Design of a Semi-Continuous Microwave System for Pretreatment of Microwave-Assisted Pyrolysis Using a Theoretical Method
Abstract
1. Introduction
2. Microwave-Assisted Pretreatment
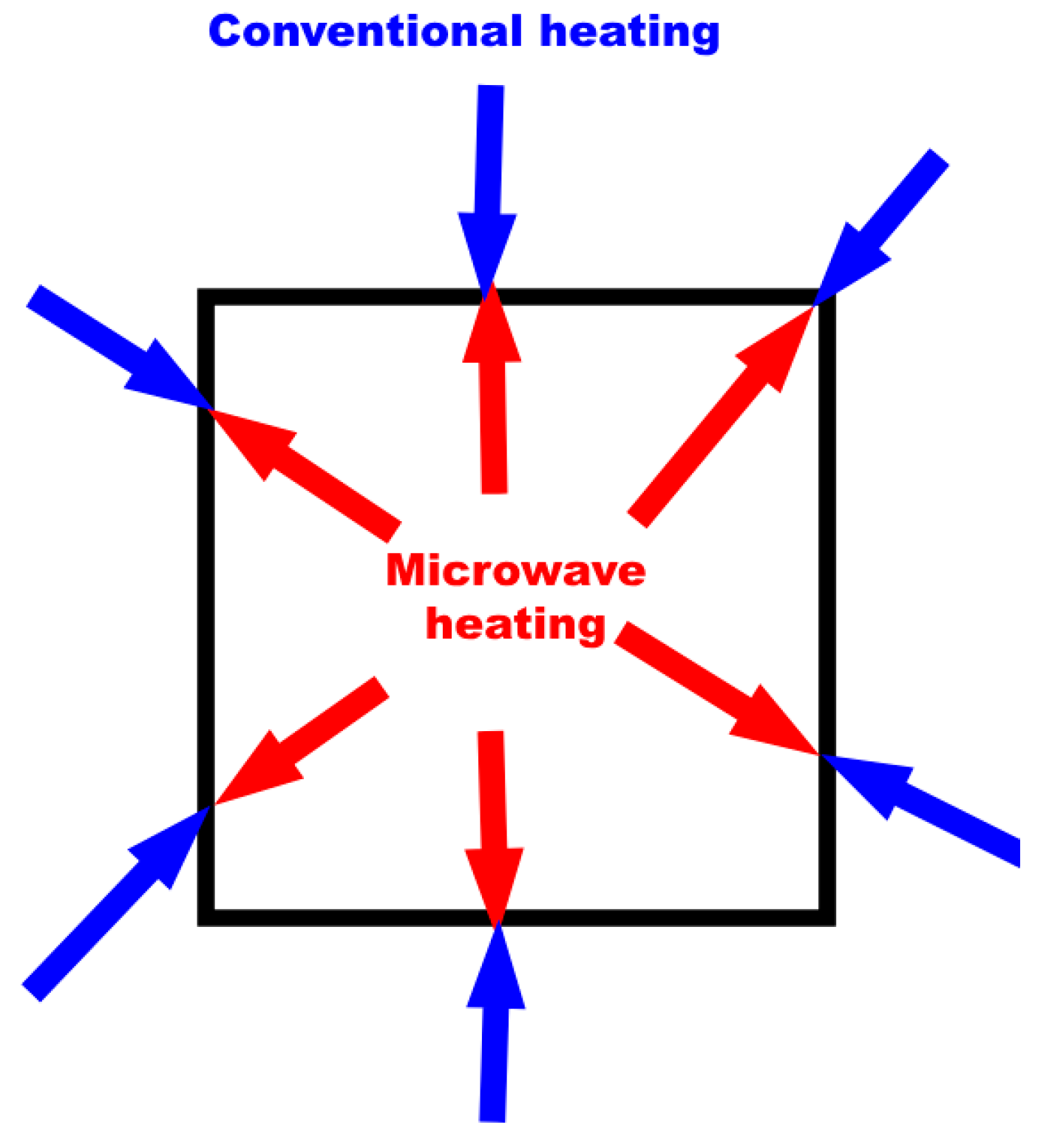
2.1. Types of Microwaves
2.2. Microwave-Assisted Pyrolysis
2.2.1. Microwave Source
2.2.2. Microwave Applicator
2.2.3. Feedstock Material
2.2.4. Microwave Absorbers
2.2.5. Product Collection System
2.2.6. Pyrolysis Reactor
2.2.7. Temperature and Pressure Control
3. Biomass Specifications
4. Designed Development
4.1. Mathematical Model
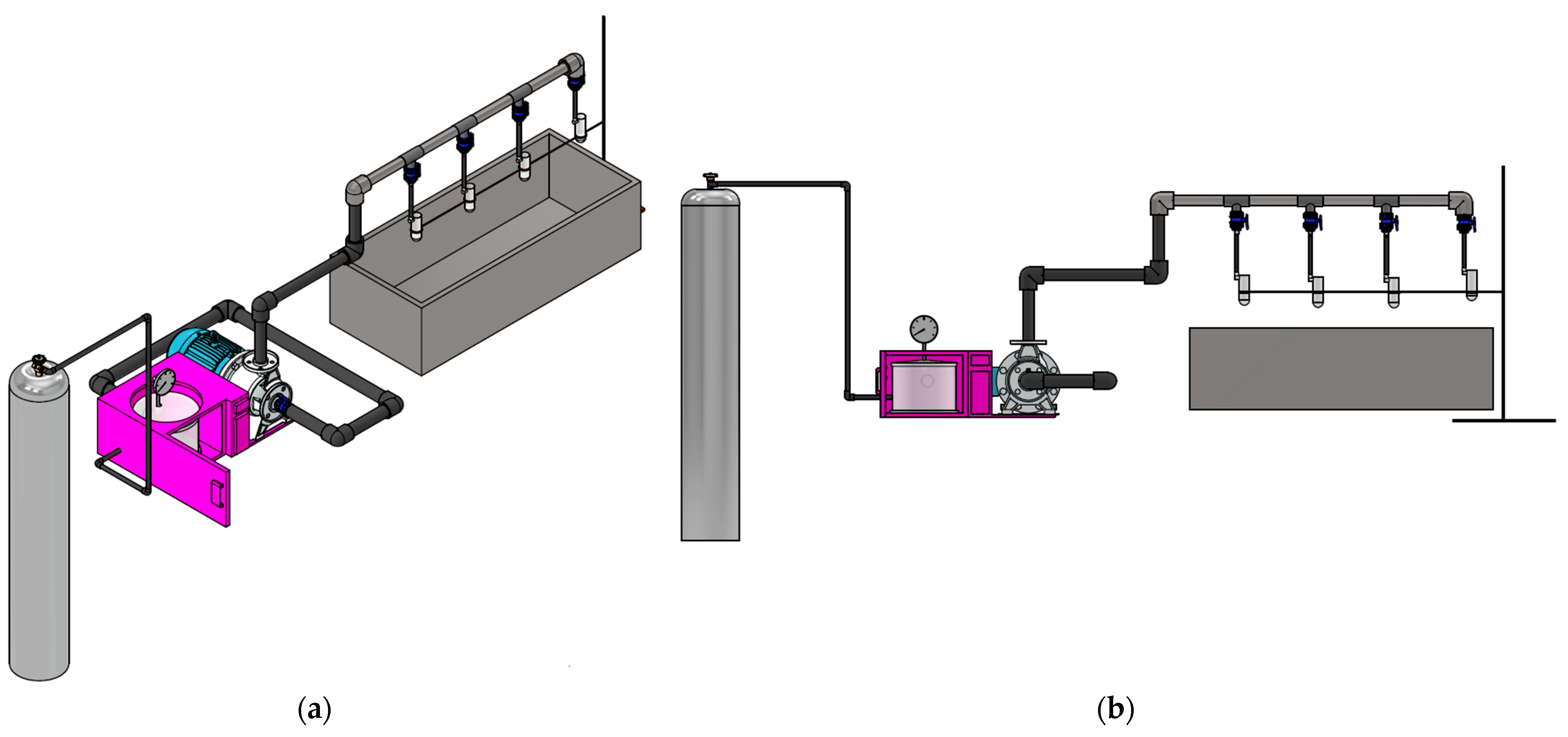
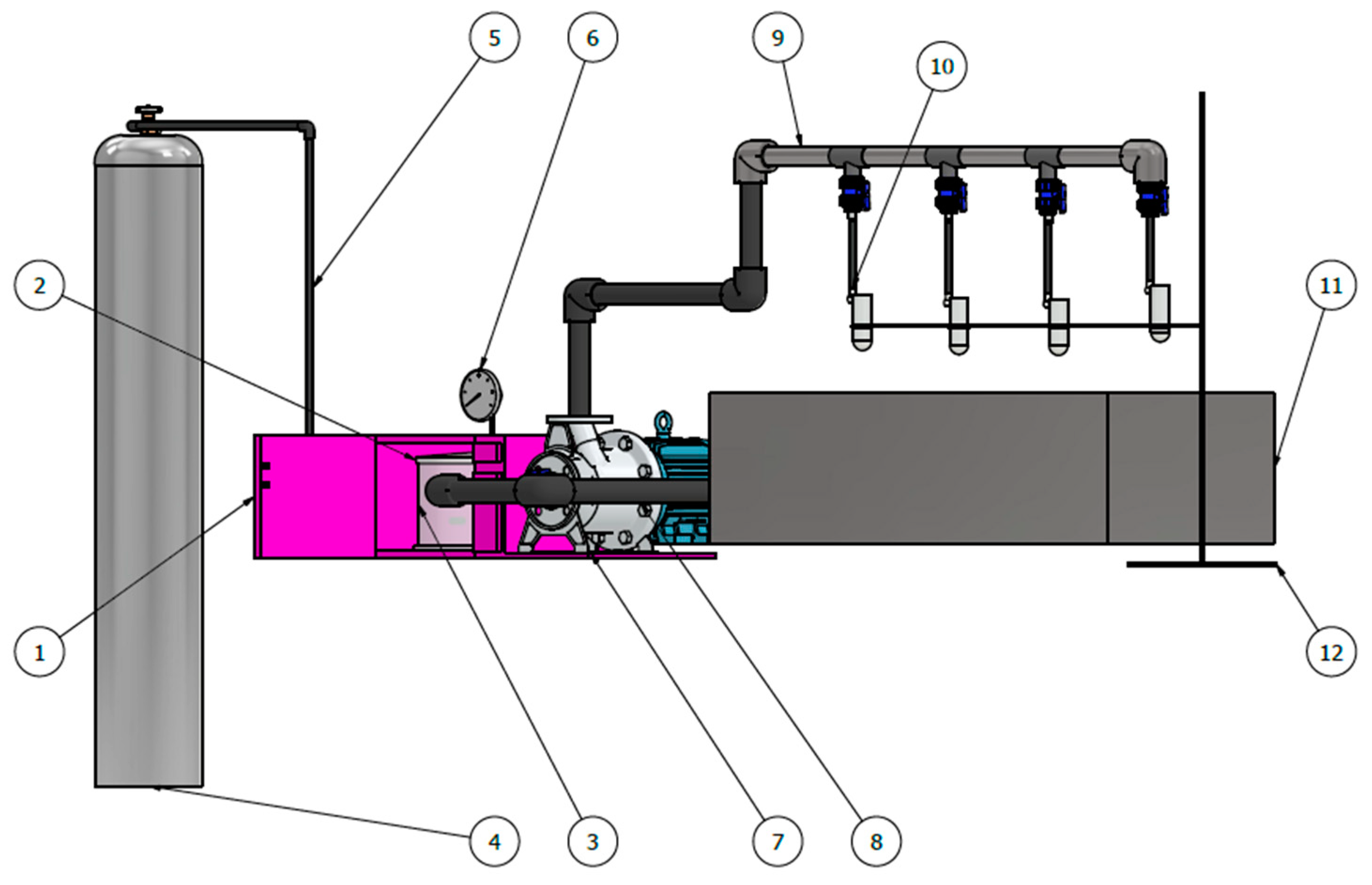
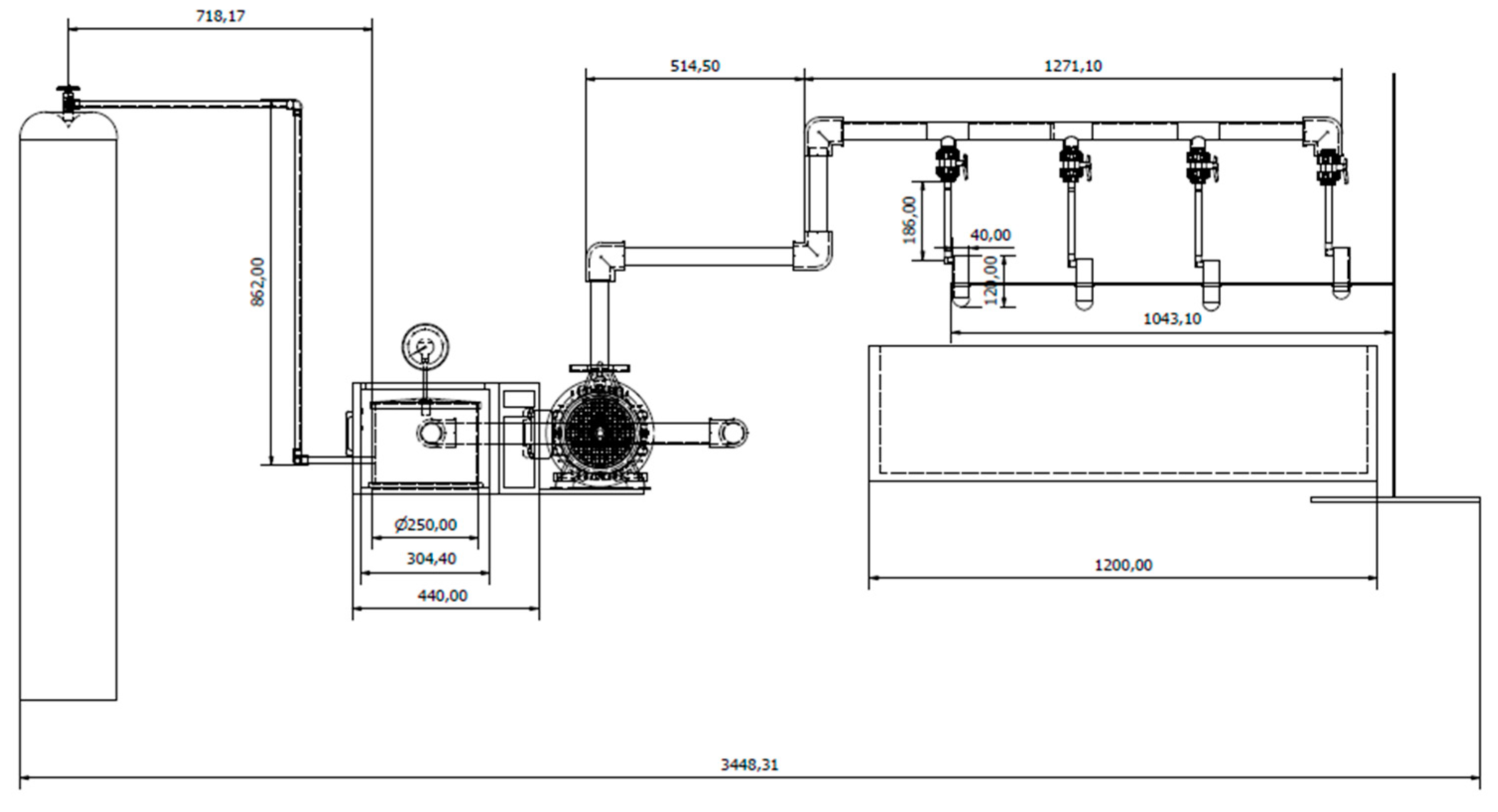
4.2. Specifications and CAD Design
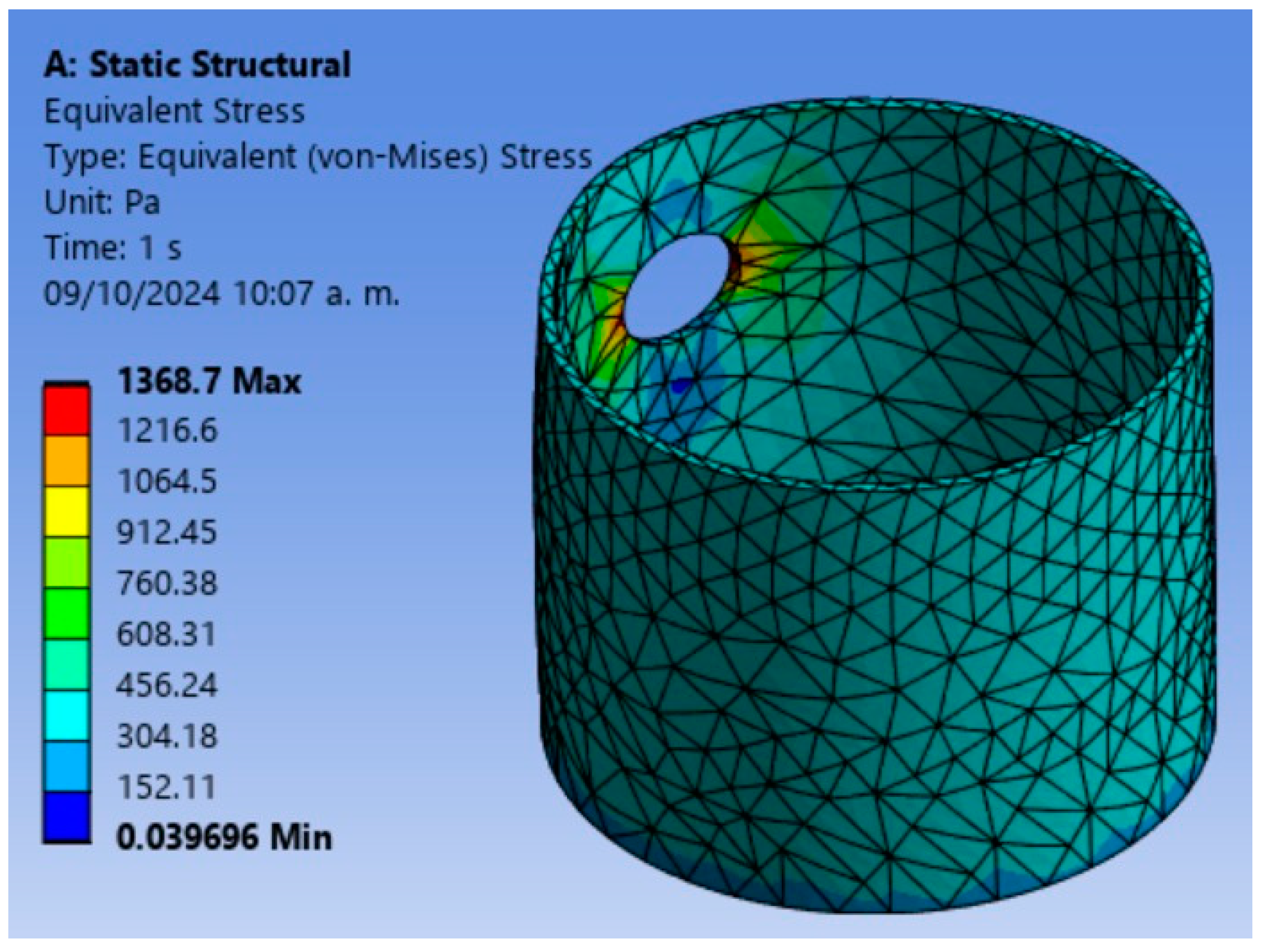
4.3. Finite Element Analysis
5. Conclusions
6. Challenges or Future Prospective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maurya, D.P.; Singla, A.; Negi, S. An overview of key pretreatment processes for biological conversion of lignocellulosic biomass to bioethanol. 3 Biotech 2015, 5, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Mahalaxmi, S.; Williford, C. Biochemical Conversion of Biomass to Fuels. In Handbook of Climate Change Mitigation and Adaptation; Springer International Publishing: Cham, Switzerland, 2017; pp. 1777–1811. [Google Scholar] [CrossRef]
- Lizundia, E.; Luzi, F.; Puglia, D. Organic waste valorisation towards circular and sustainable biocomposites. Green Chem. 2022, 24, 5429–5459. [Google Scholar] [CrossRef]
- Kalak, T. Potential Use of Industrial Biomass Waste as a Sustainable Energy Source in the Future. Energies 2023, 16, 1783. [Google Scholar] [CrossRef]
- Blasi, A.; Verardi, A.; Lopresto, C.G.; Siciliano, S.; Sangiorgio, P. Lignocellulosic Agricultural Waste Valorization to Obtain Valuable Products: An Overview. Recycling 2023, 8, 61. [Google Scholar] [CrossRef]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.U.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [PubMed]
- Shafiei, M.; Kumar, R.; Karimi, K. Pretreatment of Lignocellulosic Biomass. In Lignocellulose-Based Bioproducts; Springer: Berlin/Heidelberg, Germany, 2015; pp. 85–154. [Google Scholar] [CrossRef]
- Behera, S.; Arora, R.; Nandhagopal, N.; Kumar, S. Importance of chemical pretreatment for bioconversion of lignocellulosic biomass. Renew. Sustain. Energy Rev. 2014, 36, 91–106. [Google Scholar] [CrossRef]
- Mattanovich, D.; Sauer, M.; Gasser, B. Yeast Biotechnology: Teaching the Old Dog New Tricks. In New Microbial Technologies for Advanced Biofuels; Apple Academic Press: Williston, VT, USA, 2015; pp. 107–116. [Google Scholar] [CrossRef]
- Hu, X.; Ma, D.; Zhang, G.; Ling, M.; Hu, Q.; Liang, K.; Lu, J.; Zheng, Y. Microwave-assisted pyrolysis of waste plastics for their resource reuse: A technical review. Carbon Resour. Convers. 2023, 6, 215–228. [Google Scholar] [CrossRef]
- Mikulski, D.; Kłosowski, G. High-pressure microwave-assisted pretreatment of softwood, hardwood and non-wood biomass using different solvents in the production of cellulosic ethanol. Biotechnol. Biofuels Bioprod. 2023, 16, 19. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nižetić, S.; Ong, H.C.; Mofijur, M.; Ahmed, S.; Ashok, B.; Bui, V.T.V.; Chau, M.Q. Insight into the recent advances of microwave pretreatment technologies for the conversion of lignocellulosic biomass into sustainable biofuel. Chemosphere 2021, 281, 130878. [Google Scholar] [CrossRef]
