Effects of Passive Movement on Motor Function and Disability in Patients with Stroke: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Method
2.1. Inclusion and Exclusion Criteria
2.2. Searching the Literature
2.3. Selection of Eligible Studies and Data Extraction
2.4. Risk of Bias and Methodological Quality Assessments of the Included Studies
2.5. Data Synthesis
2.6. The Interpretation of the Evidence from the Findings
3. Results
3.1. Narrative Synthesis
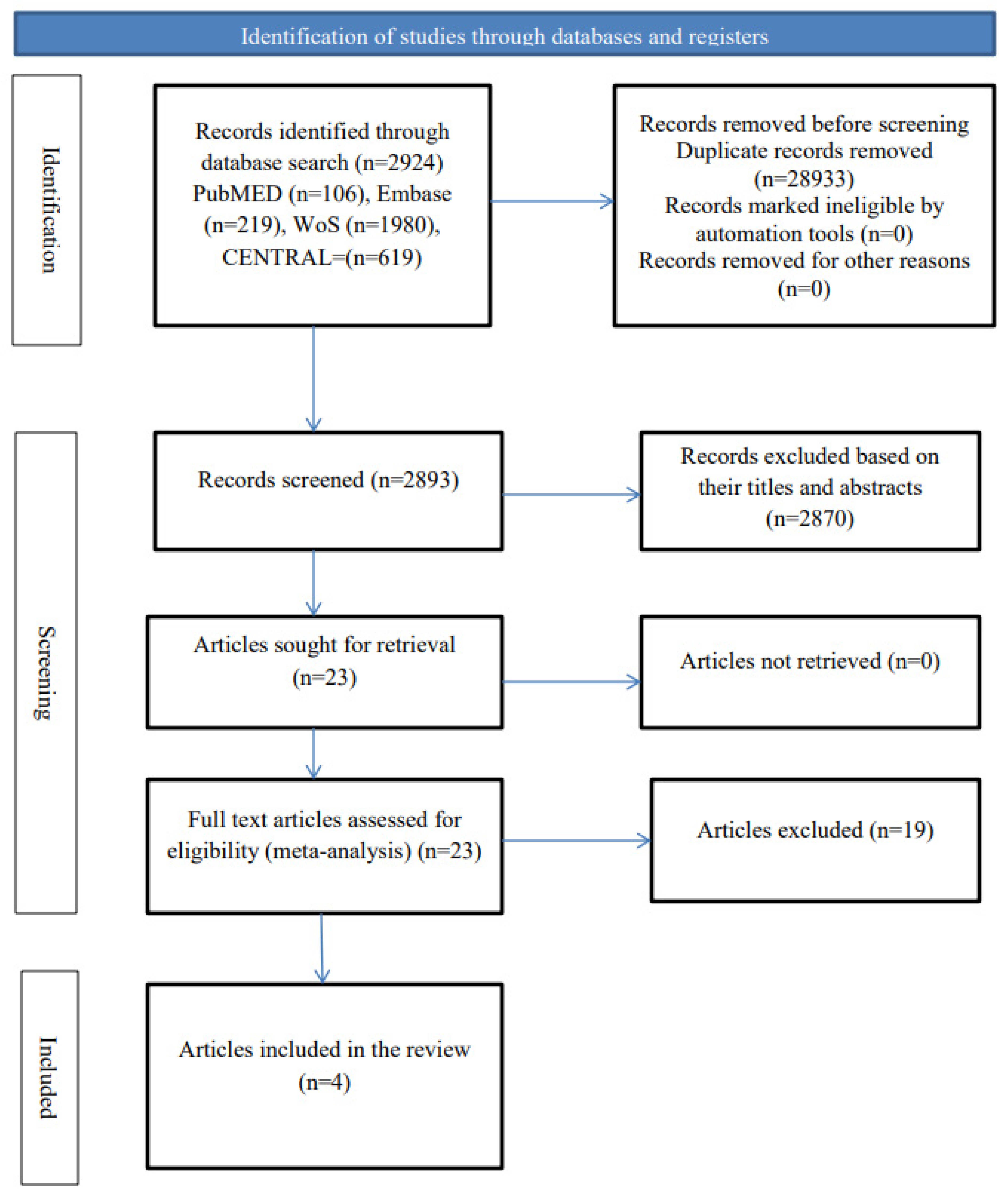
3.1.1. Selection of Eligible Studies
3.1.2. The Characteristics of the Included Studies
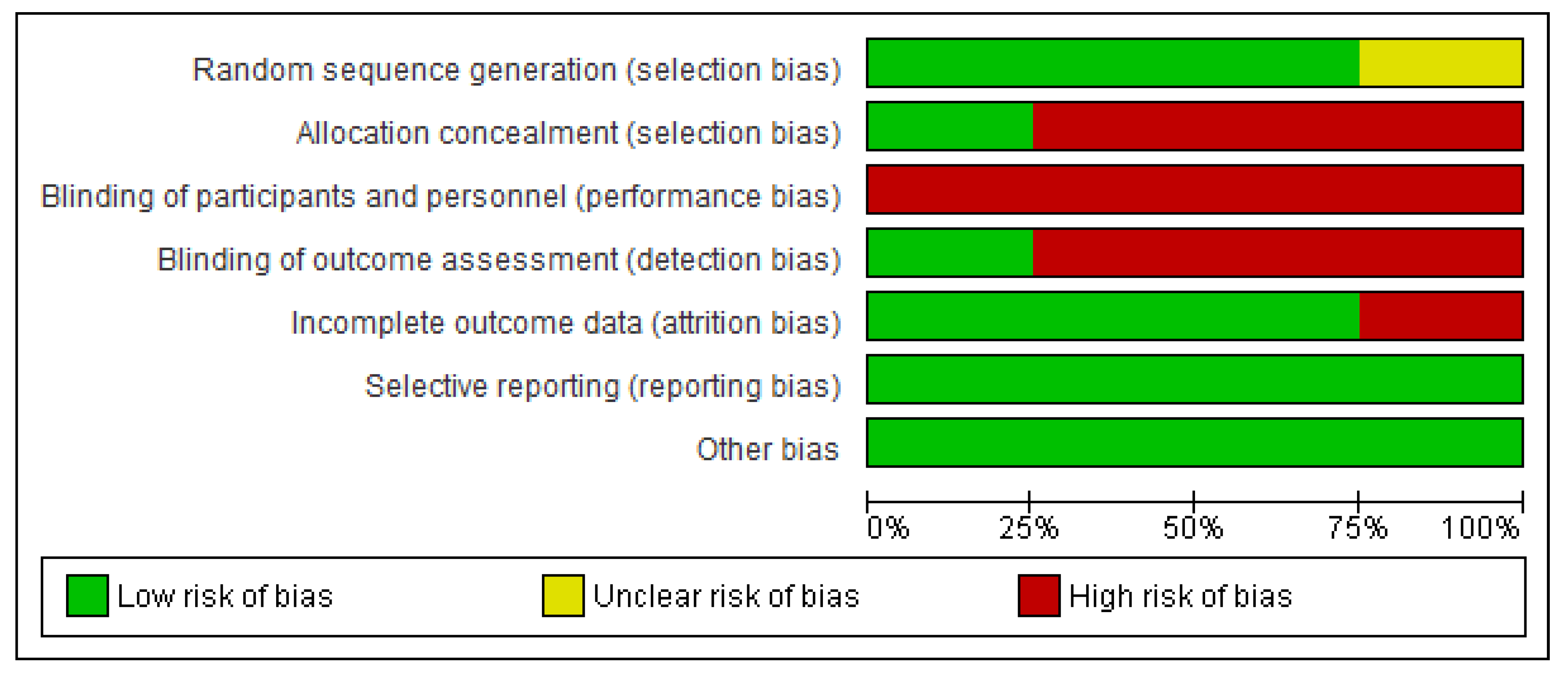
3.1.3. Methodological Quality and Risks of Bias
3.2. Quantitative Synthesis
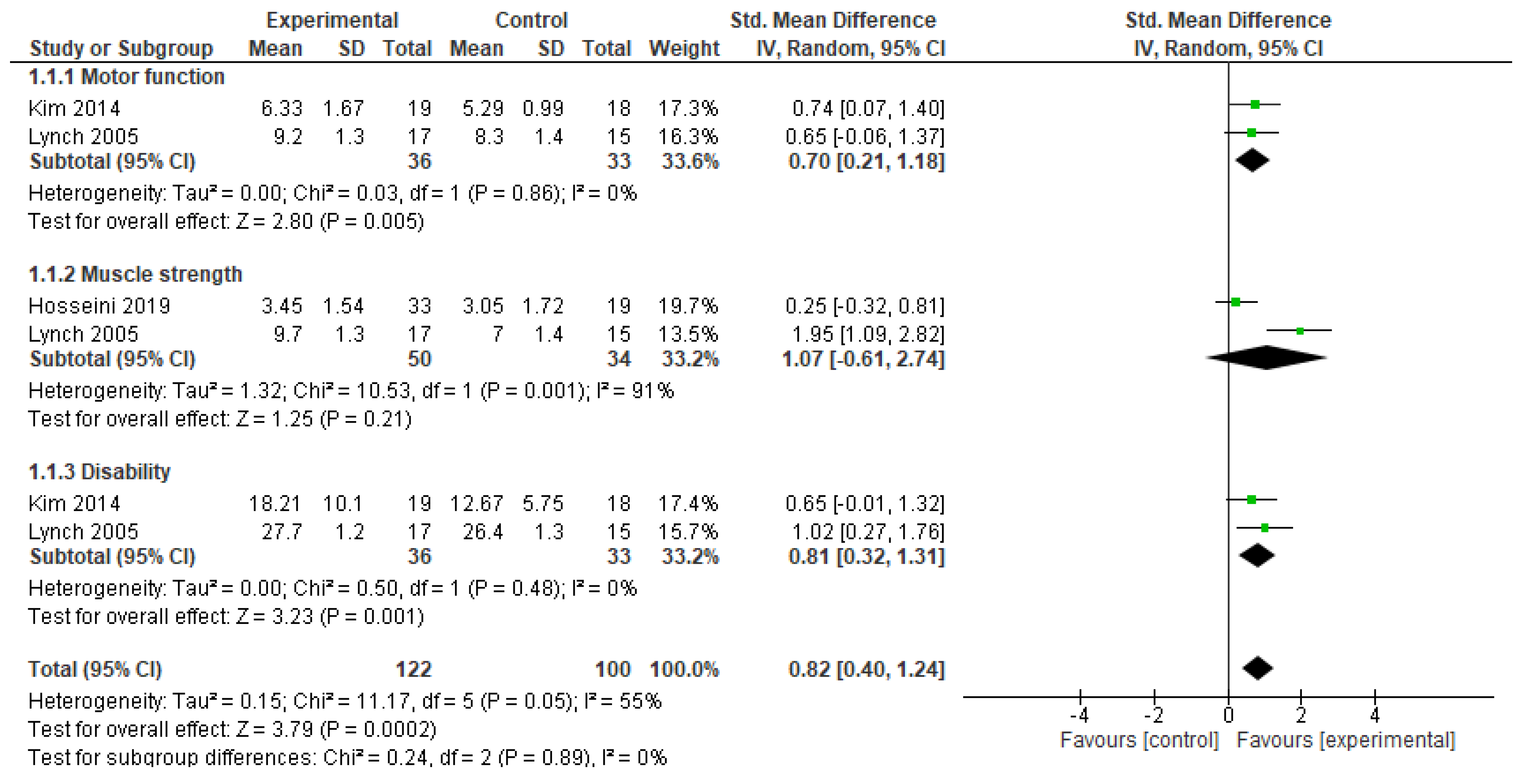
3.2.1. Recovery of Function
3.2.2. The Interpretation of the Evidence
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Appendix A
| PubMED | (((((Stroke) OR (Ischaemic stroke)) OR (Hemorrhagic stroke)) OR (Brain infarction)) AND (Exercise)) AND ((((Range of motion) OR (passive range of motion)) OR (joint range of motion)) OR (joint flexibility)) Filters: Humans: English; RCTs; Adults |
| Author Contributions: WoS | (((((Stroke) OR (Ischaemic stroke)) OR (Hemorrhagic stroke)) OR (Brain infarction)) AND (Exercise)) AND ((((Range of motion) OR (passive range of motion)) OR (joint range of motion)) OR (joint flexibility)) Filters: Stroke; Rehabilitation; English; Trials; Web of Science citation index (expanded and emerging); WoS conference citation index |
| Embase | (((((Stroke) OR (Ischaemic stroke)) OR (Hemorrhagic stroke)) OR (Brain infarction)) AND (Exercise)) AND ((((Range of motion) OR (passive range of motion)) OR (joint range of motion)) OR (joint flexibility)) Filters: English; RCTs; cerebrovascular accident |
| CENTRAL | (((((Stroke) AND (Exercise)) AND ((((Range of motion) OR (passive range of motion)) OR (joint range of motion)) Filters: CINAHL; English; Trials |
References
- Cirstea, M.C.; Ptito, A.; Levin, M.F. Arm reaching improvements with short-term practice depend on the severity of the motor deficit in stroke. Exp. Brain Res. 2003, 152, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Waddell, K.J.; Birkenmeier, R.L.; Moore, J.L.; Hornby, T.G.; Lang, C.E. Feasibility of high-repetition, task-specific training for individuals with upper-extremity paresis. Am. J. Occup. Ther. 2014, 68, 444–453. [Google Scholar] [CrossRef]
