Intensity of Resistance Exercise and Its Effects on Pain, Functionality, and Quality of Life in Adults with Fibromyalgia: A Systematic Review
Abstract
1. Introduction
2. Study Design
2.1. Search Strategy
2.2. Eligibility Criteria
- Population (P): Adults (≥18 years) of both sexes with fibromyalgia diagnosis according to the American College of Rheumatology criteria.
- Intervention (I): Resistance exercise programs lasting more than two weeks with quantifiable intensity specifications.
- Comparison (C): Control group with the same fibromyalgia pathology or healthy people subjected to different intensities of resistance exercise.
- Outcomes (O): Effects on pain, functionality, and quality of life in people with fibromyalgia.
- Study design (S): Randomized controlled trials, controlled clinical trials, and experimental studies published in English or Spanish.
2.3. Data Extraction
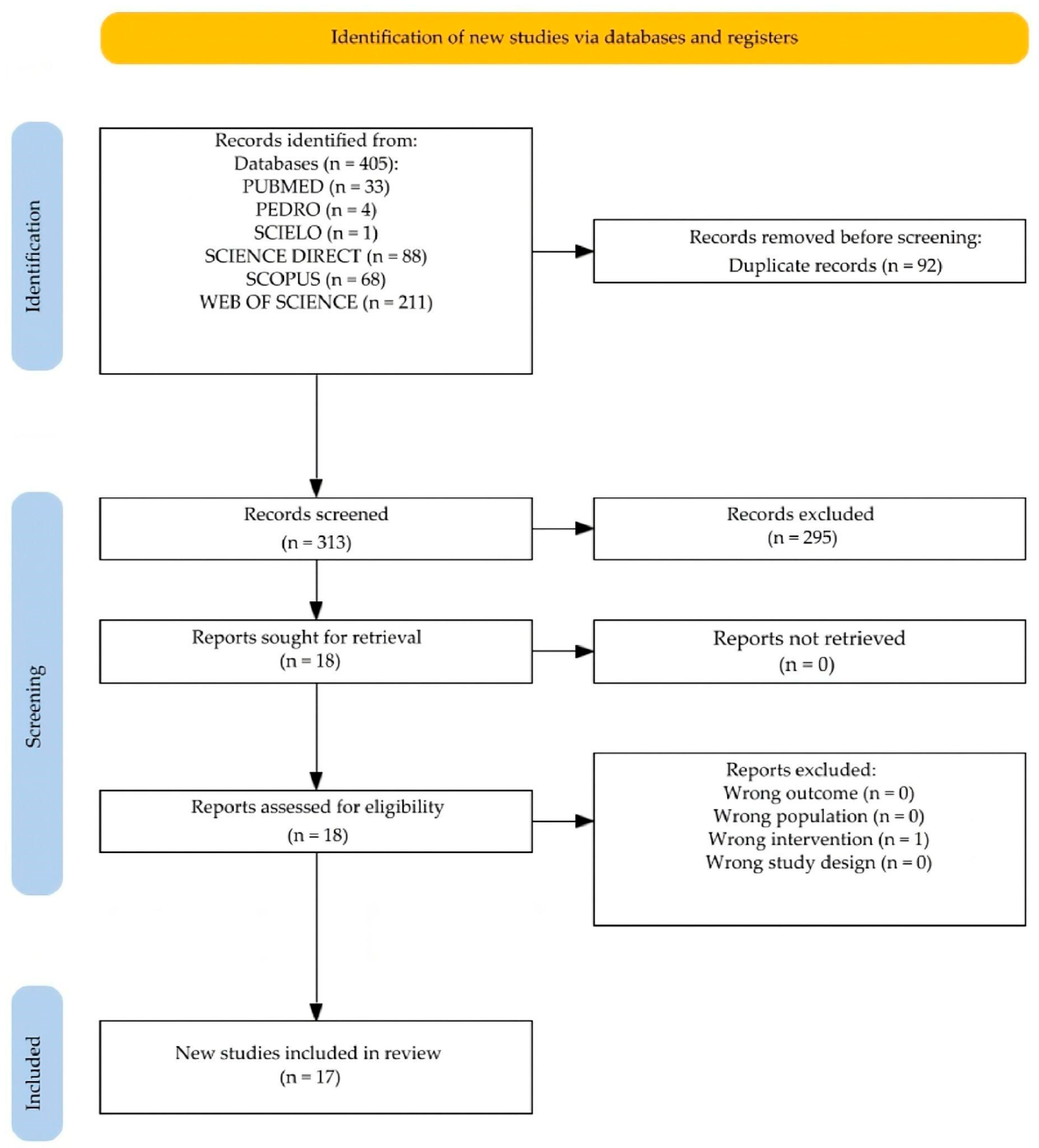
3. Results
3.1. Characteristics of Included Studies
3.2. Instruments Used to Quantify the Intensity of RE
3.3. Questionnaires Used on Pain, Functionality, and QOL
3.4. Characteristics of Interventions
3.5. Effects of Resistance Training on Pain, Functionality, and QOL
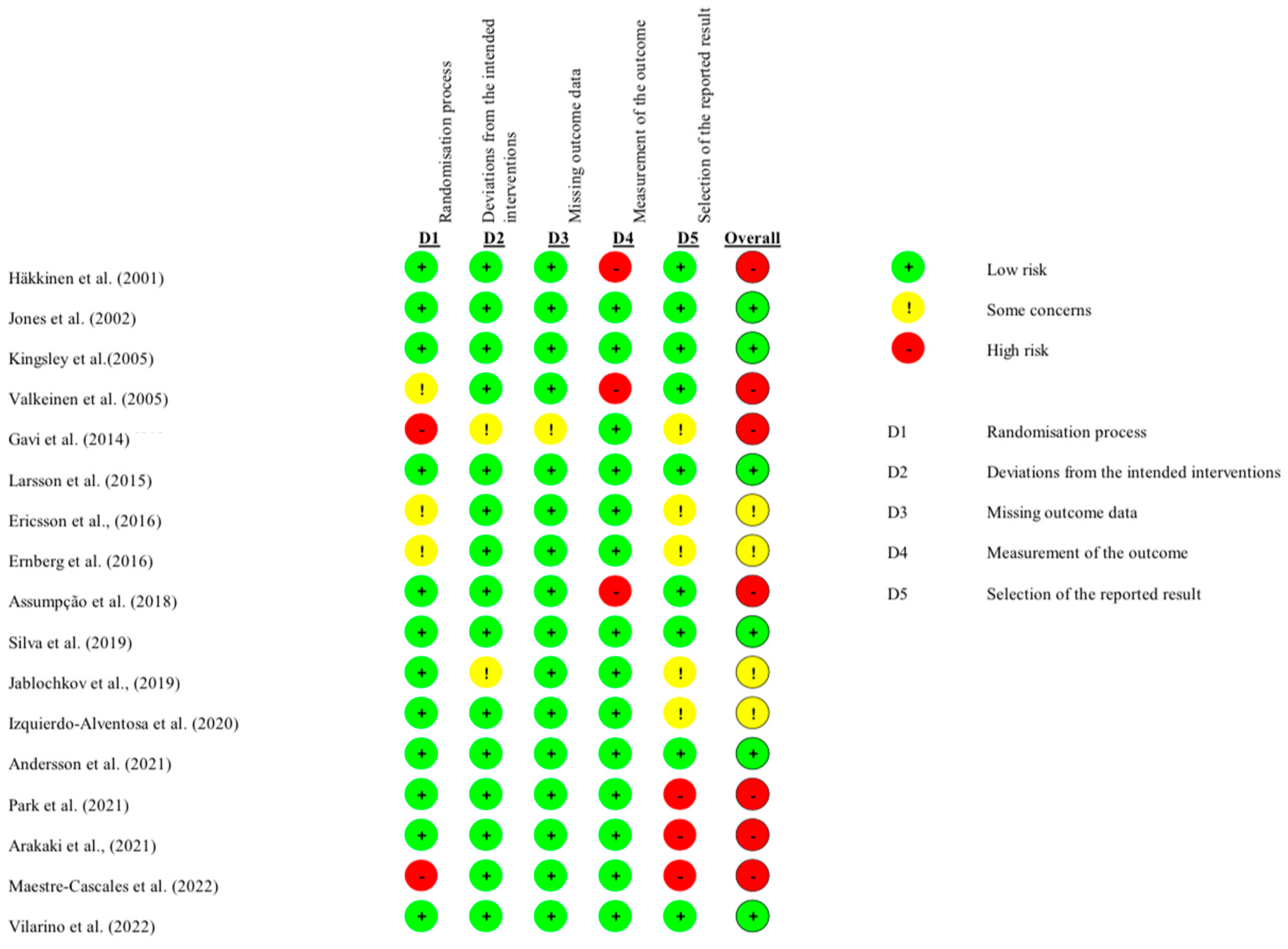
3.6. Quality of Studies and Risk of Bias
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1RM | one repetition maximum |
| BDI-FS | Beck Depression Inventory-Fast Screen |
| BPI | Brief Pain Inventory |
| BRUMS | Brunel Mood Scale |
| CG | control group |
| CS-PFP | Continuous-Scale Physical Functional Performance |
| FG | flexibility group |
| FIQ | Fibromyalgia Impact Questionnaire |
| FIQR | Fibromyalgia Impact Questionnaire-Revised |
| FM | fibromyalgia |
| HADS | Hospital Anxiety and Depression Scale |
| HAQ | Health Assessment Questionnaire |
| HC | healthy control |
| HIRE | high-intensity resistance exercise |
| LIRE | low-intensity resistance exercise |
| MIRE | medium-intensity resistance exercise |
| MVC | maximum voluntary capacity |
| OMNI-GSE | OMNI Generalized Subjective Exertion Scale |
| PIRE | progressive-intensity resistance exercise |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| PSQI | Pittsburgh Sleep Quality Index |
| QOL | quality of life |
| QOLS | Quality of Life Scale |
| RCT | randomized controlled trial |
| RE | resistance exercise |
| SF-36 | Short Form-36 Health Survey |
| STAI-S | State-Trait Anxiety Inventory |
| TP | tender point |
| VAS | Visual Analog Scale |
| WF | Weekly Frequency |
Appendix A
| PubMed—14 August 2024 | ||
| Search | Query | Items Found |
| #10 | ((Fibromyalgia [MeSH Terms]) OR (Fibromyalgia [Title/Abstract])) AND (((((Resistance Training [MeSH Terms]) OR (Resistance Training [Title/Abstract])) OR (Strength Training [MeSH Terms])) OR (Strength Training [Title/Abstract])) OR (Resistance Exercise [Title/Abstract])) | 33 |
| #9 | ((((Resistance Exercise [Title/Abstract]) OR (Strength Training [Title/Abstract])) OR (Strength Training [MeSH Terms])) OR (Resistance Training [Title/Abstract])) OR (Resistance Training [MeSH Terms]) | 7351 |
| #8 | Resistance Exercise [Title/Abstract] | 2.130 |
| #7 | Strength Training [Title/Abstract] | 1.827 |
| #6 | Strength Training [MeSH Terms] | 5.065 |
| #5 | Resistance Training [Title/Abstract] | 3.526 |
| #4 | Resistance Training [MeSH Terms] | 5.065 |
| #3 | (Fibromyalgia*[MeSH Terms]) OR (Fibromyalgia*[Title/Abstract]) | 1.124 |
| #2 | Fibromyalgia*[Title/Abstract] | 1.091 |
| #1 | Fibromyalgia*[MeSH Terms] | 1.028 |
| Scopus—14 August 2024 | ||
| Search | Query | Items Found |
| #6 | (fibromyalgia) AND (resistance AND training OR strength AND training OR resistance AND exercise) | 68 |
| #5 | (resistance AND training OR strength AND training OR resistance AND exercise) | 15.403 |
| #4 | Resistance AND Exercise | 21,266 |
| #3 | Strength AND Training | 21.366 |
| #2 | Resistance AND Training | 19.201 |