- Ludlow-Palafox, C.; Chase, H.A. Microwave-Induced Pyrolysis of Plastic Wastes. Ind. Eng. Chem. Res. 2001, 40, 4749–4756. [Google Scholar] [CrossRef]
- Pandey, S.; Erbaugh, J.T. Driving sustainable uptake: A systematic review of global literature on policies governing woody biomass for energy. Discov. Sustain. 2024, 5, 28. [Google Scholar] [CrossRef]
- Ethaib, S.; Omar, R.; Kamal, S.M.M.; Biak, D.R.A.; Zubaidi, S.L. Microwave-Assisted Pyrolysis of Biomass Waste: A Mini Review. Processes 2020, 8, 1190. [Google Scholar] [CrossRef]
- Li, Y.; Campos, L.C.; Hu, Y. Microwave pretreatment of wastewater sludge technology—A scientometric-based review. Environ. Sci. Pollut. Res. 2024, 31, 26432–26451. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, N.D.; Pauly, M.; Dama, M. Microwave Assisted Pretreatment of Szarvasi (Agropyron elongatum) Biomass to Enhance Enzymatic Saccharification and Direct Glucose Production. Front. Plant Sci. 2022, 12, 767254. [Google Scholar] [CrossRef]
- Mohabeer, C.; Guilhaume, N.; Laurenti, D.; Schuurman, Y. Microwave-Assisted Pyrolysis of Biomass with and without Use of Catalyst in a Fluidised Bed Reactor: A Review. Energies 2022, 15, 3258. [Google Scholar] [CrossRef]
- Romeiro, F.C.; Silva, S.C.; Nossol, E.; Lima, R.C. One step microwave-hydrothermal synthesis of rGO–TiO2nanocomposites for enhanced electrochemical oxygen evolution reaction. New J. Chem. 2020, 44, 6825–6832. [Google Scholar] [CrossRef]
- Volfkovich, Y.M.; Rychagov, A.Y.; Sosenkin, V.E.; Baskakov, S.A.; Kabachkov, E.N.; Shulga, Y.M. Supercapacitor Properties of rGO-TiO2 Nanocomposite in Two-component Acidic Electrolyte. Materials 2022, 15, 7856. [Google Scholar] [CrossRef]
- Guo, J.; Liu, H.; Li, D.; Wang, J.; Djitcheu, X.; He, D.; Zhang, Q. A minireview on the synthesis of single atom catalysts. RSC Adv. 2022, 12, 9373–9394. [Google Scholar] [CrossRef]
- Munnik, P.; de Jongh, P.E.; de Jong, K.P. Recent Developments in the Synthesis of Supported Catalysts. Chem. Rev. 2015, 115, 6687–6718. [Google Scholar] [CrossRef]
- Babu, S.J.; Muniyappa, M.; Rao, V.N.; Mudike, R.; Shastri, M.; Tathagata, S.; Shivaramu, P.D.; Shankar, M.V.; Ananda Kumar, C.S.; Rangappa, D. Enhanced photocatalytic hydrogen evolution from reduced graphene oxide-defect rich TiO2-x nanocomposites. Int. J. Hydrogen Energy 2022, 47, 40242–40253. [Google Scholar] [CrossRef]
- Yang, Y.; Shahbeik, H.; Shafizadeh, A.; Masoudnia, N.; Rafiee, S.; Zhang, Y.; Pan, J.; Tabatabaei, M.; Aghbashlo, M. Biomass microwave pyrolysis characterization by machine learning for sustainable rural biorefineries. Renew. Energy 2022, 201, 70–86. [Google Scholar] [CrossRef]
- Vollmer, M. Physics of the microwave oven. Phys. Educ. 2003, 39, 74–81. [Google Scholar] [CrossRef]
- Sun, J.; Wang, W.; Yue, Q. Review on Microwave-Matter Interaction Fundamentals and Efficient Microwave-Associated Heating Strategies. Materials 2016, 9, 231. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Ramanathan, S.; Basak, T. Microwave material processing-a review. AIChE J. 2011, 58, 330–363. [Google Scholar] [CrossRef]