- Sheng, Y.; Han, J. Biomechanical characteristics and neuromuscular action control mechanism of single-dual-task walking-conversion training in stroke patients. J. Back Musculoskelet. Rehabil. 2025, 12, 10538127241308215. [Google Scholar] [CrossRef]
- Oliveira, M.D.C.B.; Silva, D.R.C.; Cortez, B.V.; Coêlho, C.K.D.S.; Silva, F.M.S.E.; de Oliveira, G.B.V.P.; de Sá-Caputo, D.C.; Tavares-Oliveira, A.C.; Bernardo-Filho, M.; Moraes Silva, J. Mirror and Vibration Therapies Effects on the Upper Limbs of Hemiparetic Patients after Stroke: A Pilot Study. Rehabil. Res. Pr. 2018, 2018, 6183654. [Google Scholar] [CrossRef]
- Sañudo, B.; Taiar, R.; Furness, T.; Bernardo-Filho, M. Clinical Approaches of Whole-Body Vibration Exercises in Individuals with Stroke: A Narrative Revision. Rehabil. Res. Pract. 2018, 2018, 8180901. [Google Scholar] [CrossRef]
- Xu, S.; Xu, Y.; Wen, R.; Wang, J.; Qiu, Y.; Chan, C.C. Virtual Reality Enhanced Exercise Training in Upper Limb Function of Patients with Stroke: Meta-Analytic Study. J. Med. Internet. Res. 2025, 27, e66802. [Google Scholar] [CrossRef]
- Rozevink, S.G.; Hijmans, J.M.; Horstink, K.A.; van der Sluis, C.K. Effectiveness of task-specific training using assistive devices and task-specific usual care on upper limb performance after stroke: A systematic review and meta-analysis. Disabil. Rehabil. Assist. Technol. 2021, 18, 1245–1258. [Google Scholar] [CrossRef] [PubMed]
- Brenner, I.K.M. Effects of Passive Exercise Training in Hemiplegic Stroke Patients: A Mini-Review. Sports Med. Rehabil. J. 2018, 3, 1036. [Google Scholar]
- Park, J.; Lee, N.; Cho, M.; Kim, D.; Yang, Y. Effects of mental practice on stroke patients’ upper extremity function and daily activity performance. J. Phys. Ther. Sci. 2015, 27, 1075–1077. [Google Scholar] [CrossRef]
- Page, S.J.; Peters, H. Mental practice: Applying motor PRACTICE and neuroplasticity principles to increase upper extremity function. Stroke 2014, 45, 3454–3460. [Google Scholar] [CrossRef]
