Effects of Ankle Joint Angles and Surrounding Muscles on Hip Joint Musculature
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Electromyography (EMG) Recording
2.3. Experimental Tasks
2.4. Data Analysis
2.5. Statistical Analysis
3. Results
4. Discussion
4.1. Relationship Between Ankle Joint Angle and Hip Musculature
4.2. Relationship Between Ankle Joint Muscle Force and Hip Musculature
4.3. Clinical Implications
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lyons, K.; Perry, J.; Gronley, J.K.; Barnes, L.; Antonelli, D. Timing and relative intensity of hip extensor and abductor muscles action during level and stair ambulation. An EMG study. Phys. Ther. 1983, 63, 1597–1605. [Google Scholar] [CrossRef] [PubMed]
- Anderson, F.C.; Pandy, M.G. Individual muscle contributions to support in normal walking. Gait Posture 2003, 17, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.Q.; Anderson, F.C.; Pandy, M.G.; Delp, S.L. Muscles that support the body also modulate forward progression during walking. J. Biomech. 2006, 39, 2623–2630. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Shimada, Y.; Sato, K.; Tsutsumi, Y.; Sato, M. Gait analysis before or after varus osteotomy of the femur for hip osteoarthritis. Bio-Med. Mater. Eng. 1998, 8, 177–186. [Google Scholar]
- Zacharias, A.; Green, R.A.; Semciw, A.; English, D.J.; Kapakoulakis, T.; Pizzari, T. Atrophy of hip abductor muscles is related to clinical severity in a hip osteoarthritis population. Clin. Anat. 2018, 31, 507–513. [Google Scholar] [CrossRef]
- Turrina, A.; Martínez-González, M.A.; Stecco, C. The muscular force transmission system: Role of the intramuscular connective tissue. J. Bodyw. Mov. Ther. 2013, 17, 95–102. [Google Scholar] [CrossRef]