- Muley, P.D.; Wang, Y.; Hu, J.; Shekhawat, D. Microwave-assisted heterogeneous catalysis. In Catalysis; The Royal Society of Chemistry: London, UK, 2021; Volume 33, pp. 1–37. [Google Scholar] [CrossRef]
- Catalytic conversion of CO2 into fuels and chemicals: A green ccu option. In Industrial Catalysis and Separations; Apple Academic Press: Williston, VT, USA, 2014; pp. 186–249. [CrossRef]
- Palma, V.; Barba, D.; Cortese, M.; Martino, M.; Renda, S.; Meloni, E. Microwaves and Heterogeneous Catalysis: A Review on Selected Catalytic Processes. Catalysts 2020, 10, 246. [Google Scholar] [CrossRef]
- Jha, A. Microwave Assisted Synthesis of Organic Compounds and Nanomaterials. In Nanofibers—Synthesis, Properties and Applications; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- de la Hoz, A.; Díaz-Ortiz, A.; Prieto, P. Microwave-Assisted Green Organic Synthesis. In Alternative Energy Sources for Green Chemistry; The Royal Society of Chemistry: London, UK, 2016; pp. 1–33. [Google Scholar] [CrossRef]
- Patneedi, C.; Prasadu, D.; Sekhar, C.; Rao, V. Microwave Mediated Synthesis in Pharmaceutical Chemistry. Rasayan J. Chem. 2015, 2015, 8. [Google Scholar]
- Al-Qahtani, A.M. A Comprehensive Review in Microwave Pyrolysis of Biomass, Syngas Production and Utilisation. Energies 2023, 16, 6876. [Google Scholar] [CrossRef]
- Lew, A.; Krutzik, P.O.; Hart, M.E.; Chamberlin, A.R. Increasing Rates of Reaction: Microwave-Assisted Organic Synthesis for Combinatorial Chemistry. J. Comb. Chem. 2001, 4, 95–105. [Google Scholar] [CrossRef]
- Desai, M.; Parikh, J.; Parikh, P.A. Extraction of Natural Products Using Microwaves as a Heat Source. Sep. Purif. Rev. 2010, 39, 1–32. [Google Scholar] [CrossRef]
- Jun, C.; Chung, J.; Lee, D.; Loupy, A.; Chatti, S. Solvent-Free Chelation-Assisted Intermolecular Hydroacylation: Effect of Microwave Irradiation in the Synthesis of Ketone from Aldehyde and 1-Alkene by Rh(I) Complex. Tetrahedron Lett. 2001, 42, 4803–4805. [Google Scholar] [CrossRef]
- Muhammad, W.; Isa, M.; Habib, S.; Seah, C.; Hafriz, R.; Shamsuddin, A. Assessment of biochar, bio-oil and biogas production from lemon myrtle waste via microwave assisted catalytic pyrolysis using CaO based catalyst and zeolite catalyst. Energy Convers. Manag. X 2023, 20, 100481. [Google Scholar] [CrossRef]
- Gao, Y.; Remón, J.; Matharu, A.S. Microwave-assisted hydrothermal treatments for biomass valorisation: A critical review. Green Chem. 2021, 23, 3502–3525. [Google Scholar] [CrossRef]
- Kamath, V.; Suvarna, D.; Gayathri, D.; Rao, V. Microwave Accelerated Dilute Acid Hydrolysis of Pongamia Pressed Oil Cake for Release of Fermentable Sugars. Adv. Biores. 2018, 9, 109–118. [Google Scholar]
- Meindl, A.; Petutschnigg, A.; Schnabel, T. Microwave-Assisted Lignin Extraction—Utilizing Deep Eutectic Solvents to Their Full Potential. Polymers 2022, 14, 4319. [Google Scholar] [CrossRef] [PubMed]
- Nomanbhay, S.M.; Hussain, R.; Palanisamy, K. Microwave-Assisted Alkaline Pretreatment and Microwave Assisted Enzymatic Saccharification of Oil Palm Empty Fruit Bunch Fiber for Enhanced Fermentable Sugar Yield. J. Sustain. Bioenergy Syst. 2013, 3, 7–17. [Google Scholar] [CrossRef]