- Borges, L.R.; Fernandes, A.B.; Melo, L.P.; Guerra, R.O.; Campos, T.F. Action observation for upper limb rehabilitation after stroke. Cochrane Database Syst. Rev. 2018, 10, Cd011887. [Google Scholar] [CrossRef] [PubMed]
- Knutson, J.S.; Fu, M.J.; Sheffler, L.R.; Chae, J. Neuromuscular Electrical Stimulation for Motor Restoration in Hemiplegia. Phys. Med. Rehabil. Clin. N. Am. 2015, 26, 729–745. [Google Scholar] [CrossRef]
- Kubis, N. Non-Invasive Brain Stimulation to Enhance Post-Stroke Recovery. Front. Neural Circuits 2016, 10, 56. [Google Scholar]
- Pathak, A.; Gyanpuri, V.; Dev, P.; Dhiman, N.R. The Bobath Concept (NDT) as rehabilitation in stroke patients: A systematic review. J. Fam. Med. Prim. Care 2021, 10, 3983–3990. [Google Scholar] [CrossRef]
- Ietswaart, M.; Johnston, M.; Dijkerman, H.C.; Joice, S.; Scott, C.L.; MacWalter, R.S.; Hamilton, S.J. Mental practice with motor imagery in stroke recovery: Randomized controlled trial of efficacy. Brain 2011, 134 Pt. 5, 1373–1386. [Google Scholar]
- Wu, X.; Wang, R.; Wu, Q.; Liao, C.; Zhang, J.; Jiao, H.; Chen, B.; Wang, S.; Liu, R. The effects of combined high-frequency repetitive transcranial magnetic stimulation and cervical nerve root magnetic stimulation on upper extremity motor recovery following stroke. Front. Neurosci. 2023, 17, 1100464. [Google Scholar]
- Trinity, J.D.; Richardson, R.S. Physiological Impact and Clinical Relevance of Passive Exercise/Movement. Sports Med. 2019, 49, 1365–1381. [Google Scholar]
- Xia, W.; Dai, R.; Xu, X.; Huai, B.; Bai, Z.; Zhang, J.; Jin, M.; Niu, W. Cortical mapping of active and passive upper limb training in stroke patients and healthy people: A functional near-infrared spectroscopy study. Brain Res. 2022, 1788, 147935. [Google Scholar]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar]
- Higgins, J.P.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [PubMed]
- Herbert, R.; Moseley, A.; Sherrington, C. PEDro: A database of randomised controlled trials in physiotherapy. Health Inf. Manag. 1998, 28, 186–188. [Google Scholar] [PubMed]
- Moseley, A.M.; Herbert, R.D.; Maher, C.G.; Sherrington, C.; Elkins, M.R. Reported quality of randomized controlled trials of physiotherapy interventions has improved over time. J. Clin. Epidemiol. 2011, 64, 594–601. [Google Scholar]