- Huijing, P.A. Epimuscular myofascial force transmission between antagonistic and synergistic muscles can explain movement limitation in spastic paresis. J. Electromyogr. Kinesiol. 2007, 17, 708–724. [Google Scholar] [CrossRef]
- Shimura, K.; Kasai, T. Effects of proprioceptive neuromuscular facilitation on the initiation of voluntary movement and motor evoked potentials in upper limb muscles. Hum. Mov. Sci. 2002, 21, 101–113. [Google Scholar] [CrossRef]
- Alexandre de Assis, I.S.; Luvizutto, G.J.; Bruno, A.C.M.; Sande de Souza, L.A.P. The proprioceptive neuromuscular facilitation concept in Parkinson disease: A systematic review and meta-analysis. J. Chiropr. Med. 2020, 19, 181–187. [Google Scholar] [CrossRef]
- Mikaili, S.; Khademi-Kalantari, K.; Rezasoltani, A.; Arzani, P.; Baghban, A.A. Quadriceps force production during straight leg raising at different hip positions with and without concomitant ankle dorsiflexion. J. Bodyw. Mov. Ther. 2018, 22, 904–908. [Google Scholar] [CrossRef]
- Lim, Y.P.; Lin, Y.C.; Pandy, M.G. Muscle function during gait is invariant to age when walking speed is controlled. Gait Posture 2013, 38, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Beckman, S.M.; Buchanan, T.S. Ankle inversion injury and hypermobility: Effect on hip and ankle muscle electromyography onset latency. Arch. Phys. Med. Rehabil. 1995, 76, 1138–1143. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.L.; Davenport, T.E.; Reischl, S.F.; McPoil, T.G.; Matheson, J.W.; Wukich, D.K.; McDonough, C.M.; American Physical Therapy Association. Heel pain-plantar fasciitis: Revision 2014. J. Orthop. Sports Phys. Ther. 2014, 44, A1–A33. [Google Scholar] [CrossRef]
- Choi, J.H.; Cynn, H.S.; Yi, C.H.; Yoon, T.L.; Baik, S.M. Effect of isometric hip abduction on foot and ankle muscle activity and medial longitudinal arch during short-foot exercise in individuals with pes Planus. J. Sport Rehabil. 2020, 30, 368–374. [Google Scholar] [CrossRef]