- Das, A.; Banik, B.K. Microwave-assisted enzymatic reactions. In Microwaves in Chemistry Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 245–281. [Google Scholar] [CrossRef]
- Beluhan, S.; Mihajlovski, K.; Šantek, B.; Šantek, M.I. The Production of Bioethanol from Lignocellulosic Biomass: Pretreatment Methods, Fermentation, and Downstream Processing. Energies 2023, 16, 7003. [Google Scholar] [CrossRef]
- Pérez, A.S.L.; Castro, J.J.L.; Fajardo, C.A.G. Application of Microwave Energy to Biomass: A Comprehensive Review of Microwave-Assisted Technologies, Optimization Parameters, and the Strengths and Weaknesses. J. Manuf. Mater. Process. 2024, 8, 121. [Google Scholar] [CrossRef]
- Dertli, H.; Saloglu, D. A valorization approach of food industry wastewater using microwave-assisted extraction. In Advanced Technologies in Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2023; pp. 155–178. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; Rivas, S.; Torres, M.D.; Domínguez, H. Microwave-Assisted Extraction of Carrageenan from Sarcopeltis skottsbergii. Mar. Drugs 2023, 21, 83. [Google Scholar] [CrossRef]
- Gotama, B.; Rahman, A.K.; Ahmad, A.; Hariyadi, A. Extraction of rice bran oil using microwave-assisted extraction and green solvents. IOP Conf. Series Earth Environ. Sci. 2022, 1105, 012052. [Google Scholar] [CrossRef]
- Avelino, F.; da Silva, K.T.; de Souza Filho, M.d.S.M.; Mazzetto, S.E.; Lomonaco, D. Microwave-assisted organosolv extraction of coconut shell lignin by Brønsted and Lewis acids catalysts. J. Clean. Prod. 2018, 189, 785–796. [Google Scholar] [CrossRef]
- Avelino, F.; Marques, F.; Soares, A.K.L.; Silva, K.T.; Leitão, R.C.; Mazzetto, S.E.; Lomonaco, D.; Avelino, F.; Marques, F.; Soares, A.K.L.; et al. Microwave-Assisted Organosolv Delignification: A Potential Eco-Designed Process for Scalable Valorization of Agroindustrial Wastes. Ind. Eng. Chem. Res. 2019, 58, 10698–10706. [Google Scholar] [CrossRef]
- Goyal, H.; Chen, T.-Y.; Chen, W.; Vlachos, D.G. A review of microwave-assisted process intensified multiphase reactors. Chem. Eng. J. 2022, 430, 133183. [Google Scholar] [CrossRef]
- Zhou, N.; Zhou, J.; Dai, L.; Guo, F.; Wang, Y.; Li, H.; Deng, W.; Lei, H.; Chen, P.; Liu, Y.; et al. Syngas production from biomass pyrolysis in a continuous microwave assisted pyrolysis system. Bioresour. Technol. 2020, 314, 123756. [Google Scholar] [CrossRef]
- Bing, W.; Hongbin, Z.; Zeng, D.; Yuefeng, F.; Yu, Q.; Rui, X. Microwave fast pyrolysis of waste tires: Effect of microwave power on product composition and quality. J. Anal. Appl. Pyrolysis 2020, 155, 104979. [Google Scholar] [CrossRef]
- Robinson, J.; Binner, E.; Vallejo, D.B.; Perez, N.D.; Al Mughairi, K.; Ryan, J.; Shepherd, B.; Adam, M.; Budarin, V.; Fan, J.; et al. Unravelling the mechanisms of microwave pyrolysis of biomass. Chem. Eng. J. 2022, 430, 132975. [Google Scholar] [CrossRef]
- López-Salazar, H.; Camacho-Díaz, B.H.; Ocampo, M.L.A.; Jiménez-Aparicio, A.R. Microwave-assisted extraction of functional compounds from plants: A Review. Bioresources 2023, 18, 6614–6638. [Google Scholar] [CrossRef]
