysis of Randommized Controlled Trials. Brain Sci. 2022, 12, 698. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Wang, D.; Ba, X.; Liu, Z. Exercise sustains motor function in Parkinson’s Disease: Evidence from 109 randomized controlled trials on over 4600 patients. Front. Aging Neurosci. 2023, 15, 1071803. [Google Scholar] [CrossRef]
- Deepa, S.; Kumaresan, A.; Suganthirababu, P.; Vishnuram, S.; Srinivasan, V. A need to reconsider the rehabilitation protocol in patients with idiopatic Parkinson’s disease: Review analysis. Biomedicine 2022, 42, 657–660. [Google Scholar] [CrossRef]
- El Hayek, M.; Lobo Jofili, J.; LeLaurin, J.; Gregory, M.; Abi Nehme, A.; McCall-Junkin, P.; KLK, A.; Okun, M.; Salloum, R. Type, Timing, Frequency, and Durability of Outcome of Physical Therapy for Parkinson Disease: A systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2324860. [Google Scholar] [CrossRef]
- Mooventhan, A.; Nivethitha, L. Scientific evidence-based effects of hydrotherapy on varius systems of the body. N. Am. J. Med. Sci. 2014, 6, 199–209. [Google Scholar] [CrossRef]
- BE, B. Aquatic Therapy: Scientific foundations and clinical rehabilitation applications. PM R 2009, 1, 859–872. [Google Scholar] [CrossRef]
- Coraci, D.; Tognolo, L.; Maccarone, M.C.; Santilli, G.; Ronconi, G.; Masiero, S. Water-Based Rehabilitation in the Elderly: Data Science Approach to Support the Conduction of a Scoping Review. Appl. Sci. 2022, 12, 8999. [Google Scholar] [CrossRef]
- Wang, T.; Wang, J.; Chen, Y.; Ruan, Y.; Dai, S. Efficacy of aquatic exercise in chronic musculoskeletal disorders: A systematic review and meta-analysis of randomized controlled trials. J. Orthop. Surg. Res. 2023, 18, 942. [Google Scholar] [CrossRef]
- Faíl, L.; Marinho, D.; Marques, E.; Costa, M.; Santos, C.; Marques, M.; Izquierdo, M.; Neiva, H. Benefits of Aquatic exercise in adults with and without chronic disease-A systematic review with meta-analysis. Scand. J. Med. Sci. Sport. 2022, 32, 465–486. [Google Scholar] [CrossRef]
- Cugusi, L.; Manca, A.; Bergamin, M.; Di Blasio, A.; Monticone, M.; Deriu, F.; Mercuro, G. Aquatic Exercise improves motor impairments in people with Parkinson’s disease, with similar or greater benefits than land-based exercise: A systematic review. J. Physiother. 2019, 65, 65–74. [Google Scholar] [CrossRef]
- Methajarunon, P.; Eitivipart, C.; Diver, C.; Foongchomcheavy, A. systematic review of published studies on aquatic exercise for balance in patientes with multiple sclerosis, Parkinson’s disease, and hemiplegia. Foongchomcheavy 2016, 35, 12–20. [Google Scholar] [CrossRef]
- Plecash, A.; Leavitt, B. Aquatherapy for neurodegenerative disorders. J. Huntingtons. Dis. 2014, 3, 5–11. [Google Scholar] [CrossRef]
- Dai, S.; Yuan, H.; Wang, J.; Yang, Y.; Wen, S. Effects of aquatic exercise on the improvement of lower-extremity motor function and quality of life in patients with Parkinson’s disease: A meta-analysis. Front. Physiol. 2023, 14, 1066718. [Google Scholar] [CrossRef] [PubMed]
- Moritz, T.; Snowdon, D.; Peiris, C. combining aquatic physiotherapy with usual care physiotherapy for people with neurological conditions: A systematic review. Physiother. Res. Int. 2020, 25, e1813. [Google Scholar] [CrossRef] [PubMed]
- Terrens, A.; Soh, S.; Morgan, P. The efficacy and feasibility of aquatic physiotherapy for people with Parkinson’s disease: A systematic review. Disabil. Rehabil. 2018, 40, 2847–2856. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, M.; Laster, J.; Rogers, L.; Schultz, A.; Babl, R. The Effects of Aquatic Therapy on Gait, Balance, and Quality of Life in Patients With Parkinson’s Disease. J. Aquat. Phys. Ther. 2019, 27, 10–24. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO Strategy for the Research Question Construction and Evidence Search. Rev. Lat. Am. Enfermagem 2007, 15, 508–511. [Google Scholar] [CrossRef]
- Cabello López, J. Lectura Crítica de la Evidencia Clínica, 2nd ed.; Elsevier: Barcelona, Spain, 2021; ISBN 9788491138839. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Clerici, I.; Maestri, R.; Bonetti, F.; Ortelli, P.; Volpe, D.; Ferrazzoli, D.; Frazzitta, G. Land Plus Aquatic Therapy Versus Land-Based Rehabilitation Alone for the Treatment of Freezing of Gait in Parkinson Disease: A Randomized Controlled Trial. Phys. Ther. 2019, 99, 591–600. [Google Scholar] [CrossRef]