- Boling, M.C.; Bolgla, L.A.; Mattacola, C.G.; Uhl, T.L.; Hosey, R.G. Outcomes of a weight-bearing rehabilitation program for patients diagnosed with patellofemoral pain syndrome. Arch. Phys. Med. Rehabil. 2006, 87, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Bolgla, L.A.; Uhl, T.L. Electromyographic analysis of hip rehabilitation exercises in a group of healthy subjects. J. Orthop. Sports Phys. Ther. 2005, 35, 487–494. [Google Scholar] [CrossRef]
- Bishop, B.N.; Greenstein, J.; Etnoyer-Slaski, J.L.; Sterling, H.; Topp, R. Electromyographic analysis of gluteus maximus, gluteus medius, and tensor fascia latae during therapeutic exercises with and without elastic resistance. Int. J. Sports Phys. Ther. 2018, 13, 668–675. [Google Scholar] [CrossRef]
- Griffin, L.; Cafarelli, E. Transcranial magnetic stimulation during resistance training of the tibialis anterior muscle. J. Electromyogr. Kinesiol. 2007, 17, 446–452. [Google Scholar] [CrossRef]
- Soma, M.; Murata, S.; Kai, Y.; Nakae, H.; Satou, Y.; Murata, J.; Miyazaki, J. Comparison of toe grip strength and muscle activities during maximal toe grip strength exertion according to the presence/absence of an ankle immobilization belt. J. Phys. Ther. Sci. 2015, 27, 3081–3084. [Google Scholar] [CrossRef]
- Arnold, C.M.; Warkentin, K.D.; Chilibeck, P.D.; Magnus, C.R. The reliability and validity of handheld dynamometry for the measurement of lower-extremity muscle strength in older adults. J. Strength Cond. Res. 2010, 24, 815–824. [Google Scholar] [CrossRef]