| #1 | Fibromyalgia | 6969 |
| SciELO—14 August 2024 | ||
| Search | Query | Items Found |
| #6 | (fibromyalgia) AND (resistance AND training OR strength AND training OR resistance AND exercise) | 1 |
| #5 | (resistance AND training OR strength AND training OR resistance AND exercise) | 275 |
| #4 | Resistance AND Exercise | 870 |
| #3 | Strength AND Training | 1.208 |
| #2 | Resistance AND Training | 1.047 |
| #1 | Fibromyalgia | 394 |
| Web of Science—14 August 2024 | ||
| Search | Query | Items Found |
| #6 | (fibromyalgia) AND (resistance AND training OR strength AND training OR resistance AND exercise) | 211 |
| #5 | (resistance AND training OR strength AND training OR resistance AND exercise) | 133,093 |
| #4 | Resistance AND Exercise | 41,022 |
| #3 | Strength AND Training | 68,197 |
| #2 | Resistance AND Training | 59,017 |
| #1 | Fibromyalgia | 12,073 |
| Science Direct—14 August 2024 | ||
| Search | Query | Items Found |
| #6 | (fibromyalgia) AND (resistance AND training OR strength AND training OR resistance AND exercise) | 88 |
| #5 | (resistance AND training OR strength AND training OR resistance AND exercise) | 2576 |
| #4 | Resistance AND Exercise | 4164 |
| #3 | Strength AND Training | 9494 |
| #2 | Resistance AND Training | 4014 |
| #1 | Fibromyalgia | 1078 |
| PEDro—14 August 2024 | ||
| Search | Query | Items Found |
| #6 | (fibromyalgia) AND (resistance AND training OR strength AND training OR resistance AND exercise) | 4 |
| #5 | (resistance AND training OR strength AND training OR resistance AND exercise) | 1236 |
| #4 | Resistance AND Exercise | 3.835 |
| #3 | Strength AND Training | 5059 |
| #2 | Resistance AND Training | 3.727 |
| #1 | Fibromyalgia | 669 |
References
- Siracusa, R.; Paola, R.D.; Cuzzocrea, S.; Impellizzeri, D. Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int. J. Mol. Sci. 2021, 22, 3891. [Google Scholar] [CrossRef]
- Vilarino, G.T.; Andreato, L.V.; de Souza, L.C.; Branco, J.H.L.; Andrade, A. Effects of resistance training on the mental health of patients with fibromyalgia: A systematic review. Clin. Rheumatol. 2021, 40, 4417–4425. [Google Scholar] [CrossRef] [PubMed]
- Sarzi-Puttini, P.; Giorgi, V.; Marotto, D.; Atzeni, F. Fibromyalgia: An update on clinical characteristics, aetiopathogenesis and treatment. Nat. Rev. Rheumatol. 2020, 16, 645–660. [Google Scholar] [CrossRef]
- García, D.; Abud, C. Physiopathology of fibromyalgia. Reumatol. Clin. 2020, 16, 191–194. [Google Scholar] [CrossRef]
- Ardila, D.; Alejandro, C. Fibromialgia Una Enfermedad Sin Prevención Ni Promoción. Master’s Thesis, Universidad Nacional de Colombia, Bogotá, Colombia, 2024. [Google Scholar]
- Latorre-Santiago, D.; Torres-Lacomba, M. Fibromyalgia and therapeutic exercise: A qualitative systematic review. Rev. Int. Med. Cienc. Act. Fis. Deporte 2017, 17, 183–204. [Google Scholar]
- Busch, A.J.; Webber, S.C.; Richards, R.S.; Bidonde, J.; Schachter, C.L.; Schafer, L.A.; Danyliw, A.; Sawant, A.; Dal Bello-Haas, V.; Rader, T.; et al. Resistance exercise training for fibromyalgia. Cochrane Database Syst. Rev. 2013, 12, 1465–1858. [Google Scholar] [CrossRef]
- Bennie, J.A.; Shakespear-Druery, J.; De Cocker, K. Muscle-strengthening Exercise Epidemiology: A New Frontier in Chronic Disease Prevention. Sports Med. Open 2020, 6, 40. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports Medicine. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334. [Google Scholar] [CrossRef]
- Ocampo, N.; Ramírez, J. Effects of muscular strength training programs on functional performance: Systematic review. Rev. Fac. Med. 2018, 66, 399–410. [Google Scholar] [CrossRef]
- Schoenfeld, B.J.; Grgic, J.; Ogborn, D.; Krieger, J.W. Strength and Hypertrophy Adaptations Between Low- vs. High-Load Resistance Training: A Systematic Review and Meta-analysis. J. Strength. Cond. Res. 2017, 31, 3508–3523. [Google Scholar] [CrossRef] [PubMed]