- Yao, J.; Karlsson, M.; Lawoko, M.; Odelius, K.; Hakkarainen, M. Microwave-assisted organosolv extraction for more native-like lignin and its application as a property-enhancing filler in a light processable biobased resin. RSC Sustain. 2023, 1, 1211–1222. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass—Convers. Biorefin. 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Aguilar-Reynosa, A.; Romaní, A.; Rodríguez-Jasso, R.M.; Aguilar, C.N.; Garrote, G.; Ruiz, H.A. Microwave heating processing as alternative of pretreatment in second-generation biorefinery: An overview. Energy Convers. Manag. 2017, 136, 50–65. [Google Scholar] [CrossRef]
- Xie, Q.; Chen, Z.; Zhou, Y.; Pan, T.; Duan, Y.; Yu, S.; Liang, X.; Wu, Z.; Ji, W.; Nie, Y. Efficient treatment of oily sludge via fast microwave-assisted pyrolysis, followed by thermal plasma vitrification. Molecules 2023, 28, 4036. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.S.; Russell, A.D.; Lee, C.L.; Chase, H.A. Microwave-heated pyrolysis of waste automotive engine oil: Influence of operation parameters on the yield, composition, and fuel properties of pyrolysis oil. Fuel 2012, 92, 327–339. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Ren, S.; Wang, L.; Holladay, J.; Zhang, Q.; Tang, J.; Ruan, R. Phenol and phenolics from lignocellulosic biomass by catalytic microwave pyrolysis. Bioresour. Technol. 2011, 102, 7004–7007. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Kuan, W.; Lo, S.; Lin, C. Total recovery of resources and energy from rice straw using microwave-induced pyrolysis. Bioresour. Technol. 2008, 99, 8252–8258. [Google Scholar] [CrossRef]
- Du, Z.; Li, Y.; Wang, X.; Wan, Y.; Chen, Q.; Wang, C.; Lin, X.; Liu, Y.; Chen, P.; Ruan, R. Microwave-assisted pyrolysis of microalgae for biofuel production. Bioresour. Technol. 2011, 102, 4890–4896. [Google Scholar] [CrossRef]
- Chemat, F.; Poux, M. Microwave assisted pyrolysis of urea supported on graphite under solvent-free conditions. Tetrahedron Lett. 2001, 42, 3693–3695. [Google Scholar] [CrossRef]
- Hu, Z.; Ma, X.; Chen, C. A study on experimental characteristic of microwave-assisted pyrolysis of microalgae. Bioresour. Technol. 2012, 107, 487–493. [Google Scholar] [CrossRef]
- Azwar, E.; Mahari, W.A.W.; Rastegari, H.; Tabatabaei, M.; Peng, W.; Tsang, Y.F.; Park, Y.-K.; Chen, W.-H.; Lam, S.S. Progress in thermochemical conversion of aquatic weeds in shellfish aquaculture for biofuel generation: Technical and economic perspectives. Bioresour. Technol. 2021, 344, 126202. [Google Scholar] [CrossRef]
- Kurian, V.; Gill, M.; Dhakal, B.; Kumar, A. Recent trends in the pyrolysis and gasification of lignocellulosic biomass. In Biofuels and Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 511–552. [Google Scholar] [CrossRef]
- Lam, S.S.; Liew, R.K.; Jusoh, A.; Chong, C.T.; Ani, F.N.; Chase, H.A. Progress in waste oil to sustainable energy, with emphasis on pyrolysis techniques. Renew. Sustain. Energy Rev. 2016, 53, 741–753. [Google Scholar] [CrossRef]
- Sumnu, G.; Sahin, S. Recent Developments in Microwave Heating. In Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2005; pp. 419–444. [Google Scholar] [CrossRef]
- Yang, C.; Shang, H.; Li, J.; Fan, X.; Sun, J.; Duan, A. A Review on the Microwave-Assisted Pyrolysis of Waste Plastics. Processes 2023, 11, 1487. [Google Scholar] [CrossRef]
- Li, J.; Lin, L.; Ju, T.; Meng, F.; Han, S.; Chen, K.; Jiang, J. Microwave-assisted pyrolysis of solid waste for production of high-value liquid oil, syngas, and carbon solids: A review. Renew. Sustain. Energy Rev. 2024, 189, 113979. [Google Scholar] [CrossRef]