- Flack, N.A.M.S.; Nicholson, H.D.; Woodley, S.J. A review of the anatomy of the hip abductor muscles, gluteus medius, gluteus minimus, and tensor fascia lata. Clin. Anat. 2012, 25, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.A. Kinesiology of the hip: A focus on muscular actions. J. Orthop. Sports Phys. Ther. 2010, 40, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Besomi, M.; Salomoni, S.E.; Cruz-Montecinos, C.; Stecco, C.; Vicenzino, B.; Hodges, P.W. Distinct displacement of the superficial and deep fascial layers of the iliotibial band during a weight shift task in runners: An exploratory study. J. Anat. 2022, 240, 579–588. [Google Scholar] [CrossRef] [PubMed]
- Huijing, P.A. Muscle as a collagen fiber reinforced composite: A review of force transmission in muscle and whole limb. J. Biomech. 1999, 32, 329–345. [Google Scholar] [CrossRef]
- Topp, K.S.; Boyd, B.S. Structure and biomechanics of peripheral nerves: Nerve responses to physical stresses and implications for physical therapist practice. Phys. Ther. 2006, 86, 92–109. [Google Scholar] [CrossRef]
- Sunderland, S. Nerves and Nerve Injuries, 2nd ed.; Churchill Livingstone: London, UK, 1978. [Google Scholar]
- Negro, F.; Farina, D. Factors influencing the estimates of correlation between motor unit activities in Humans Negro. PLoS ONE 2012, 7, e44894. [Google Scholar] [CrossRef]
- Chon, S.C.; Chang, K.Y.; You, J.S.H. Effect of the abdominal draw-in manoeuvre in combination with ankle dorsiflexion in strengthening the transverse abdominal muscle in healthy young adults: A preliminary, randomised, controlled study. Physiotherapy 2010, 96, 130–136. [Google Scholar] [CrossRef]
- Saito, A.; Nakagawa, K.; Masugi, Y.; Nakazawa, K. Inter-muscle differences in modulation of motor evoked potentials and posterior root-muscle reflexes evoked from lower-limb muscles during agonist and antagonist muscle contractions. Exp. Brain Res. 2021, 239, 463–474. [Google Scholar] [CrossRef]