- Shariat, A.; Cleland, J.A.; Danaee, M.; Alizadeh, R.; Sangelaji, B.; Kargarfard, M.; Ansari, N.N.; Sepehr, F.H.; Tamrin, S.B.M. Borg CR-10 scale as a new approach to monitoring office exercise training. Work 2018, 60, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Takarada, Y.; Nozaki, D. Shouting strengthens maximal voluntary force and is associated with augmented pupillary dilation. Sci. Rep. 2021, 11, 18419. [Google Scholar] [CrossRef]
- Diong, J.; Kishimoto, K.C.; Butler, J.E.; Héroux, M.E. Muscle electromyographic activity normalized to maximal muscle activity, not to Mmax, better represents voluntary activation. PLoS ONE 2022, 17, e0277947. [Google Scholar] [CrossRef]
- Haff, G.; Triplett, N. Essentials of Strength Training and Conditioning, 4th ed.; Human Kinetics: Champaign, IL, USA, 2015. [Google Scholar]
- Vilarino, G.T.; Branco, J.H.L.; de Souza, L.C.; Andrade, A. Effects of resistance training on the physical symptoms and functional capacity of patients with fibromyalgia: A systematic review and meta-analysis of randomized clinical trials. Ir. J. Med. Sci. 2023, 192, 2001–2014. [Google Scholar] [CrossRef]
- Moreno-Ligero, M.; Moral-Munoz, J.A.; Salazar, A.; Failde, I. Health Intervention for Improving Pain, Quality of Life, and Functional Disability in Patients With Chronic Pain: Systematic Review. JMIR Mhealth Uhealth 2023, 11, e40844. [Google Scholar] [CrossRef]
- Albuquerque, M.L.L.; Monteiro, D.; Marinho, D.A.; Vilarino, G.T.; Andrade, A.; Neiva, H.P. Effects of different protocols of physical exercise on fibromyalgia syndrome treatment: Systematic review and meta-analysis of randomized controlled trials. Rheumatol. Int. 2022, 42, 1893–1908. [Google Scholar] [CrossRef] [PubMed]
- Estévez-López, F.; Maestre-Cascales, C.; Russell, D.; Álvarez-Gallardo, I.C.; Rodriguez-Ayllon, M.; Hughes, C.M.; Davison, G.W.; Sañudo, B.; McVeigh, J.G. Effectiveness of Exercise on Fatigue and Sleep Quality in Fibromyalgia: A Systematic Review and Meta-analysis of Randomized Trials. Arch. Phys. Med. Rehabil. 2021, 102, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Vilarino, G.T.; Coimbra, D.R.; Bevilacqua, G.G.; Diotaiuti, P.; Falese, L.; Andrade, A. Can different degrees of resistance training improve mood states in patients with fibromyalgia? A randomized controlled trial. Reumatismo 2022, 74, 152–164. [Google Scholar] [CrossRef]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef]
- Ouzzani, M.; Hammady, H.; Fedorowicz, Z.; Elmagarmid, A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016, 5, 210. [Google Scholar] [CrossRef]
- Flemyng, E.; Dwan, K.; Moore, T.H.; Page, M.J.; Higgins, J.P. Risk of Bias 2 in Cochrane Reviews: A phased approach for the introduction of new methodology. Cochrane Database Syst. Rev. 2020, 10, ED000148. [Google Scholar] [CrossRef]
- Häkkinen, A.; Hakkinen, K.; Hannonen, P.; Alen, M. Strength training induced adaptations in neuromuscular function of premenopausal women with fibromyalgia: Comparison with healthy women. Ann. Rheum. Dis. 2001, 60, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Maestre-Cascales, C.; Castillo-Paredes, A.; Romero-Parra, N.; Adsuar, J.C.; Carlos-Vivas, J. Gradual Strength Training Improves Sleep Quality, Physical Function and Pain in Women with Fibromyalgia. Int. J. Environ. Res. Public Health 2022, 19, 15662. [Google Scholar] [CrossRef]
- Valkeinen, H.; Häkkinen, K.; Pakarinen, A.; Hannonen, P.; Häkkinen, A.; Airaksinen, O.; Niemitukia, L.; Kraemer, W.J.; Alén, M. Muscle hypertrophy, strength development, and serum hormones during strength training in elderly women with fibromyalgia. Scand. J. Rheumatol. 2005, 34, 309–314. [Google Scholar] [CrossRef]
- Park, H.K.; Song, M.K.; Kim, D.J.; Choi, I.S.; Han, J.Y. Comparison of core muscle strengthening exercise and stretching exercise in middle-aged women with fibromyalgia. Medicine 2021, 100, e27854. [Google Scholar] [CrossRef] [PubMed]