- da Costa, B.R.; Hilfiker, R.; Egger, M. PEDro’s bias: Summary quality scores should not be used in meta-analysis. J. Clin. Epidemiol. 2013, 66, 75–77. [Google Scholar]
- Guyatt, G.H.; Oxman, A.D.; Vist, G.E.; Kunz, R.; Falck-Ytter, Y.; Alonso-Coello, P.; Schünemann, H.J.; GRADE Working Group. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008, 336, 924–926. [Google Scholar] [PubMed]
- Lynch, D.; Ferraro, M.; Krol, J.; Trudell, C.M.; Christos, P.; Volpe, B.T. Continuous passive motion improves shoulder joint integrity following stroke. Clin. Rehabil. 2005, 19, 594–599. [Google Scholar]
- Kim, H.J.; Lee, Y.; Sohng, K.Y. Effects of bilateral passive range of motion exercise on the function of upper extremities and activities of daily living in patients with acute stroke. J. Phys. Ther. Sci. 2014, 26, 149–156. [Google Scholar]
- Hosseini, Z.S.; Peyrovi, H.; Gohari, M. The Effect of Early Passive Range of Motion Exercise on Motor Function of People with Stroke: A Randomized Controlled Trial. J. Caring Sci. 2019, 8, 39–44. [Google Scholar]
- Cho, K.H.; Park, S.J. Effects of joint mobilization and stretching on the range of motion for ankle joint and spatiotemporal gait variables in stroke patients. J. Stroke Cerebrovasc. Dis. 2020, 29, 104933. [Google Scholar]
- Shin, D.S.; Song, R.; Shin, E.K.; Seo, S.J.; Park, J.E.; Han, S.Y.; Jung, H.Y.; Ryu, C.J. Effects of passive upper arm exercise on range of motion, muscle strength, and muscle spasticity in hemiplegic patients with cerebral vascular disease. J. Korean Acad. Nurs. 2012, 42, 783–790. [Google Scholar]
- Vér, C.; Emri, M.; Spisák, T.; Berényi, E.; Kovács, K.; Katona, P.; Balkay, L.; Menyhárt, L.; Kardos, L.; Csiba, L. The Effect of Passive Movement for Paretic Ankle-Foot and Brain Activity in Post-Stroke Patients. Eur. Neurol. 2016, 76, 132–142. [Google Scholar] [PubMed]
- Rydwik, E.; Eliasson, S.; Akner, G. The effect of exercise of the affected foot in stroke patients—A randomized controlled pilot trial. Clin. Rehabil. 2006, 20, 645–655. [Google Scholar] [PubMed]
- Katz-Leurer, M.; Sender, I.; Keren, O.; Dvir, Z. The influence of early cycling training on balance in stroke patients at the subacute stage. Results of a preliminary trial. Clin. Rehabil. 2006, 20, 398–405. [Google Scholar] [CrossRef]