- Kurt, E.; Büyükturan, B.; Büyükturan, Ö.; Erdem, H.; Tuncay, F. Effects of Ai Chi on balance, quality of life, functional mobility, and motor impairment in patients with Parkinson’s disease. Disabil. Rehabil. 2018, 40, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Cruz, S. Mental health in Parkinson’s disease after receiving aquatic therapy: A clinical trial. Acta Neurol. Belg. 2019, 119, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Pérez-de la Cruz, S. A bicentric controlled study on the effects of aquatic Ai Chi in Parkinson disease. Complement. Ther. Med. 2018, 36, 147–153. [Google Scholar] [CrossRef]
- Pérez de la Cruz, S. Effectiveness of aquatic therapy for the control of pain and increased functionality in people with Parkinson’s disease. Eur. J. phys. Rehabil. Med. 2017, 53, 825–832. [Google Scholar] [CrossRef]
- Shahmohammadi, R.; Sharifi, G.; Melvin, J.; Sadeghi-Demned, E. A comparison between aquatic and land-based physical exercise on postural sway and quality of life in people with Parkinson’s disease: A randomized controlled pilot study. Sport Sci. Health 2017, 13, 341–348. [Google Scholar] [CrossRef]
- Terrens, A.; Soh, S.; Morgan, P. The safety and feasibility of a Halliwick style of aquatic physiotherapy for falls and balance dysfunction in people with Parkinson’s Disease: A single blind pilot trial. PLoS ONE 2020, 15, e0236391. [Google Scholar] [CrossRef]
- Volpe, D.; Giantin, M.; Manuela, P.; Filippetto, C.; Pelosin, E.; Abbruzzese, G.; Anttonini, A. Water-based vs. non-water-based physiotherapy for rehabilitation of postural deformities in Parkinson’s disease: A randomized controlled pilot study. Clin. Rehabil. 2017, 31, 1107–1115. [Google Scholar] [CrossRef]
- Volpe, D.; Giatin, M.; Giantin, M.; Maestri, R.; Frazzitta, G. Comparing the effects of hydrotherapy and land-based therapy on balance in patients with Parkinson’s disease: A randomized controlled pilot study. Clin. Rehabil. 2014, 28, 1210–1217. [Google Scholar] [CrossRef]
- Loureiro, A.; Burkot, J.; Oliveira, J.; Barbosa, J. WATSU therapy for individuals with Parkinson’s disease to improve quality of sleep and quality of life: A randomized controlled study. Complement. Ther. Med. Pract. 2022, 46, 101523. [Google Scholar] [CrossRef]
- Nogueira, A.; dos Santos, M.; Passos-Monteiro, E.; Wolffenbuttel, M.; Gimenes, R.; Zimmermann, M.; Janner, A.; Palmeiro, L.; Gomez, L.; Gomes, F.; et al. The effects of Brazilian dance, deep-water exercise and nordic walking, pre- and post-12 weeks, on functional-motor and non-motor symptoms in trained PwPD. Arch. Gerontol. Geriatr. 2024, 118, 105285. [Google Scholar] [CrossRef]
- Nowak, I. The Effect of Land-Based and Aquatic Exercise Interventions in the Management of Parkinson’s Disease Patients; University of Johannesburg: Johannesburg, South Africa, 2018. [Google Scholar]
- Palamara, G.; Gotti, F.; Maestri, R.; Bera, R.; Gargantini, R.; Bossio, F.; Zivi, I.; Volpe, D.; Ferrazzoli, D.; Frazzitta, G.; et al. Land Plus Aquatic Therapy Versus Land-Based Rehabilitation Alone for the Treatment of Balance Dysfunction in Parkinson Disease: A Randomized Controlled Study With 6-Month Follow-Up. Phys. Ther. 2017, 98, 1077–1085. [Google Scholar] [CrossRef] [PubMed]
- Association, A.P. Australian Guidelines for Aquatic Physiotherapist Working in and/or Managing Hydrotherapy Pools. 2015. Available online: https://docslib.org/doc/11521132/australian-guidelines-for-aquatic-physiotherapists-working-in-and-or-managing-hydrotherapy-pools (accessed on 23 February 2025).
- Port, R.; Rumsby, M.; Brown, G.; Harrison, I.; Amjad, A.; Bale, C. People with Parkinson’s Disease: What Symptoms Do They Most Want to Improve and How Does This Change with Disease Duration? J. Park. Dis. 2021, 11, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Samoudi, G.; Jivegard, M.; Mulavara, A.; Bergquist, F. Effects of Stochastic Vestibular Galvanic Stimulation and LDOPA on Balance and Motor Symptoms in Patients with Parkinson’s Disease. Brain Stimul. 2015, 8, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Huang, M.; Liao, Y.; Xie, X.; Zhu, P.; Liu, Y.; Tan, C. Long-term efficacy of hydrotherapy on balance function in patients with Parkinson’s disease: A systematic review and meta-analysis. Front. Aging Neurosci. 2023, 15, 1320240. [Google Scholar] [CrossRef]
- Dunlap, E.; Lambeck, J.; Gobert, D. ai Chi for Balance, Pain, Functional Mobility, and Quality of Life in Adults: A scoping Review. J. Aquat. Phys. Ther. 2021, 29, 14–28. [Google Scholar] [CrossRef]
- Rutz, D.; Benninger, D. Physical Therapy for Freezing of Gait and Gait Impairments in Parkinson Disease: A systematic Review. PM R 2020, 12, 1140–1156. [Google Scholar] [CrossRef]
- Volpe, D.; Pavan, D.; Morris, M.; Guiotto, A.; Lansek, R.; Fortuna, S.; Frazzitta, G.; Sawacha, Z. under water gait analysis in Parkinson’s disease. Gait Posture 2017, 52, 87–94. [Google Scholar] [CrossRef]