- Jones, K.D.; Burckhardt, C.S.; Clark, S.R.; Bennett, R.M.; Potempa, K.M. A randomized controlled trial of muscle strengthening versus flexibility training in fibromyalgia. J. Rheumatol. 2002, 29, 1041–1048. [Google Scholar] [PubMed]
- Kingsley, J.D.; Panton, L.B.; Toole, T.; Sirithienthad, P.; Mathis, R.; McMillan, V. The Effects of a 12-Week Strength-Training Program on Strength and Functionality in Women With Fibromyalgia. Arch. Phys. Med. Rehabil. 2005, 86, 1713–1721. [Google Scholar] [CrossRef]
- Izquierdo-Alventosa, R.; Inglés, M.; Cortés-Amador, S.; Gimeno-Mallench, L.; Chirivella-Garrido, J.; Kropotov, J.; Serra-Añó, P. Low-Intensity Physical Exercise Improves Pain Catastrophizing and Other Psychological and Physical Aspects in Women with Fibromyalgia: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3634. [Google Scholar] [CrossRef]
- Andersson, U.M.; Åberg, A.C.; von Koch, L.; Palstam, A. Women with Fibromyalgia Prefer Resistance Exercise with Heavy Loads—A Randomized Crossover Pilot Study. Int. J. Environ. Res. Public Health 2021, 18, 6276. [Google Scholar] [CrossRef]
- Ericsson, A.; Palstam, A.; Larsson, A.; Löfgren, M.; Bileviciute-Ljungar, I.; Bjersing, J.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Resistance exercise improves physical fatigue in women with fibromyalgia: A randomized controlled trial. Arthritis Res. Ther. 2016, 18, 176. [Google Scholar] [CrossRef]
- Ernberg, M.; Christidis, N.; Ghafouri, B.; Bileviciute-Ljungar, I.; Löfgren, M.; Larsson, A.; Palstam, A.; Bjersing, J.; Mannerkorpi, K.; Kosek, E. Effects of 15 weeks of resistance exercise on pro-inflammatory cytokine levels in the vastus lateralis muscle of patients with fibromyalgia. Arthritis Res. Ther. 2016, 18, 137. [Google Scholar] [CrossRef]
- Jablochkova, A.; Bäckryd, E.; Kosek, E.; Mannerkorpi, K.; Ernberg, M.; Gerdle, B.; Ghafouri, B. Unaltered low nerve growth factor and high brain-derived neurotrophic factor levels in plasma from patients with fibromyalgia after a 15-week progressive resistance exercise. J. Rehabil. Med. 2019, 51, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Assumpção, A.; Matsutani, L.A.; Yuan, S.L.; Santo, A.S.; Sauer, J.; Mango, P.; Marques, A.P. Muscle stretching exercises and resistance training in fibromyalgia: Which is better? A three-arm randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2018, 54, 663–670. [Google Scholar] [CrossRef]
- Silva, H.J.; Júnior, J.; Oliveira, F.S.; Oliveira, J.M.; Dantas, G.A.; Lins, C.A.; de Souza, M.C. Sophrology versus resistance training for treatment of women with fibromyalgia: A randomized controlled trial. J. Bodyw. Mov. Ther. 2019, 23, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Larsson, A.; Palstam, A.; Löfgren, M.; Ernberg, M.; Bjersing, J.; Bileviciute-Ljungar, I.; Gerdle, B.; Kosek, E.; Mannerkorpi, K. Resistance exercise improves muscle strength, health status and pain intensity in fibromyalgia—A randomized controlled trial. Arthritis Res. Ther. 2015, 17, 161. [Google Scholar] [CrossRef]
- Gavi, M.B.R.O.; Vassalo, D.V.; Amaral, F.T.; Macedo, D.C.F.; Gava, P.L.; Dantas, E.M.; Valim, V. Strengthening Exercises Improve Symptoms and Quality of Life but Do Not Change Autonomic Modulation in Fibromyalgia: A Randomized Clinical Trial. PLoS ONE 2014, 9, e90767. [Google Scholar] [CrossRef]
- Arakaki, J.S.; Jennings, F.; Estrela, G.Q.; Martinelli, V.G.C.; Natour, J. Strengthening exercises using swiss ball improve pain, health status, quality of life and muscle strength in patients with fibromyalgia: A randomized controlled trial. Reumatismo 2021, 73, 15–23. [Google Scholar] [CrossRef]
- Macfarlane, G.J.; Kronisch, C.; Dean, L.E.; Atzeni, F.; Häuser, W.; Flub, E.; Choy, E.; Kosek, E.; Amris, K.; Branco, J.; et al. EULAR revised recommendations for the management of fibromyalgia. Ann. Rheum. Dis. 2017, 76, 318–328. [Google Scholar] [CrossRef]