- Nam, S.J.; Oh, D.W. Is it appropriate to use external loads during side-lying hip abduction exercise for weakened gluteus medius? J. Back Musculoskelet. Rehabil. 2021, 34, 1057–1062. [Google Scholar] [CrossRef]
- Ebert, J.R.; Edwards, P.K.; Fick, D.P.; Janes, G.C. A systematic review of rehabilitation exercises to progressively load the gluteus medius. J. Sport Rehabil. 2017, 26, 418–436. [Google Scholar] [CrossRef]
- Monselli, C.; Bianco Prevot, L.; Accetta, R.; Tronconi, L.P.; Bolcato, V.; Basile, G. State of the art in rehabilitation strategies after hip arthroscopy for femoroacetabular impingement syndrome: A systematic review. J. Clin. Med. 2024, 13, 7302. [Google Scholar] [CrossRef]
- Kim, E.H.; Lim, T.H.; Park, S.H.; Kim, C.S.; Jang, S.H.; Cho, Y.W.; Kim, K.J.; Choi, H.S.; Ahn, S.H. Effect of hip abduction exercise with manual pelvic fixation on recruitment of deep trunk muscles. Am. J. Phys. Med. Rehabil. 2015, 94, 201–210. [Google Scholar] [CrossRef]
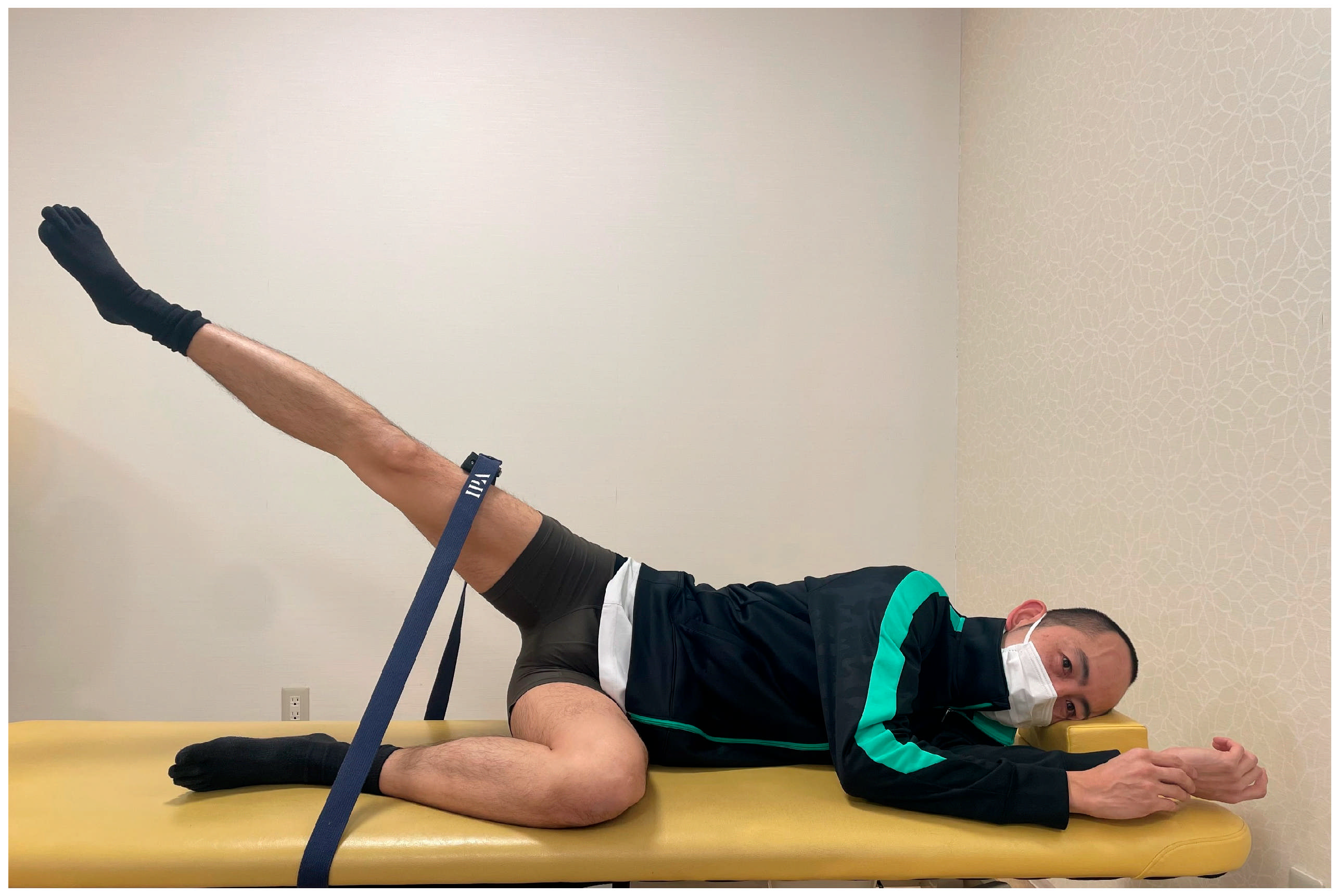

| Dorsiflexion Position | Neutral Position | Plantarflexion Position | |||||||
|---|---|---|---|---|---|---|---|---|---|
| DFX | NMS | PFX | DFX | NMS | PFX | DFX | NMS | PFX | |
| M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | M ± SD | |
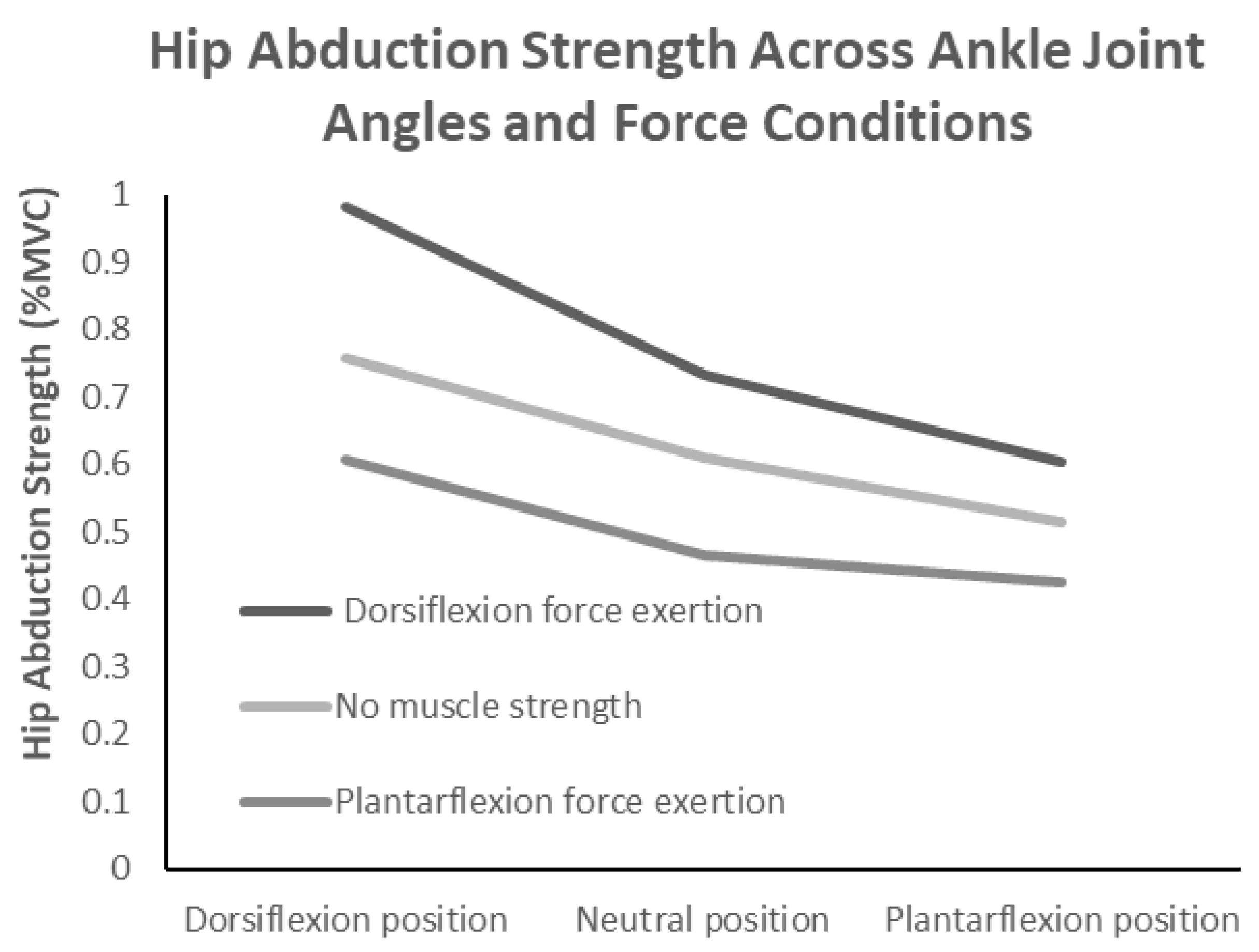
| Hip abductor strength (%) | 1.0 ± 0.0 * | 0.7 ± 0.1 * | 0.6 ± 0.1 * | 0.8 ± 0.1 * | 0.6 ± 0.1 * | 0.5 ± 0.1 * | 0.6 ± 0.1 * | 0.5 ± 0.1 * | 0.4 ± 0.1 |
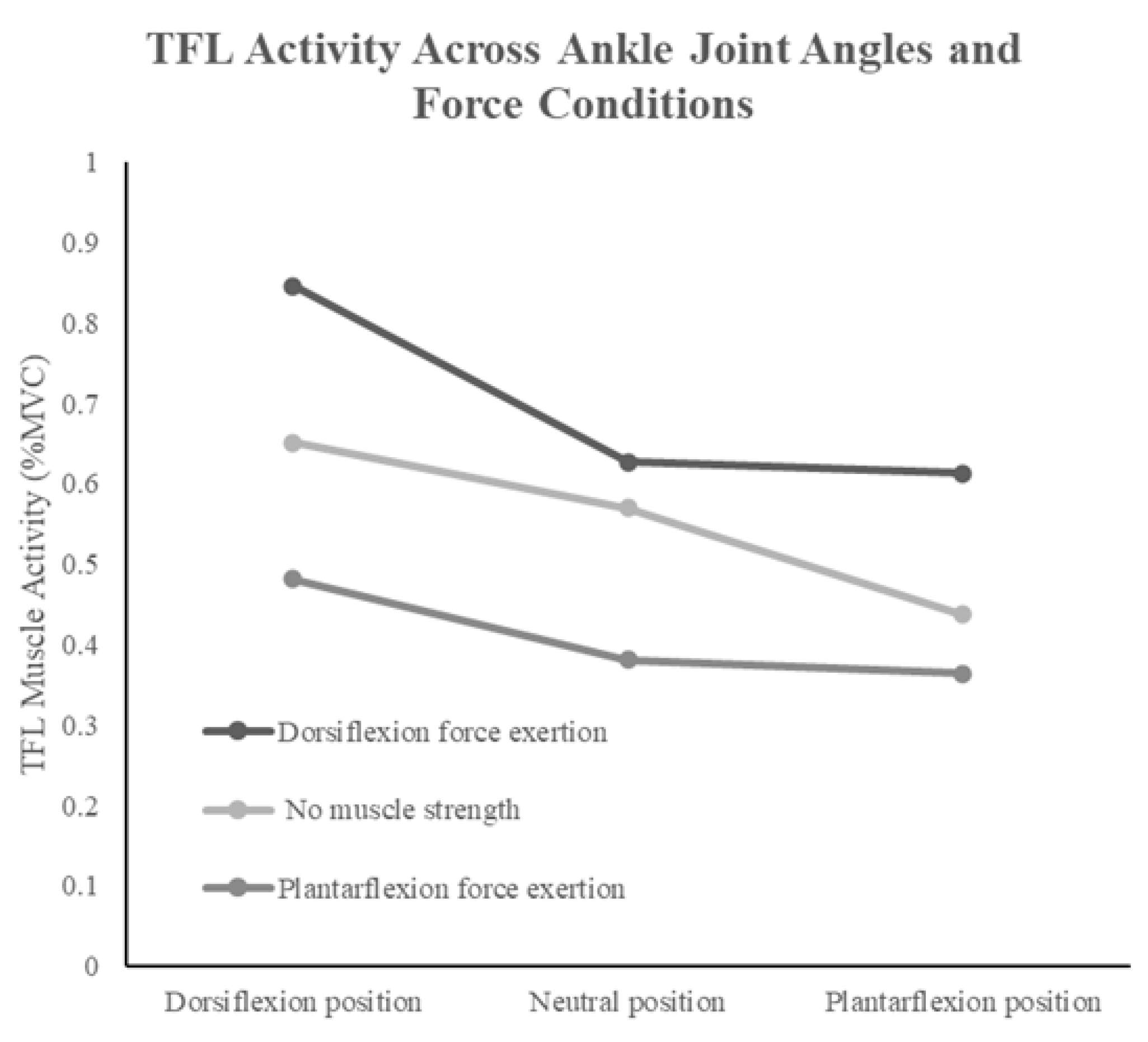
| Tensor fasciae latae (%) | 0.8 ± 0.1 * | 0.7 ± 0.1 * | 0.5 ± 0.1 | 0.6 ± 0.2 * | 0.6 ± 0.2 * | 0.4 ± 0.1 | 0.6 ± 0.1 * | 0.4 ± 0.2 | 0.4 ± 0.2 |
| Gluteus medius (%) | 0.9 ± 0.1 * | 0.7 ± 0.1 * | 0.6 ± 0.1 * | 0.7 ± 0.1 * | 0.6 ± 0.1 * | 0.5 ± 0.1 * | 0.6 ± 0.2 * | 0.5 ± 0.2 * | 0.4 ± 0.1 |