- Nutt, J.; Bloem, B.; Giladi, N.; Hallet, M.; Horak, F.; Nieuwboew, A. Freezing of gait: Moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011, 10, 734–744. [Google Scholar] [CrossRef]
- Huh, Y.; Hwang, S.; Kim, K.; Chung, W.; Youn, J.; Cho, J. Postural sensory correlates of freezing of gait in Parkinson’s Disease. Park. Relat. Disord. 2016, 25, 72–77. [Google Scholar] [CrossRef]
- Gomes Neto, M.; Pontes, S.; Almeida, L.; Da Silva, C.; da Conceicao Sena, C.; Saquetto, M. Effects of water-based exercise on functioning and quality of life in people with Parkinson’s disease: A systematic review and meta-analysis. Clin. Rehabil. 2020, 34, 1425–1435. [Google Scholar] [CrossRef]
- Liposcki, D.; da Silva, I.; Silvano, G.; Zanella, K.; Schneider, R. Influence of a Pilates exercise program on the quality of life of sedentary elderly people: A randomized clinical trial. J. Bodyw. Mov. Ther. 2019, 23, 390–393. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.; Tortelli, L.; Motta, J.; Menguer, L.; Marinano, S.; Tasca, G.; Silveira, G.; Pinho, R.; Silveira, P. Effects of aquatic exercise on mental health, functional autonomy and oxidative stress in depressed elderly individuals: A randomized clínical trial. Clinics 2019, 74, e322. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.; Menguer, L.; Doyenart, R.; Boeira, D.; Milhomens, Y.; Dieke, B.; Volpato, A.; Thirupathi, A.; Silveira, P. Effect of aquatic exercise on menthal health, functional autonomy, and oxidative damages in diabetes elderly individuals. Int. J. Enviorn. Health Res. 2022, 32, 2098–2111. [Google Scholar] [CrossRef]
- Yaremchuk, K. Sleep Disorders in Elderly. Clin. Geriatr. Med. 2018, 34, 205–216. [Google Scholar] [CrossRef]
- Frohnhofen, H.; Popp, R.; Stieglitz, S.; Netzer, N.; Danker-Hopfe, H. Assessment of sleep and sleep disorders in geriatric patients. Z. Gerontol. Geriatr. 2020, 53, 100–104. [Google Scholar] [CrossRef]
- Martins, D.; Brito, R.; Stramosk, J.; Batisti, A.; Madeira, F.; Turnes, B.; Mazzardo-Martins, L.; Santos, A.; Piovezan, A. Peripheral neurobiologic mechanisms of antiallodynic effect of warm water immersion therapy on persistent inflamatory pain. J. Neurosci. Res. 2015, 93, 157–166. [Google Scholar] [CrossRef]
- Bayraktar, D.; Guclu-Gunduz, A.; Yazici, G.; Lambeck, J.; Batur-Caglayan, H.; Irkec, C.; Nazliel, B. Effects of Ai-Chi on balance, functional movility, strength and fatigue in patients with multiple sclerosis: A pilot study. NeuroRehabilitation 2013, 33, 431–437. [Google Scholar] [CrossRef]
- Prado, A.; Reichert, T.; Conceiçao, M.; Delevatti, R.; Kanitz, A.; Kruel, L. Effects of Aquatic Exercise on Muscle Strength in Young and Elderly Adults: A Systematic Review and Meta-Analysis of Ranzomized Trials. J. Strength Cond. Res. 2022, 36, 1468–1483. [Google Scholar] [CrossRef]
- Scalzo, P.; Kümmer, A.; Bretas, T.L.; Cardoso, F.; Teixeira, A.L. Serum levels of brain-derived neurotrophic factor correlate with motor impairment in Parkinson’s disease. J. Neurol. 2010, 257, 540–545. [Google Scholar] [CrossRef]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef]
- Cefis, M.; Chaney, R.; Wirtz, J.; Méloux, A.; Quirié, A.; Leger, C.; Prigent-Tessier, A.; Garnier, P. Molecular mechanisms underlying physical exercise-induced brain BDNF overproduction. Front. Mol. Neurosci. 2023, 16, 1275924. [Google Scholar] [CrossRef] [PubMed]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise Induces Hippocampal BDNF through a PGC-1α/FNDC5 Pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.W.; Bressel, E.; Kim, D.Y. Effects of aquatic exercise on insulin-like growth factor-1, brain-derived neurotrophic factor, vascular endothelial growth factor, and cognitive function in elderly women. Exp. Gerontol. 2020, 132, 110842. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, M.A.; van Wegen, E.E.H.; Newman, M.A.; Heyn, P.C. Exercise-induced increase in brain-derived neurotrophic factor in human Parkinson’s disease: A systematic review and meta-analysis. Transl. Neurodegener. 2018, 7, 7. [Google Scholar] [CrossRef]
- Farinha, C.; Ferreira, J.P.; Serrano, J.; Santos, H.; Oliveiros, B.; Silva, F.M.; Cascante-Rusenhack, M.; Teixeira, A.M. The impact of aquatic exercise programs on the systemic hematological and inflammatory markers of community dwelling elderly: A randomized controlled trial. Front. Physiol. 2022, 13, 838580. [Google Scholar] [CrossRef]
- Ortega, E.; Bote, M.E.; Giraldo, E.; García, J.J.; Ortega, E.; Bote, M.E.; Giraldo, E.; García, J.J. Aquatic exercise improves the monocyte pro- and anti-inflammatory cytokine production balance in fibromyalgia patients. Scand. J. Med. Sci. Sport. 2012, 22, 104–112. [Google Scholar] [CrossRef]
| Study | Item | Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | ||