- Cadenas-Sánchez, C.; Ruiz-Ruiz, J. Effect of a physical activity programme in patients with fibromyalgia: A systematic review. Med. Clin. 2014, 143, 548–553. [Google Scholar] [CrossRef]
- Andrade, A.; Azevedo, R.; Sieczkowska, S.M.; Peyré, L.A.; Vilarino, G. A systematic review of the effects of strength training in patients with fibromyalgia: Clinical outcomes and design considerations. Adv. Rheumatol. 2018, 58, 36. [Google Scholar] [CrossRef]
- Noetel, M.; Sanders, T.; Gallardo-Gómez, D.; Taylor, P.; Del Pozo Cruz, B.; Van Den Hoek, D.; Smith, J.J.; Mahoney, J.; Spathis, J.; Moresi, M.; et al. Effect of exercise for depression: Systematic review and network meta-analysis of randomised controlled trials. BMJ 2024, 384, e075847. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.P.; Santo, R.C.; Ramis, T.R.; Portes, J.K.; Chakr, R.M.; Xavier, R.M. The effects of resistance training with blood flow restriction on muscle strength, muscle hypertrophy and functionality in patients with osteoarthritis and rheumatoid arthritis: A systematic review with meta-analysis. PLoS ONE 2021, 16, e0259574. [Google Scholar] [CrossRef] [PubMed]
- Bastos, A.C.; Vilarino, G.T.; Souza, L.C.; Dominski, F.H.; Branco, J.H.; Andrade, A. Effects of resistance training on sleep of patients with fibromyalgia: A systematic review. J. Health Psychol. 2023, 28, 1072–1084. [Google Scholar] [CrossRef] [PubMed]
| Authors | Pain | Physical Functional Capacity | Quality Life | Sleep Quality | Depression |
|---|---|---|---|---|---|
| Häkkinen et al. [24] | VAS | HAQ | - | - | BDI-FS |
| Jones et al. [28] | TP, FIQ | - | QOLS | - | BDI-FS |
| Kingsley et al. [29] | TP, FIQ | CS-PFP | - | - | - |
| Valkeinen et al. [26] | VAS | - | - | - | - |
| Gavi et al. [38] | VAS | SF-36 | FIQ, SF-36 | - | BDI-FS |
| Larsson et al. [37] | FIQ | - | FIQ | PSQI | HADS |
| Ericsson et al. [32] | FIQ | - | FIQ | - | HADS |
| Ernberg et al. [33] | VAS | - | SF-36 | - | HADS |
| Assumpção et al. [35] | VAS, FIQ | FIQ | FIQ, SF-36 | - | FIQ |
| Silva et al. [36] | VAS | SF-36 | SF-36 | - | SF-36 |
| Jablochkova et al. [34] | VAS | FIQ | SF-36 | - | HADS |
| Izquierdo-Alventosa et al. [30] | VAS | FIQ | FIQR | - | BDI-FS |
| Andersson et al. [31] | VAS | - | - | PSQI | - |
| Park et al. [27] | VAS | FIQ | FIQ | - | FIQ |
| Arakaki et al. [39] | VAS | SF-36 | FIQ, SF-36 | - | SF-36 |
| Maestre-Cascales et al. [25] | BPI | Senior Fitness Test | - | FIQ | STAI-S |
| Vilarino et al. [20] | - | - | - | - | BRUMS |
| Reference; Study | Type of Study | Sample Size | Age in Years | Intervention | Intensity |
|---|---|---|---|---|---|
| Häkkinen et al., 2001 [24] | RCT | RE: 11 CG: 10 HC: 12 | RE: 39 ± 6 CG: 37 ± 5 HC: 37 ± 6 | 21 weeks: WF: 2 week Exercises: Isotonic exercises including supine, squats, extension, and flexion of knees and trunk. | PIRE Weeks 1–3: 1 set of 15–20 reps at 40–60% 1RM Weeks 4–7: 1 set of 10–12 reps at 60–70% 1RM Weeks 8–14: 1 set of 8–12 reps at 60–80% 1RM Weeks 15–21: 1 set of 5–10 reps at 70–80% 1RM |
| Jones et al., 2002 [28] | RCT | RE: 28 FG: 28 | RE: 49.2 ± 6.36 FG: 46.4 ± 8.56 | 12 weeks: WF: 2 week Exercises: The resistance program used free weights and elastic bands, targeting three regions: trunk stabilizers (abdominal and paraspinal muscles), lower-limb muscles (ankle, knee, and hip muscle groups), and upper-body musculature (thoracic, scapular, and arm muscles). Specific exercises were not detailed. | LIRE Using hand weights (1–3 pounds) and resistance bands based on the patient’s tolerance, while staying aware of their body’s signals. |