- Meloni, E.; Iervolino, G.; Palma, V. Basics of Microwave Heating and Recent Advances. In Advances in Microwave-Assisted Heterogeneous Catalysis; Royal Society of Chemistry: London, UK, 2023; pp. 1–24. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Chou, T.-W. Microwave processing: Fundamentals and applications. Compos. Part A Appl. Sci. Manuf. 1999, 30, 1055–1071. [Google Scholar] [CrossRef]
- Huang, Y.-F.; Chiueh, P.-T.; Kuan, W.-H.; Lo, S.-L. Microwave pyrolysis of lignocellulosic biomass: Heating performance and reaction kinetics. Energy 2016, 100, 137–144. [Google Scholar] [CrossRef]
- Samsonov, S.V.; Denisov, G.G.; Bogdashov, A.A.; Gachev, I.G. Gyro-TWT and Gyro-BWO with a Microwave Circuit in the Form of Zigzag Quasi-optical Transmission Line. In Proceedings of the 2021 Photonics & Electromagnetics Research Symposium (PIERS), Hangzhou, China, 21–25 November 2021; pp. 2790–2799. [Google Scholar]
- Prapuchanay, E.; Puangngernmak, N.; Chalermwisutkul, S. A single mode cylindrical microwave cavity for heating applications of liquid material. In Proceedings of the 2012 9th International Conference on Electrical Engineering/Electronics, Computer, Telecommunications and Information Technology (ECTI-CON 2012), Phetchaburi, Thailand, 16–18 May 2012; pp. 1–4. [Google Scholar]
- Mehdizadeh, M. Microwave Multimode Cavities for Material Heating. In Microwave/RF Applicators and Probes for Material Heating, Sensing, and Plasma Generation; Elsevier: Amsterdam, The Netherlands, 2010; pp. 151–182. [Google Scholar] [CrossRef]
- Okawa, T.; Utaki, H.; Takada, T. The application of microwave ceramics. In Proceedings of the 1994 IEEE International Symposium on Applications of Ferroelectrics, University Park, PA, USA, 7–10 August 1994; pp. 367–371. [Google Scholar]
- Abdullah, S.; You, K.Y.; Chong, C.Y.; Ali, M.S.M. Milk Pasteurization and Characterization Using Mono-Mode Microwave Reactor and Slotted Coaxial Antenna. In Handbook of Research on Energy-Saving Technologies for Environmentally-Friendly Agricultural Development; IGI Global: Hershey, PA, USA, 2020; pp. 107–138. [Google Scholar] [CrossRef]
- Mondal, A.; Agrawal, D.; Upadhyaya, A. Microwave Sintering of Refractory Metals/alloys: W, Mo, Re, W-Cu, W-Ni-Cu and W-Ni-Fe Alloys. J. Microw. Power Electromagn. Energy 2010, 44, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Andrade, N.M.; Hooten, S.; Fortuna, S.A.; Han, K.; Yablonovitch, E.; Wu, M.C. Inverse design optimization for efficient coupling of an electrically injected optical antenna-LED to a single-mode waveguide. Opt. Express 2019, 27, 19802–19814. [Google Scholar] [CrossRef]
- Frediani, M.; Frediani, P.; Innocenti, G.; Mellone, I.; Simoni, R.; Oteri, G. Microwave-Assisted Pyrolysis Process: From a Laboratory Scale to an Industrial Plant. In Recent Microwave Technologies; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Knight, D.R.; Goldsworthy, M.; Smith, P. Are biomass feedstocks sustainable? A systematic review of three key sustainability metrics. GCB Bioenergy 2024, 16, e13187. [Google Scholar] [CrossRef]