| Clerici et al. [31] 2019 | Yes | Yes | Yes | No | Yes | Yes | B | p < 0.05 | Yes | Yes | No | 9 |
| Kurt et al. [32] 2018 | Yes | Yes | Yes | No | Yes | Yes | B | p < 0.05 | Yes | Yes | Yes | 10 |
| Loureiro et al. [40] 2022 | Yes | Yes | Yes | No | Yes | Yes | B | p < 0.05 | Yes | No | Yes | 9 |
| Nogueira et al. [41] 2024 | Yes | Yes | Yes | No | Yes | Yes | S | p < 0.05 | Yes | Yes | No | 8 |
| Nowak [42] 2018 | Yes | Yes | Yes | No | Yes | Yes | M | p < 0.05 | Yes | Yes | No | 9 |
| Palamara et al. [43] 2017 | Yes | Yes | Yes | No | Yes | Yes | B | p < 0.05 | Yes | Yes | No | 9 |
| Pérez de la Cruz [35] 2017 | Yes | Yes | Yes | No | Yes | Yes | S | p < 0.001 | Yes | Yes | Yes | 9 |
| Pérez de la Cruz [34] 2018 | Yes | Yes | Yes | No | Yes | Yes | S | p < 0.001 | Yes | Yes | Yes | 9 |
| Pérez de la Cruz [33] 2019 | Yes | Yes | Yes | No | Yes | Yes | S | p < 0.001 | Yes | Yes | Yes | 9 |
| Shahmohammadi et al. [36] 2017 | Yes | Yes | Yes | No | Yes | Yes | M | p < 0.05 | Yes | Yes | Yes | 10 |
| Terrens et al. [37] 2020 | Yes | Yes | Yes | No | No | Yes | B | p < 0.05 | Yes | Yes | No | 8 |
| Volpe et al. [39] 2014 | Yes | Yes | Yes | No | Yes | Yes | B | p < 0.05 | Yes | Yes | Yes | 10 |
| Volpe et al. [38] 2016 | Yes | Yes | Yes | No | Yes | Yes | M | p < 0.05 | Yes | Yes | Yes | 10 |
| First Author, Year, and Country of Publication | Study Desing | Participants (Baseline Sample Side and Characteristics) | Intervention | Outcomes | Results |
|---|---|---|---|---|---|
| Clerici et al. [31], 2019 Italy | RCT | ni = 60 (8 dropouts → nf = 52); 13 ♀ and 39 ♂. 2–3 H&Y stage. Medication ON IG: ni = 30 (3 dropouts → nf = 27); 8 ♀ and 19 ♂. Age (mean ± SD) = 67 ± 8 years CG: ni = 30 (5 dropouts → nf = 25); 5 ♀ and 20 ♂. Age (mean ± SD) = 67 ± 11 years | IG: 4-week aquatic exercise program and intensive multidisciplinary rehabilitation program. CG: 4-week intensive multidisciplinary rehabilitation program. | Balance: BBS Gait: TUG and FOGQ Aerobic capacity: 6MWT QoL: UPDRS II and UPDRS III | IG: Changes from baseline (d = 0.8) BBS: MD = 7.33 points; p < 0.0001 TUG: MD = −3.88 s; p < 0.0001 FOGQ: MD = −5.48 points; p < 0.0001 6MWT: MD = 86 m; p < 0.0001 UPDRS II: MD = −4.85 points; p < 0.0001 UPDRS III: MD = −6.26 points; p < 0.0001 IG vs. CG (d = 0.8) BBS: MD = −0.23 points; p = 0.88 TUG: MD = −0.63 s; p = 0.57 FOGQ: MD = 0.36 points; p = 0.58 6MWT: MD = 22 m; p = 0.19 UPDRS II: MD = −0.49 points; p = 0.41 UPDRS III: MD = −0.58 points; p = 0.42 |
| Kurt et al. [32] 2018 Turkey | RCT | ni = 40 (0 dropouts → nf = 40); 16 ♀ and 24 ♂. 2–3 H&Y stage. Medication ON IG: ni = 20 (0 dropouts → nf = 20); 9 ♀ and 11 ♂. Age (mean ± SD) = 62.41 ± 6.76 years GC: ni = 20 (0 dropouts → nf = 20); 7 ♀ and 13 ♂. Age (mean ± SD) = 63.61 ± 7.18 years | IG: 5-week Ai Chi program. CG: 5-week land-based exercise program. | Balance: Biodex (API, MLI, OBI) and BBS Gait: TUG QoL: PDQ-39 and UPDRS-III | IG: Changes from baseline (d = 0.8) API: MD = −0.5; p < 0.001 MLI: MD = −0.3; p < 0.001 OBI: MD = −0.5; p < 0.001 BBS: MD = 4.41 points; p < 0.001 TUG: MD = −5.01 s; p < 0.001 PDQ-39: MD = −4 points; p < 0.001 UPDRS-III: MD = −3.29 points; p < 0.001 IG vs. CG (d = 0.8) API: MD = −0.4; p < 0.001 MLI: MD = −0.15; p < 0.001 OBI: MD = −0.62; p < 0.001 BBS: MD = 2.5 points; p < 0.001 TUG: MD = −3.96 s; p < 0.001 PDQ-39: MD = −3 points; p < 0.001 UPDRS-III: MD = −1.41 points; p < 0.001 |
| Loureiro et al. [40] 2022 Brazil | RCT | ni = 35 (7 dropouts → nf = 28); 12 ♀ and 16 ♂. 1–3 H&Y stage. Medication ON IG: ni = 18 (4 dropouts → nf = 14); 6 ♀ and 8 ♂. Age (median (IQR)) = 69.0 (11.0) years CG: ni = 17 (3 dropouts → nf = 14); 6 ♀ and 8 ♂. Age (median (IQR)) = 63.0 (5.8) years | IG: 9-week WATSU program and land-based exercise program. CG: 9-week land-based exercise program. | QoL: NHP Sleep quality: PSQI | IG: Changes from baseline NHP: MD = 12 points; p = 0.001; d = 0.87 PDQI: MD = 6 points; p = 0.001; d = 0.85 IG vs. CG NHP: MD = 13 points; p < 0.001; d = 0.68 PSQI: MD = 5.5 points; p < 0.01; d = 0.78 |
| Nogueira et al. [41] 2024 Brazil and Ireland | RCT | ni = 94 (11 dropouts → nf = 83); 33 ♀ and 50 ♂. 1–3 H&Y stage. Medication ON IG: ni = 22 (1 dropout → nf = 21); 4 ♀ and 17 ♂. Age (mean ± SD) = 66.76 ± 8.97 years CG: ni = 37 (6 dropout → nf = 31); 8 ♀ and 23 ♂. Age (mean ± SD) = 67.87 ± 11.20 years | IG: 12-week aquatic exercise program. CG: 12-week Nordic walking program. | Falls: FES Gait: TUG Aerobic capacity: 6MWT Strength: STS and manual dynamometry QoL: PDQ-39 and UPDRS III | IG: Changes from baseline FES: MD = 2.19 points; p > 0.05; d = 0.23 TUG: MD = 0.46 s; p > 0.05; d = 0.07 6MWT: MD = 22.37 m; p > 0.05; d = 0.24 STS: MD = −1.27 s; p > 0.05; d = 0.16 Manual dynamometry: MD = −0.81 kg; p > 0.05; d = 0.04 PDQ-39: MD = 0.86 points; p > 0.05; d = 0.05 UPDRS III: MD = 1.53 points; p > 0.05; d = 0.25 IG vs. CG FES: MD = 0.06 points TUG: MD = 0.16 s 6MWT: MD = −3.41 m STS: MD = −2.36 s Manual dynamometry: MD = 0.43 kg PDQ-39: MD = 4.96 points UPDRS III: MD = 1.04 points |