- Lindberg, P.; Schmitz, C.; Forssberg, H.; Engardt, M.; Borg, J. Effects of passive-active movement training on upper limb motor function and cortical activation in chronic patients with stroke: A pilot study. J. Rehabil. Med. 2004, 36, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Szameitat, A.J.; Shen, S.; Conforto, A.; Sterr, A. Cortical activation during executed, imagined, observed, and passive wrist movements in healthy volunteers and stroke patients. Neuroimage 2012, 62, 266–280. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takeda, K.; Koyama, S.; Morishima, K.; Hirakawa, Y.; Motoya, I.; Sakurai, H.; Kanada, Y.; Kawamura, N.; Kawamura, M.; et al. Relationship between upper limb motor function and activities of daily living after removing the influence of lower limb motor function in subacute patients with stroke: A cross-sectional study. Hong Kong J. Occup. Ther. 2020, 33, 12–17. [Google Scholar] [CrossRef]
- Jigjid, E.; Kawashima, N.; Ogata, H.; Nakazawa, K.; Akai, M.; Eto, F.; Haga, N. Effects of Passive Leg Movement on the Oxygenation Level of Lower Limb Muscle in Chronic Stroke Patients. Neurorehabilit. Neural Repair 2008, 22, 40–49. [Google Scholar] [CrossRef]
- Wu, C.L.; Huang, M.H.; Lee, C.L.; Liu, C.W.; Lin, L.J.; Chen, C.H. Effect on spasticity after performance of dynamic-repeated-passive ankle joint motion exercise in chronic stroke patients. Kaohsiung J. Med. Sci. 2006, 22, 610–617. [Google Scholar] [CrossRef]
- Selles, R.W.; Li, X.; Lin, F.; Chung, S.G.; Roth, E.J.; Zhang, L.Q. Feedback-controlled and programmed stretching of the ankle plantarflexors and dorsiflexors in stroke: Effects of a 4-week intervention program. Arch. Phys. Med. Rehabil. 2005, 86, 2330–2336. [Google Scholar]
- Kawahira, K.; Shimodozono, M.; Ogata, A.; Tanaka, N. Addition of intensive repetition of facilitation exercise to multidisciplinary rehabilitation promotes motor functional recovery of the hemiplegic lower limb. J. Rehabil. Med. 2004, 36, 159–164. [Google Scholar]
- Abdullahi, A.; Shehu, S.; Dantani, I.B. Feasibility of high repetition of task practice in constraint induced movement therapy in an acute stroke patient. Int. J. Ther. Rehabil. 2014, 21, 190–195. [Google Scholar] [CrossRef]