- Fodah, A.E.M.; Ghosal, M.K.; Behera, D. Microwave-assisted pyrolysis of agricultural residues: Current scenario, challenges, and future direction. Int. J. Environ. Sci. Technol. 2021, 19, 2195–2220. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Liu, S.; Peng, P.; Min, M.; Cheng, Y.; Anderson, E.; Zhou, N.; Fan, L.; Liu, C.; et al. Effects of feedstock characteristics on microwave-assisted pyrolysis—A review. Bioresour. Technol. 2017, 230, 143–151. [Google Scholar] [CrossRef]
- Ellison, C.R.; Hoff, R.; Mărculescu, C.; Boldor, D. Investigation of microwave-assisted pyrolysis of biomass with char in a rectangular waveguide applicator with built-in phase-shifting. Appl. Energy 2019, 259, 114217. [Google Scholar] [CrossRef]
- Bartoli, M.; Frediani, M.; Briens, C.; Berruti, F.; Rosi, L. An Overview of Temperature Issues in Microwave-Assisted Pyrolysis. Processes 2019, 7, 658. [Google Scholar] [CrossRef]
- Nzediegwu, C.; Naeth, M.A.; Chang, S.X. Biochar production from lignocellulosic and nonlignocellulosic biomass using conventional and microwave heating. In Biochar in Agriculture for Achieving Sustainable Development Goals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 85–95. [Google Scholar] [CrossRef]
- Motasemi, F.; Afzal, M.T.; Salema, A.A.; Mouris, J.; Hutcheon, R. Microwave dielectric characterization of switchgrass for bioenergy and biofuel. Fuel 2014, 124, 151–157. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Basak, T.; Srinivasan, R. Microwave heating characteristics of graphite based powder mixtures. Int. Commun. Heat Mass Transf. 2013, 48, 22–27. [Google Scholar] [CrossRef]
- Wen, B.; Zhang, J.; Yang, G.; Jing, D.; Yin, X.; Fan, L.; Nasir, M.S.; Ding, S. Optimal electrical conductivity and interfacial polarization induced by loaded nanoparticles on carbon nanotubes for excellent electromagnetic wave absorption performance. J. Colloid Interface Sci. 2022, 626, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Elmahaishi, M.F.; Azis, R.S.; Ismail, I.; Muhammad, F.D. A review on electromagnetic microwave absorption properties: Their materials and performance. J. Mater. Res. Technol. 2022, 20, 2188–2220. [Google Scholar] [CrossRef]
- Shao, J.; Yan, R.; Chen, H.; Yang, H.; Lee, D.H. Catalytic effect of metal oxides on pyrolysis of sewage sludge. Fuel Process. Technol. 2010, 91, 1113–1118. [Google Scholar] [CrossRef]
- Tursi, A. A review on biomass: Importance, chemistry, classification, and conversion. Biofuel Res. J. 2019, 6, 962–979. [Google Scholar] [CrossRef]
- Mati, A.; Buffi, M.; Dell’orco, S.; Lombardi, G.; Ramiro, P.M.R.; Kersten, S.R.A.; Chiaramonti, D. Fractional Condensation of Fast Pyrolysis Bio-Oil to Improve Biocrude Quality towards Alternative Fuels Production. Appl. Sci. 2022, 12, 4822. [Google Scholar] [CrossRef]
- Anukam, A.; Mamphweli, S.; Okoh, O.; Reddy, P. Influence of Torrefaction on the Conversion Efficiency of the Gasification Process of Sugarcane Bagasse. Bioengineering 2017, 4, 22. [Google Scholar] [CrossRef]
- Brassard, P.; Godbout, S.; Lévesque, V.; Palacios, J.H.; Raghavan, V.; Ahmed, A.; Hogue, R.; Jeanne, T.; Verma, M. Biochar for soil amendment. In Char and Carbon Materials Derived from Biomass; Elsevier: Amsterdam, The Netherlands, 2019; pp. 109–146. [Google Scholar] [CrossRef]