| Kingsley et al., 2005 [29] | RCT | RE: 15 (8 completed the study) CG: 14 (12 completed the study) | RE: 45 ± 9 CG: 47 ± 4 | 12 weeks: WF: 2 week Exercises: The program included 11 isotonic exercises that targeted the following muscle groups: chest, leg extensors, leg flexors, shoulders, lumbar extensors, abdominals, biceps, triceps, back, and lower limbs. The exercises used nautilus machines, cable machines, and body weight. | PIRE Exercise intensity progressed from 40% to 60% 1RM (upper body) and 80% 1RM (lower body). When 12 proper reps were achieved, weight increased by 2.3–4.5 kg. |
| Valkeinen et al., 2005 [26] | RCT | RE: n = 13 GC: n = 13 | RE: 60 ± 2 GC: 59 ± 4 | 12 weeks: WF: 2 week Exercises: The program included 6–7 isotonic exercises that are full-body exercises with a greater focus on the upper and lower extremities. | PIRE The program began at an intensity of 40% of 1RM and progressed to 80% of 1RM. Subjects kept an exercise diary to record the loads used. |
| Gavi et al., 2014 [38] | RCT | RE: n = 35 FG: n = 31 | RE: 44.34 ± 7.94 FG: 48.65 ± 7.60 | 16 weeks: WF: 2 week Exercises: Supervised progressive weight training, 8 muscle groups (quads, hamstrings, biceps, triceps, pecs, calves, deltoids, and lats), 12 exercises, 3 × 12 reps. | PIRE RE progressed from 40% to 80% of 1RM over 15 weeks. Load increases are assessed every 3–4 weeks. A total of 42 participants (62.7%) reached 80% 1RM, and 7 (10.4%) reached 60% 1RM. |
| Larsson et al., 2015 [37] | RCT | RE: n = 67 GC: n = 63 | RE: 50.81 ± 9.05 GC: 52.10 ± 9.78 años | 15 weeks: WF: 2 week Exercises: The protocol included 12 isotonic resistance exercises targeting 8 major muscle groups using weight machines, free weights, and body weight. Key exercises included leg presses, knee extensions, biceps curls, and heel raises. Explosive strength exercises, like rapid heel raises and knee extensions, were added in the later weeks. | PIRE Weeks 1–2: 40% 1RM, 15–20 repetitions, 1–2 sets Weeks 3–5: 60% 1RM, 10–12 repetitions, 1–2 sets Weeks 6–15: 80% 1RM, 5–8 repetitions, 1–2 sets |
| Ericsson et al., 2016 [32] | RCT | RE: n = 67 RE: n = 63 | RE: 50.81 ± 9.05 RE: 52.10 ± 9.78 | 15 weeks: WF: 2 week Exercises: The resistance exercise program targeted major muscle groups using a combination of weight machines and free weights, including leg press, knee extension/flexion, biceps curl, hand grip, heel raises, core stability, chest press, triceps extensions, and shoulder exercises. | PIRE Progressive, starting at 40% of 1RM and progressing up to 80% of 1RM. |
| Ernberg et al., 2016 [33] | RCT | RE: n= 24 GC: n = 27 | >18 | 15 weeks: WF: 2 week Each session started with 10 min of bicycling to warm up and was then followed by 50 min of resistance exercise (major muscle groups). | PIRE The exercise was initiated at low loads at 40% of the maximum voluntary capacity (MVC) and successively progressed up to 70–80% of MVC. |
| Assumpção et al., 2018 [35] | RCT | RE: n = 16 FG: n = 14 GC: n = 14 | RE: 45.7 ± 7.7 FG: 47.9 ± 5.3 GC: 46.9 ± 6.5 | 12 weeks: WF: 2 week Exercises: 40 min sessions: The stretching group did active stretches and isometrics; the resistance group used weights and isometric holds, both targeting full-body muscles. | PIRE Started without a load in the first two sessions Then, increased by 0.5 kg each week if the effort was perceived as “slightly intense” on the Borg scale. Progression was based on perceived effort, not on a percentage of 1RM. Performed 1 set of 8 repetitions for each exercise |
| Silva et al., 2019 [36] | RCT | RE: n = 30 RE: n = 30 | GE: 49.40 ± 8.30 RE: 44.93 ± 10.30 | 12 weeks: WF: 2 week Exercises: The protocol consisted of resistance training targeting the upper body (arm, chest, and shoulder muscles), and lower extremity muscles (knee and hip musculature), performing 3 sets of 12 repetitions. The control group underwent relaxation sessions with music in a temperature-controlled environment. | PIRE StaREing intensity: 60% of 1RM in the first month Progressive increase: 70% of 1RM in the second month 80% of 1RM in the third month |