- Grewal, A.; Kataria, H.; Dhawan, I. Literature search for research planning and identification of research problem. Indian J. Anaesth. 2016, 60, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.; Salanti, G.; Morton, S.C.; Riley, R.D.; Chu, H.; Kimmel, S.E.; Chen, Y. Testing small study effects in multivariate meta-analysis. Biometrics 2020, 76, 1240–1250. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.; Thompson, S.; Deeks, J.; Altman, D. Statistical heterogeneity in systematic reviews ofclinical trials: A critical appraisal of guidelines and practice. J. Health Serv. Res. Policy 2002, 7, 51–61. [Google Scholar] [CrossRef]
| PICOS Category | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| P (population) | Studies on males or females with stroke who are 18 years or older, within any stage of stroke | Studies not on patients with stroke |
| I (intervention) | Passive movement of joints | Unclearly described passive movement |
| C (comparators) | Any intervention or sham other than passive movement | Unclearly described intervention |
| O (outcomes) | Recovery outcomes such as motor function and disability | Outcomes that are not reported to be valid and reliable |
| S (study design) | Randomized controlled trial (RCT) | Study designs other than RCTs |
| References | N | Stroke Duration | Mean Age (Years) | Intervention | Outcomes | Findings | Adverse Events |
|---|---|---|---|---|---|---|---|
| Lynch et al. [26] | N = 32; experimental (n = 17, females = 12); control (n = 15, females = 7) | Experimental = 14.0 + 2.0 days; control = 12.0 + 2.0 days | Experimental = 61.0 + 3.0; control = 71.0 + 3.0 | Participants in both groups received standard interdisciplinary therapy for 3.5 hrs per day. Experimental = received 25 min daily continuous passive motion comprising shoulder elevation, external rotation, and abduction, 5 times a week. Control 2 = performed group self-range of motion focused on goal-directed movements of the shoulder. | Level of motor impairment (FMA); motor function of shoulder and elbow (MSS); muscle power of wrist and hand (MPS); disability (FIM); joint pain (FMA pain index); shoulder joint stability; muscle tone (MAS). | No significant difference between groups in the outcomes of interest post intervention (at discharge). | Not reported |