| Nowak [42] 2018 South Africa | RCT | ni = 43 (8 dropouts → nf = 35). 1–3 H&Y stage. Medication ON Age (mean ± SD) = 65.2 ± 9.85 years IG: ni = 23 (6 dropouts → nf = 17) CG: ni = 20 (2 dropouts → nf = 18) | IG: 12-week aquatic exercise program. CG: 12-week land-based exercise program. | Posture: kyphosis Balance: BBS Gait: TUG and speed 10 m Strength: STS, knee and ankle isometric, manual dynamometry, knee isokinetic (quadriceps-hamstring relation) Flexibility: SLRT and shoulder ROM Mental health: MHC, BDI and MBCBA. QoL: UPDRS | IG: Changes from baseline Kyphosis: MD = 1; p > 0.05 BBS: MD = 2 points; p = 0.003 TUG: MD = 1 s; p < 0.001 Speed 10 m: MD = 0.3 m/s; p < 0.001 STS: MD = 2 reps; p < 0.001 Knee isometric: MD = 1.3 kg; p = 0.091 Ankle isometric: MD = 2.6 kg; p = 0.004 Manual dynamometry: MD = 2.5 kg; p = 0.021 Knee isokinetic: MD = 8%; p = 0.036 SLRT: MD = 7°; p < 0.001 Shoulder ROM: p > 0.05 flexion and extension MHC: p > 0.05 in all sections BDI: MD = −4 points; p = 0.003 MBCBA: p > 0.05 in all sections UPDRS: MD = −13 points; p < 0.001 IG vs. CG Kyphosis: MD = −4; p > 0.05 BBS: MD = 0 points; p = 0.352 TUG: MD = 0 s; p = 0.998 Speed 10 m: MD = 0 m/s; p = 0.999 STS: MD = −1 reps; p = 0.971 Knee isometric: MD = −0.1 kg; p = 0.116 Ankle isometric: MD = −0.4 kg; p = 0.663 Manual dynamometry: MD = 2.9 kg; p = 0.603 Knee isokinetic: MD = 1%; p = 0.363 SLRT: MD = 3°; p = 0.015 Shoulder ROM: p > 0.05 flexion and extension MHC: p > 0.05 in all sections BDI: MD = −1 points; p = 0.771 MBCBA: p > 0.05 in all sections UPDRS: MD = 0; p = 0.629 |
| Palamara et al. [43] 2017, Italy | RCT | ni = 34 (0 dropouts → nf = 34); 14 ♀ and 20 ♂. 2,5–3 H&Y stage. Medication ON IG: ni = 17 (0 dropouts → nf = 17); 8 ♀ and 9 ♂. Age (mean ± SD) = 70.9 ± 5.7 years CG: ni = 17 (0 dropouts → nf = 17); 6 ♀ and 11 ♂. Age (mean ± SD) = 70.8 ± 5.3 years | IG: 4-week aquatic exercise program and intensive, multidisciplinary rehabilitation program. CG: 4-week intensive multidisciplinary rehabilitation program. | Balance: BBS Gait: TUG QoL: UPDRS II and UPDRS III | IG: Changes from baseline (d = 0.8) BBS: MD = 7.8 points; p = 0.0001 TUG: MD = −3.45 s; p = 0.001 UPDRS II: MD = −5.1 points; p = 0.0005 UPDRS III: MD = −6 points; p = 0.0009 IG vs. CG (d = 0.8) BBS: MD = 0.5 points; p = 0.99 TUG: MD = −1 s; p = 0.99 UPDRS II: MD = 1 points; p = 0.88 UPDRS III: MD = 1 points; p = 0.99 |
| Pérez de la Cruz [35] 2017 Spain | RCT | ni = 30 (0 dropouts → nf = 30); 16 ♀ and 14 ♂. 1–3 H&Y stage. Medication OFF IG: ni = 15 (0 dropouts → nf = 15). Age (mean ± SD) = 66.80 ± 5.26 years CG: ni = 15 (0 dropouts → nf = 15). Age (mean ± SD) = 67.53 ± 9.89 years | IG: 10-week Ai Chi program. CG: 10-week land-based exercise program. | Pain: VAS Balance: BBS Gait: TUG and Tinetti Strength: STS QoL: UPDRS | IG: Changes from baseline VAS: MD = −1.4 points; p < 0.001; d = 0.487 BBS: MD = 4.1 points; p < 0.001; d = 0.412 TUG: MD = −2.5 s; p < 0.001; d = 0.295 Tinetti: MD = 2.6 points; p < 0.001; d = 0.314 STS: MD = −1.7 s; p < 0.001; d = 0.225 UPDRS: MD = 0 points; p < 0.001; d = 0.516 IG vs. CG VAS: MD = −0.9 points; p = 0.005; d = 0.233 BBS: MD = 4.1 points; p < 0.001; d = 0.412 TUG: MD = −2.5 s; p < 0.001; d = 0.295 Tinetti: MD = 2.9 points; p < 0.001; d = 0.418 STS: MD = −1.6 s; p = 0.006; d = 0.177 UPDRS: MD = 0.4 points; p < 0.001; d = 0.453 |
| Pérez de la Cruz [34] 2018 Spain | RCT | ni = 29 (0 dropouts → nf = 29); 17 ♀ and 12 ♂. 1–3 H&Y stage. Medication OFF IG: ni = 14 (0 dropouts → nf = 14); 9 ♀ and 5 ♂. Age (mean ± SD) = 65.87 ± 7.09 years CG: ni = 15 (0 dropouts → nf = 15); 8 ♀ and 7 ♂. Age (mean ± SD) = 66.44 ± 5.72 years | IG: 11-week Ai Chi program. CG: 11-week land-based exercise program. | Pain: VAS Balance: monopodal balance Gait: TUG Strength: STS Mental Health: GDS QoL: PDQ-39 | IG: Changes from baseline VAS: MD = −1.4 points; p < 0.001; d = 0.489 Right monopodal balance; MD = 4.2 s; p < 0.001; d = 0.495 Left monopodal balance: MD = 2.94 s; p < 0.001; d = 0.392 TUG: MD = −2 s; p < 0.001; d = 0.284 STS: MD = −1.6 s; p = 0.001; d = 0.233 GDS: MD = −0.14 points; p = 0.001; d = 0.279 PDQ-39: p > 0.05 in all sections except social support IG vs. CG VAS: MD = −1 point; p = 0.005; d = 0.248 Right monopodal balance: MD = 4.27 s; p < 0.001; d = 0.516 Left monopodal balance: MD = 3 s; p < 0.001; d = 0.390 TUG: MD = −1.8 s; p < 0.001; d = 0.288 STS: MD = −1.4 s; p = 0.006; d = 0.186 GDS: MD = 0.06 points; p = 0.002; d = 0.240 PDQ-39: p > 0.05 in all sections except social support |