| Jablochkova et al., 2019 [34] | RCT | RE: n = 41 RE: n = 34 HC: n = 25 | 20–65 | 15 weeks: WF: 2 week Exercises: The resistance exercise group performed sessions twice a week, which included 10 min of warm-up followed by 50 min of strength training, focusing mainly on the lower limbs. Meanwhile, the relaxation group participated in 25 min sessions twice a week, consisting of guided relaxation therapy with mental exercises, relaxation, and autosuggestion, ending with stretching exercises. | PIRE Intensity progressed from an initial 40% of maximum voluntary capacity (MVC) to 70–80% of MVC. |
| Izquierdo-Alventosa et al., 2020 [30] | RCT | RE: n = 16 GC: n = 16 | RE: 53.06 ± 8.4 GC: 55.13 ± 7.35 | 8 weeks: WF: 2 week Exercises: They performed 60 min resistance and coordination training sessions, including warm-up, main workout, and cool-down. The training involved walking and a circuit of 10 exercises targeting muscles of the upper and lower limbs: biceps, shoulders, pectorals, quadriceps, hip abductors, and calves. Each exercise was performed for 15 to 25 repetitions | LIRE Training intensity was adjusted using the Borg CR-10 scale. Initially, weak effort (1–2) was sought, later increasing to moderate (3–4). Weights of 0.5–2 kg for arms and 1–3 kg for legs were used, plus soft elastic bands. The intensity was adapted based on each participant’s pain and effort. |
| Andersson et al., 2021 [31] | RCT | RE (80% 1RM): n = 10 RE (50% 1RM): n = 10 | RE: 22–46 | 2 to 3 weeks: WF: 2 week Exercises: The study utilized two resistance exercise protocols, each consisting of six main exercises including bench press, lunges, and squats. | HIRE The light/moderate load protocol was performed at 50% of 1RM with 20–30 repetitions, while the heavy load protocol was executed at 80% of 1RM with 7–8 repetitions. Both protocols were applied in separate sessions with a rest period between them. |
| Park et al., 2021 [27] | RCT | RE: 15 FG:15 | RE: 52.8 ± 7.1 FG: 50.5 ± 7.1 | 4 weeks: WF: 2 week Exercises: The program combined core stabilization exercises (drawing-in maneuver, bridges, and bird dog) and strengthening movements (sit-ups with/without weights, crunches, and back extensions). The stretching protocol included 2 sets of 3 repetitions (30 s hold) for 8–9 exercises targeting pain areas | MIRE Initially perceived as moderate, with a score of 12.1 (Borg scale), corresponding to approximately 50–60% of maximum effort. |
| Arakaki et al., 2021 [39] | RCT | RE: n = 30 FG: n = 30 | RE: 47.4 ± 9.0 FG: 47.3 ± 8.7 | 12 weeks: WF: 3 week Exercises: Swiss ball group: 8 strengthening exercises targeting major muscle groups, 3 sets of 12 repetitions Stretching group: Stretches targeting the same muscles as the Swiss ball group, 3 sets of 30 s per stretch | MIRE 60% de 1RM |
| Maestre-Cascales et al., 2022 [25] | EXP | RE: n = 41 | RE: 56.36 ± 8.72 | 24 weeks: WF: 2 week Exercises: Three progressive phases: Free weights and bodyweight exercises (5 weeks) Added elastic bands (7 weeks) Added external loads (12 weeks) Exercises targeted upper and lower limb muscles and trunk muscles. | PIRE Weeks 1–5: 3–4 on the OMNI-GSE scale Weeks 6–12: 4–5 on the OMNI-GSE scale Weeks 13–24: 6–8 on the OMNI-GSE scale |
| Vilarino et al., 2022 [20] | RCT | RE (Low intensity): n = 9 RE (High intensity): n = 7 RE (Preferred intensity) n = 10 HC: n = 27 | RE (Low intensity): n = 9 RE (High intensity): n = 7 RE (Preferred intensity): n = 10 HC: n = 27 | 8 weeks: WF: 2 week Exercises: Isotonic: standing calf raise, leg press, squat, low row, shoulder press, and bench press. | PIRE RE (Low intensity): 2 sets of 12 repetitions, 1 min rest between sets. RE (High intensity): 4 sets of 6 maximum repetitions, 2 min rest between sets RE (Preferred intensity): 3 sets of 8–12 repetitions based on tolerance, 1 min rest between sets |
| Authors | Intensity | Pain | Physical Functional Capacity | Depression | Sleep Quality | Quality Life |
|---|---|---|---|---|---|---|