| Gluteus maximus (%) | 0.6 ± 0.2 * | 0.4 ± 0.1 * | 0.3 ± 0.2 | 0.4 ± 0.2 * | 0.3 ± 0.1 * | 0.3 ± 0.3 | 0.4 ± 0.2 * | 0.3 ± 0.2 | 0.3 ± 0.2 |
| Main Effect | Interaction Effect | ||||||||
| Range | Muscle Strength | Range × Muscle Strength | |||||||
| F | p | η2 | F | p | η2 | F | p | η2 | |
| 174.25 | <0.001 * | 0.926 | 196.08 | <0.001 * | 0.933 | 8.37 | <0.001 * | 0.374 | |
| 29.93 | <0.001 * | 0.681 | 86.32 | <0.001 * | 0.86 | 3.56 | 0.012 * | 0.203 | |
| 57.61 | <0.001 * | 0.805 | 121.22 | <0.001 * | 0.896 | 3.25 | 0.018 * | 0.188 | |
| 10.99 | <0.001 * | 0.44 | 27.33 | <0.001 * | 0.661 | 1.86 | 0.131 | 0.117 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murata, Y.; Kida, N.; Jiromaru, T.; Wachi, M.; Yoshikawa, K.; Noguchi, S.; Onishi, H. Effects of Ankle Joint Angles and Surrounding Muscles on Hip Joint Musculature. J. Funct. Morphol. Kinesiol. 2025, 10, 110. https://doi.org/10.3390/jfmk10020110
Murata Y, Kida N, Jiromaru T, Wachi M, Yoshikawa K, Noguchi S, Onishi H. Effects of Ankle Joint Angles and Surrounding Muscles on Hip Joint Musculature. Journal of Functional Morphology and Kinesiology. 2025; 10(2):110. https://doi.org/10.3390/jfmk10020110
Chicago/Turabian StyleMurata, Yuta, Noriyuki Kida, Takumi Jiromaru, Michio Wachi, Kohei Yoshikawa, Shinichi Noguchi, and Hitoshi Onishi. 2025. "Effects of Ankle Joint Angles and Surrounding Muscles on Hip Joint Musculature" Journal of Functional Morphology and Kinesiology 10, no. 2: 110. https://doi.org/10.3390/jfmk10020110
APA StyleMurata, Y., Kida, N., Jiromaru, T., Wachi, M., Yoshikawa, K., Noguchi, S., & Onishi, H. (2025). Effects of Ankle Joint Angles and Surrounding Muscles on Hip Joint Musculature. Journal of Functional Morphology and Kinesiology, 10(2), 110. https://doi.org/10.3390/jfmk10020110