| Pérez de la Cruz [33] 2019 Spain | RCT | ni = 30 (0 dropouts → nf = 30); 15 ♀ and 15 ♂. 1–3 H&Y stage. Medication OFF IG: ni = 15 (0 dropouts → nf = 15); 8 ♀ and 7 ♂. Age (mean ± SD) = 64.40 ± 5.17 years CG: ni = 15 (0 dropouts → nf = 15); 7 ♀ and 8 ♂. Age (mean ± SD) = 65.83 ± 8.92 years | IG: 10-week Ai Chi program. CG: 10-week land-based exercise program. | Pain: VAS Mental health: GDS QoL: SF-36 | IG: Changes from baseline VAS: MD = −1.4 points; p < 0.001 GDS: MD = −0.14 points; p < 0.001 SF-36: p ≤ 0.01 in all sections IG vs. CG VAS: MD = −1 points; p = 0.005 GDS: MD = 0.06 points; p = 0.002 SF-36: p ≤ 0.01 in all sections |
| Shahmohammadi et al. [36] 2017 Iran and United Kingdom | RCT | ni = 22 (2 dropouts → nf = 20); 20 ♂. 2–3 H&Y stage. Medication ON IG: ni = 11 (1 dropouts → nf = 10); 10 ♂. Age (mean ± SD) = 60.50 ± 5.44 years CG: ni = 11 (1 dropouts → nf = 10); 10 ♂. Age (mean ± SD) = 63.20 ± 4.94 years | IG: 8-week aquatic exercise program. CG: 8-week land-based exercise program. | Balance: Postural sway evaluation in a Kistler force plate (sway range, mean speed, sway area, and mean frequency) QoL: PDQL | IG: Changes from baseline (d = 0.65) Sway range: MD = 21.35 mm; p = 0.055 Mean speed: MD = −6.54 mm/s; p = 0.001 Sway area: MD = 11.11 mm2/s; p = 0.001 Mean frequency: MD = −0.11 Hz; p = 0.003 PDQL: MD = 21 points; p < 0.001 IG vs. CG (d = 0.65) Sway range: MD = 8.9 mm; p = 0.52 Mean speed; MD = −3.6 mm/s; p = 0.01 Sway area: MD = 12.88 mm2/s; p = 0.33 Mean frequency: MD = −0.13 HZ; p = 0.59 PDQL: MD = 10.8 points; p < 0.001 |
| Terrens et al. [37] Australia | RCT | ni = 30 (5 dropouts → nf = 25); 6 ♀ and 24 ♂. 1–3 H&Y stage. Medication ON IG 1: ni = 11 (2 dropouts → nf = 9); 1 ♀ and 10 ♂. Age (mean ± SD) = 74.1 ± 6.6 years IG 2: ni = 10 (1 dropouts → nf = 9); 3 ♀ and 7 ♂. Age (mean ± SD) = 65.6 ± 7.7 years CG: ni = 9 (2 dropouts → nf = 7); 2 ♀ and 7 ♂. Age (mean ± SD) = 76.4 ± 7.4 years | IG 1: 12-week Halliwik program. IG 2: 12-week aquatic exercise program. CG: 12-week land-based exercise program. | Balance: BBS and Mini BESTest. Falls: mFES QoL: UPDRS III | IG 1: Changes from baseline BBS: MedD = 0 points; p > 0.05 Mini-best: MedD = 8 points; p = 0.011 mFES: MedD = 0.5 points; p > 0.05 UPDRS III: MedD = −1 points; p > 0.05 IG 1 vs. CG BBS: MedD = −1 points; p > 0.05 Mini-Best: MedD = 10 points; p > 0.05 mFES: MedD = −1 points; p > 0.05 UPDRS III: MedD = 5 points; p > 0.05 IG 2: Changes from baseline. BBS: MedD = −1 points; p > 0.05 Mini-Best: MedD: −3 points; p > 0.05 mFES: MedD: 0.25 points; p > 0.05 UPDRS III: MedD: 5 points; p > 0.05 IG 2 vs. CG BBS: MedD = 0 points; p > 0.05 Mini-best: MedD = −1 points; p > 0.05 mFES: MedD = −1.25 points; p > 0.05 UPDRS III: MedD = 14 points; p > 0.05 |
| Volpe et al. [39] 2914 Italy | RCT | ni = 34 (0 dropouts → nf = 34). 2,5–3 H&Y stage. Medication ON IG: ni = 17 (0 dropouts → nf = 17). Age (mean ± SD) = 68 ± 7 years CG: ni = 17 (0 dropouts → nf = 17). Age (mean ± SD) = 66 ± 8 years | IG: 8-week aquatic exercise program. CG: 8-week land-based exercise program. | Balance: evaluation of the COP sway area with open and closed eyes. BBS and ABC Falls: FES y falls diary. Gait: TUG QoL: PDQ-39, UPDRS II and III | IG: Changes from baseline (d = 0.8) Sway area open eyes: MD = 49.7 mm2; p = 0.002 Sway area closed eyes: MD = 45.4 mm2; p = 0.010 BBS: MD = 9.9 points; p < 0.0001 ABC: MD = 16.8 points; p < 0.0001 FES: MD = −5.9 points; p < 0.0001 Falls diary: MD = −2.4; p < 0.0001 TUG: MD = −2.0 s; p < 0.0001 PDQ-39: MD = −18.4 points; p < 0.0001 UPDRS II: MD = −4.3 points; p < 0.0001 UPDRS III: MD = −8.3 points; p < 0.0001 IG vs. CG (d = 0.8) Sway area open eyes: MD = 24.3 mm2; p = 0.2871 Sway area closed eyes: MD = 38.5 mm2; p = 0.0480 BBS: MD = 3.9 points; p = 0.0046 ABC: MD = 12.7 points; p = 0.0001 FES: MD = −4 points; p = 0.0026 Falls diary: MD = −2; p = 0.0010 TUG: MD = −0.9 s; p = 0.151 PDQ-39: MD = −10.4 points; p = 0.0063 UPDRS II: MD = 0.8 points; p = 0.4336 UPDRS III: MD = 0.1 points; p = 0.9381 |