| Häkkinen et al. [24] | PIRE | ↑ | ↑ | ↑ | - | - |
| Jones et al. [28] | LIRE | ↑ | - | ↑ | - | ↔ |
| Kingsley et al. [29] | PIRE | ↔ | ↑ | - | - | - |
| Valkeinen et al. [26] | PIRE | ↔ | - | - | - | - |
| Gavi et al. [38] | PIRE | ↑ | ↑ | ↑ | - | ↑ |
| Larsson et al. [37] | PIRE | ↑ | - | ↑ | ↑ | ↑ |
| Ericsson et al. [32] | PIRE | ↔ | - | ↔ | - | ↔ |
| Ernberg et al. [33] | PIRE | ↑ | - | ↔ | - | ↑ |
| Assumpção et al. [35] | PIRE | ↑ | ↑ | ↑ | - | ↑ |
| Silva et al. [36] | PIRE | ↑ | ↑ | ↑ | - | ↑ |
| Jablochkova et al. [34] | PIRE | ↑ | ↑ | ↔ | - | ↔ |
| Izquierdo-Alventosa et al. [30] | LIRE | ↑ | ↑ | ↑ | - | ↑ |
| Andersson et al. [31] | HIRE | ↔ | - | - | ↑ | - |
| Park et al. [27] | MIRE | ↑ | ↑ | ↔ | - | ↔ |
| Arakaki et al. [39] | MIRE | ↑ | ↑ | ↑ | - | ↑ |
| Maestre-Cascales et al. [25] | PIRE | ↑ | ↑ | ↔ | ↑ | - |
| Vilarino et al. [20] | PIRE | - | - | ↔ | - | - |
| Study | D1: Randomization | D2: Deviations | D3: Missing Data | D4: Measurement | D5: Selection | Overall Risk |
|---|---|---|---|---|---|---|
| Izquierdo-Alventosa et al. (2020) [30] | Low | Low | Low | Low | Some concerns | Some concerns |
| Assumpção et al. (2018) [35] | Low | Low | Low | High | Low | High |
| Silva et al. (2019) [36] | Low | Low | Low | Low | Low | Low |
| Vilarino et al. (2022) [20] | Low | Low | Low | Low | Low | Low |
| Larsson et al. (2015) [37] | Low | Low | Low | Low | Low | Low |
| Häkkinen et al. (2001) [24] | Low | Low | Low | High | Low | High |
| Jones et al. (2002) [28] | Low | Low | Low | Low | Low | Low |
| Kingsley et al. (2005) [29] | Low | Low | Low | Low | Low | Low |
| Valkeinen et al. (2005) [26] | Some concerns | Low | Low | High | Low | High |
| Gavi et al. (2014) [38] | High | Some concerns | Some concerns | Low | Some concerns | High |
| Ericsson et al. (2016) [32] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Ernberg et al. (2016) [33] | Some concerns | Low | Low | Low | Some concerns | Some concerns |
| Jablochkova et al. (2019) [34] | Low | Low | Low | Low | Some concerns | Some concerns |
| Park et al. (2021) [27] | Low | Low | Low | Low | High | High |
| Arakaki et al. (2021) [39] | Low | Low | Low | Low | High | High |
| Maestre-Cascales et al. (2022) [25] | High | Low | Low | Low | High | High |
| Study | D1: Randomization | D2: Deviations | D3: Missing Data | D4: Measurement | D5: Selection | D6: Carry-Over Effects | D7: Wash-Out Period | Overall Risk |
|---|---|---|---|---|---|---|---|---|
| Andersson et al. (2021) [31] | Low | Low | Low | Low | Low | Low | Low | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guachizaca Moreno, K.P.; Flores-Santy, L.F.; Vinueza Fernández, I. Intensity of Resistance Exercise and Its Effects on Pain, Functionality, and Quality of Life in Adults with Fibromyalgia: A Systematic Review. J. Funct. Morphol. Kinesiol. 2025, 10, 121. https://doi.org/10.3390/jfmk10020121
Guachizaca Moreno KP, Flores-Santy LF, Vinueza Fernández I. Intensity of Resistance Exercise and Its Effects on Pain, Functionality, and Quality of Life in Adults with Fibromyalgia: A Systematic Review. Journal of Functional Morphology and Kinesiology. 2025; 10(2):121. https://doi.org/10.3390/jfmk10020121
Chicago/Turabian StyleGuachizaca Moreno, Kevin Paúl, Lucía Fernanda Flores-Santy, and Israel Vinueza Fernández. 2025. "Intensity of Resistance Exercise and Its Effects on Pain, Functionality, and Quality of Life in Adults with Fibromyalgia: A Systematic Review" Journal of Functional Morphology and Kinesiology 10, no. 2: 121. https://doi.org/10.3390/jfmk10020121
APA StyleGuachizaca Moreno, K. P., Flores-Santy, L. F., & Vinueza Fernández, I. (2025). Intensity of Resistance Exercise and Its Effects on Pain, Functionality, and Quality of Life in Adults with Fibromyalgia: A Systematic Review. Journal of Functional Morphology and Kinesiology, 10(2), 121. https://doi.org/10.3390/jfmk10020121