| Kim et al. [27] | N = 37 experimental (n = 19, females = 5); control (n = 18, females = 10) | Not reported | Experimental = 59.2 ± 14.1; control = 63.0 ± 16.2 | Experimental = performed 15 min bilateral passive ROM exercise of the upper limb joints twice a day, 5 days per week for 4 weeks. Each movement was repeated 10 times during the session. Control = performed same exercise as in the experimental group, but only after 2 weeks. | Upper extremity oedema (tape measure); ROM (goniometry); motor function (MFT); disability | All outcomes improved more significantly in the experimental group compared to the control post intervention. | Not reported |
| Hosseini et al. [28] | N = 52; experimental (n = 33, females = 16); control (n = 19, females = 10) | Not reported | Experimental = 30–90; control = 30–90 | Experimental = performed 15–40 min passive ROM exercise of the upper limb joints 6 to 8 times. Control = received only routine care during the period. | Muscle strength (MRC scale). | Significant improvement in muscle strength in the experimental group. | Not reported |
| Cho and Park [29] | N = 45; experimental 1 (n = 15, females = 5); experimental 2 (n = 15, females = 5); control (n = 15, females = 2) | Experimental 1 = 64.53 + 8.05; experimental 2 = 64.66 + 5.32; control = 63.40 + 7.09 | Experimental 1 = 3rd stage of the joint mobilization (gliding) was performed to induce dorsiflexion of the ankle joint repeatedly for 15 min. Control = performed active stretching of the ankle joint, repeated for 15 min. Experimental 2 = received a combined joint mobilization in experimental group 1 and the active stretching in the control group for 15 min. Intervention was done for 6 weeks. | ROM (goniometer); cadence, gait velocity and stride length (Gait–Walk). | All outcomes improved in the experimental groups. However, the improvement was more significant in the experimental group 2 (a combination of group experimental 1 and control group intervention). | Not reported |