| Volpe et al. [38] 2016 Italy | RCT | ni = 30 (6 dropouts → nf = 24); 11 ♀ and 19 ♂. 1–3 H&Y stage. Medication ON IG: ni = 15 (2 dropouts → nf = 13); 6 ♀ and 9 ♂. Age (mean ± SD) = 70.6 ± 7.8 years CG: ni = 15 (4 dropouts → nf = 11); 5 ♀ and 10 ♂. Age (mean ± SD) = 70 ± 7.8 years | IG: 8-week aquatic exercise program. CG: 8-week land-based exercise program. | Posture: dorsal and cervical BAK and shoulder symmetry Balance: BBS and ABC. Falls: FES Gait: TUG QoL: PDQ-39 and UPDRS III | IG: Changes from baseline Dorsal BAK: MD = −22.5°; p = 0.008 Cervical BAK: MD = −62.2°; p < 0.001 Shoulder symmetry: MD = −2.3°; p = 0.002 BBS: MD = 3.5 points; p < 0.001 ABC: MD = 8.1%; p = 0.02 FES: MD = −2.3 points; p = 0.027 TUG: MD = −1.4 s; p = 0.036 PDQ-39: MD = −9,6 points; p < 0.001 UPDRS III: MD = −6.1 points; p = 0.001 IG vs. CG Dorsal BAK: MD = −16°; p = 0.046 Cervical BAK: MD = −66.9°; p = 0.024 Shoulder symmetry: MD = −2.6°; p = 0.047 BBS: MD = −3.4 points; p > 0.05 ABC: MD = 5.7%; p > 0.05 FES: MD = −1 points; p > 0.05 TUG: MD = 1.8 s; p > 0.05 PDQ-39: MD = −5.4 points; p = 0.001 UPDRS: MD = −1.1 points; p > 0.05 |
| Author and Year | Exercises | Volume and Intensity | Tª (°C) | Frecuency (Days/Week) | Time (Minutes/Session) | Duration (Weeks) | Supervision |
|---|---|---|---|---|---|---|---|
| Clerici et al. [31] 2019 | IG: Warm-up (walking in different directions, with heels, counter-resistance, and with eyes closed); Principal (proprioception, dual-task walking, obstacles and turns); Cool down (walking and stretching). All submerged in water. + CG intervention | 70–80% reserve HR | 33–34 | 3 AT + CG (AT replaces session 1) | 60 | 4 | Yes |
| CG: Session 1 (cardiovascular warm-up, stretching, ROM work, and core and posture work). Session 2 (work on gait, balance, endurance, and motor control). Session 3 (autonomy in ADL). Session 4 (speech therapy). Day 6 (training with devices). | 70–80% reserve HR | - | 5 (4 daily sessions) + 1 session with devices | 60 | 4 | Yes | |
| Kurt et al. [32] 2018 | IG: Warm-up (mobility exercises); Principal (Ai Chi, 16 exercises with slow movements and deep breathing to work on balance, strength, flexibility, and breathing). Cool-down (walking and stretching). All submerged in water. | Not specified | 32 | 5 | 60 | 5 | Yes |
| CG: Warm-up (light aerobic exercise); Principal (stretching, gait, and balance work); Cool-down (slow walking and breathing exercises). | Not specified | - | 5 | 60 | 5 | Yes | |
| Loureiro et al. [40] 2022 | IG: Warm-up (recreational activities in the pool); Principal (WATSU, 12 exercises mainly in supine position); Cool-down (massage therapy in pool) + CG intervention | Not specified | 34.4 - 36 | 2 WATSU + 2 land-based | 30 | 9 | Yes |
| CG: Warm-up (mobility exercises); Principal (exercises with a wide ROM, postural control, and balance); Cool-down (stretching). | Not specified | - | 2 | 30 | 9 | Yes | |
| Nogueira et al. [41] 2024 | IG: Warm-up (mobility exercises and stretching); Principal (running, strength exercises, balance, postural control, coordination, dual task, and diving); Cool-down (stretching and relaxation). All submerged in water. | Individualized | - | 2 | 60 | 12 | Yes |
| CG: Warm-up (coordination); Principal (Nordic walking); Cool-down (stretching). | Individualized | - | 2 | 60 | 12 | Yes | |
| Nowak [42] 2018 | IG: Warm-up (walking); Principal (gait work, joint mobility, balance, and strength exercises); Cool-down (walking and stretching). All submerged in water. | Not specified | - | 2 | 60 | 12 | Yes |
| CG: Warm-up (static bicycle), Principal (gait work, joint mobility, balance, and strength exercises); Cool-down (walking and stretching) | Not specified | - | 2 | 60 | 12 | Yes | |
| Palamara et al. [43] 2017 | IG: Warm-up (walking in different directions, with heels, counter-resistance, and with eyes closed); Principal (proprioception, dual-task walking, obstacles, and turns); Cool-down (walking and stretching). All submerged in water. + CG intervention | 70–80% reserve HR | - | 3 AT + CG (AT replaces session 1) | 60 | 4 | Yes |
| CG: Session 1 (cardiovascular warm-up, stretching, ROM work, and core and posture work). Session 2 (work on gait, balance, endurance, and motor control). Session 3 (autonomy in ADL). Session 4 (speech therapy). Day 6 (training with devices). | 70–80% reserve HR | - | 5 (4 daily sessions) + 1 session with devices | 60 | 4 | Yes | |
| Pérez de la Cruz [35] 2017 | IG: Warm-up (recreational exercises); Principal (Ai Chi, 19 exercises emphasizing reach and postural responses, trunk rotation, bipodal, and monopodal balance); Cool-down. | Not specified | 30 | 2 | 45 | 10 | Yes |
| CG: Warm-up (mobility exercises and walking); Principal (aerobic and strength exercises); Cool-down (functional ADL exercises, balance, proprioception, facial exercises, and stretching) | Not specified | - | 2 | 45 | 10 | Yes | |
| Pérez de la Cruz [34] 2018 | IG: Warm-up (recreational exercises); Principal (Ai Chi, 19 exercises); Cool-down. | Not specified | 30 | 2 | 45 | 11 | Yes |
| CG: Warm-up (mobility exercises and walking); Principal (aerobic and strength exercises); Cool-down (functional ADL exercises, balance, proprioception, facial exercises, and stretching) | Not specified | - | 2 | 45 | 11 | Yes | |
| Pérez de la Cruz [33] 2019 | IG: Warm-up (recreational exercises); Principal (Ai Chi, 10 exercises “Contemplating”, “Floating”, “Uplifting”, “Enclosing”, “Folding”, “Soothing”, “Gathering”, “Freeing”, “Shifting”, “Accepting”); Cool-down. | Not specified | 30 | 2 | 45 | 10 | Yes |
| CG: Warm-up (mobility exercises and gaiting); Principal (aerobic and strength exercises); Cool down (functional ADL exercises, balance, proprioception, facial exercises, and stretching) | Not specified | - | 2 | 45 | 10 | Yes | |
| Shahmohammadi et al. [36] 2017 | IG: Warm-up (walking); Principal (gait work, walking in different directions, with heels, tiptoeing, and throwing a ball in different directions); Cool-down (stretching). All submerged in water. | 2 series of 10–20 repetitions | 30 | 3 | 60 | 8 | Yes |
| CG: Warm-up (walking); Principal (gait work, walking in different directions, with heels, tiptoeing, and throwing a ball in different directions); Cool down (stretching). | 2 series of 10–20 repetitions | - | 3 | 60 | 8 | Yes | |
| Terrens et al. [37] 2020 | IG 1: Warm-up (walking); Principal (Halliwick, balance, trunk mobility, core work, and rotations); Cool-down (stretching) | 13–14 Borg Scale | 34,7 | 1 | 60 | 12 | Yes |
| IG 2: Warm-up (walking); Principal (balance, aerobic, and strength exercises); Cool-down (stretching). All submerged in water. | 13–14 Borg Scale | 34,7 | 1 | 60 | 12 | Yes | |
| CG: Warm-up (walking); Principal (balance, aerobic, and strength exercises); Cool-down (stretching). | 13–14 Borg Scale | - | 1 | 60 | 12 | Yes | |
| Volpe et al. [39] 2014 | IG: Warm-up (aerobic exercises and stretching); Principal (balance training with external disturbances, functional reach, postural responses, and strength exercises); Cool down. All submerged in water. | Not specified | 30 | 5 | 60 | 8 | Yes |
| CG: Warm-up (aerobic exercises and stretching); Principal (balance training with external disturbances, functional reach, postural responses, and strength exercises); Cool-down. | Not specified | - | 5 | 60 | 8 | Yes | |
| Volpe et al. [38] 2016 | IG: Warm-up; Principal (balance training with external disturbances); Cool-down (relax exercises). All submerged in water. | Not specified | - | 5 | 60 | 8 | Yes |
| CG: Warm-up (aerobic exercises and stretching); Principal (balance training with external disturbance); Cool-down (relax exercises) | Not specified | - | 5 | 60 | 8 | Yes |
| Study | Item | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| Clerici et al. [31] 2019 |  |  |  |  |  |  |  |
| Kurt et al. [32] 2018 |  |  |  |  |  |  |  |
| Loureiro et al. [40] 2022 |  |  |  |  |  |  |  |
| Nogueira et al. [41] 2024 |  |  |  |  |  |  |  |
| Nowak [42] 2018 |  |  |  |  |  |  |  |
| Palamara et al. [43] 2017 |  |  |  |  |  |  |  |
| Pérez de la Cruz [35] 2017 |  |  |  |  |  |  |  |
| Pérez de la Cruz [34] 2018 |  |  |  |  |  |  |  |
| Pérez de la Cruz [33] 2019 |  |  |  |  |  |  |  |
| Shahmohammadi et al. [36] 2017 |  |  |  |  |  |  |  |
| Terrens et al. [37] 2020 |  |  |  |  |  |  |  |
| Volpe et al. [39] 2014 |  |  |  |  |  |  |  |
| Volpe et al. [38] 2017 |  |  |  |  |  |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santamaría, G.; Fernández-Gorgojo, M.; Gutiérrez-Abejón, E.; García Gómez, B.; Molina, Á.; Fernández-Lázaro, D. Aquatic Therapy Versus Land-Based Therapy in Patients with Parkinson’s Disease: A Systematic Review. J. Funct. Morphol. Kinesiol. 2025, 10, 170. https://doi.org/10.3390/jfmk10020170
Santamaría G, Fernández-Gorgojo M, Gutiérrez-Abejón E, García Gómez B, Molina Á, Fernández-Lázaro D. Aquatic Therapy Versus Land-Based Therapy in Patients with Parkinson’s Disease: A Systematic Review. Journal of Functional Morphology and Kinesiology. 2025; 10(2):170. https://doi.org/10.3390/jfmk10020170
Chicago/Turabian StyleSantamaría, Gema, Mario Fernández-Gorgojo, Eduardo Gutiérrez-Abejón, Blanca García Gómez, Ángela Molina, and Diego Fernández-Lázaro. 2025. "Aquatic Therapy Versus Land-Based Therapy in Patients with Parkinson’s Disease: A Systematic Review" Journal of Functional Morphology and Kinesiology 10, no. 2: 170. https://doi.org/10.3390/jfmk10020170
APA StyleSantamaría, G., Fernández-Gorgojo, M., Gutiérrez-Abejón, E., García Gómez, B., Molina, Á., & Fernández-Lázaro, D. (2025). Aquatic Therapy Versus Land-Based Therapy in Patients with Parkinson’s Disease: A Systematic Review. Journal of Functional Morphology and Kinesiology, 10(2), 170. https://doi.org/10.3390/jfmk10020170