| Study | Eligibility Criteria Specified | Random Allocation | Concealed Allocation | Comparable Subjects | Blind Subjects | Blind Therapists | Blind Assessors | Adequate Follow-Up | Intention to Treat Analysis | Between Group Comparison | Point Estimation and Variability | Total Score |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lynch et al. [26] | Yes | 1 | 0 | 1 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 5/10 |
| Kim et al. [27] | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7/10 |
| Hosseini et al. [28] | Yes | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 5/10 |
| Cho and Park [29 | Yes | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 7/10 |
| Number of Participants | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Outcome | Number of Studies | Risks of Bias | Inconsistency | Indirectness | Imprecision | Experimental | Control | Effect Size (95% CI) | Overall Certainty of the Evidence |
| Motor function | 2 | Serious | Very serious a | Not serious | Serious b | 36 | 33 | 0.70 (0.21 to 1.18) | ⨁⨁◯◯ Moderate |
| Muscle strength | 2 | Serious | Very serious a | Not serious | Serious b | 50 | 34 | 1.07 (−0.61 to 2.74) | ⨁⨁◯◯ Moderate |
| Disability | 2 | Serious | Very serious a | Not serious | Serious b | 36 | 33 | 0.81 (0.32 to 1.31) | ⨁⨁◯◯ Moderate |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdullahi, A.; Wong, T.W.L.; Ng, S.S.M. Effects of Passive Movement on Motor Function and Disability in Patients with Stroke: A Systematic Review and Meta-Analysis. J. Funct. Morphol. Kinesiol. 2025, 10, 117. https://doi.org/10.3390/jfmk10020117
Abdullahi A, Wong TWL, Ng SSM. Effects of Passive Movement on Motor Function and Disability in Patients with Stroke: A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology. 2025; 10(2):117. https://doi.org/10.3390/jfmk10020117
Chicago/Turabian StyleAbdullahi, Auwal, Thomson W. L. Wong, and Shamay S. M. Ng. 2025. "Effects of Passive Movement on Motor Function and Disability in Patients with Stroke: A Systematic Review and Meta-Analysis" Journal of Functional Morphology and Kinesiology 10, no. 2: 117. https://doi.org/10.3390/jfmk10020117
APA StyleAbdullahi, A., Wong, T. W. L., & Ng, S. S. M. (2025). Effects of Passive Movement on Motor Function and Disability in Patients with Stroke: A Systematic Review and Meta-Analysis. Journal of Functional Morphology and Kinesiology, 10(2), 117. https://doi.org/10.3390/jfmk10020117