- Escalante, J.; Chen, W.-H.; Tabatabaei, M.; Hoang, A.T.; Kwon, E.E.; Lin, K.-Y.A.; Saravanakumar, A. Pyrolysis of lignocellulosic, algal, plastic, and other biomass wastes for biofuel production and circular bioeconomy: A review of thermogravimetric analysis (TGA) approach. Renew. Sustain. Energy Rev. 2022, 169, 112914. [Google Scholar] [CrossRef]
- Ren, X.; Ghazani, M.S.; Zhu, H.; Ao, W.; Zhang, H.; Moreside, E.; Zhu, J.; Yang, P.; Zhong, N.; Bi, X. Challenges and opportunities in microwave-assisted catalytic pyrolysis of biomass: A review. Appl. Energy 2022, 315, 118970. [Google Scholar] [CrossRef]
- Parvez, A.M.; Afzal, M.T.; Jiang, P.; Wu, T. Microwave-assisted biomass pyrolysis polygeneration process using a scaled-up reactor: Product characterization, thermodynamic assessment and bio-hydrogen production. Biomass Bioenergy 2020, 139, 105651. [Google Scholar] [CrossRef]
- Schena, E.; Tosi, D.; Saccomandi, P.; Lewis, E.; Kim, T. Fiber Optic Sensors for Temperature Monitoring during Thermal Treatments: An Overview. Sensors 2016, 16, 1144. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zuo, W.; Ren, Z.; Chen, D. Estimation of a novel method to produce bio-oil from sewage sludge by microwave pyrolysis with the consideration of efficiency and safety. Bioresour. Technol. 2010, 102, 2053–2061. [Google Scholar] [CrossRef]
- Irfan, M.; Saleem, R.; Shoukat, B.; Hussain, H.; Shukrullah, S.; Naz, M.Y.; Rahman, S.; Ghanim, A.A.J.; Nawalany, G.; Jakubowski, T. Production of combustible fuels and carbon nanotubes from plastic wastes using an in-situ catalytic microwave pyrolysis process. Sci. Rep. 2023, 13, 9057. [Google Scholar] [CrossRef]
- Aishwarya, K.N.; Sindhu, N. Microwave Assisted Pyrolysis of Plastic Waste. Procedia Technol. 2016, 25, 990–997. [Google Scholar] [CrossRef]
- Idris, S.S.; Rahman, N.A.; Ismail, K.; Yunus, M.F.M.; Hakimi, N.I.N.M. Microwave-Assisted Pyrolysis of Oil Palm Biomass: Multi-Optimisation of Solid Char Yield and Its Calorific Value Using Response Surface Methodology. Front. Chem. Eng. 2022, 4, 864589. [Google Scholar] [CrossRef]
- Ahmed, A.S. Microwave Assisted Pyrolysis of Moringa Seed and Karanja for Bio-Oil Production. Int. J. Renew. Energy Resour. 2023, 13, 14–24. [Google Scholar] [CrossRef]
- Huang, Y.; Kuan, W.; Lo, S.; Lin, C. Hydrogen-rich fuel gas from rice straw via microwave-induced pyrolysis. Bioresour. Technol. 2010, 101, 1968–1973. [Google Scholar] [CrossRef]
- Arshad, H.; A Sulaiman, S.; Hussain, Z.; Naz, Y.; Basrawi, F. Microwave assisted pyrolysis of plastic waste for production of fuels: A review. MATEC Web Conf. 2017, 131, 02005. [Google Scholar] [CrossRef]
- Syed, N.R.; Zhang, B.; Mwenya, S.; Aldeen, A.S. A Systematic Review on Biomass Treatment Using Microwave-Assisted Pyrolysis under PRISMA Guidelines. Molecules 2023, 28, 5551. [Google Scholar] [CrossRef]
- Fodah, A.E.M.; Abdelwahab, T.A.M. Process optimization and technoeconomic environmental assessment of biofuel produced by solar powered microwave pyrolysis. Sci. Rep. 2022, 12, 12572. [Google Scholar] [CrossRef] [PubMed]
- Klinger, J.L.; Westover, T.L.; Emerson, R.M.; Williams, C.L.; Hernandez, S.; Monson, G.D.; Ryan, J.C. Effect of biomass type, heating rate, and sample size on microwave-enhanced fast pyrolysis product yields and qualities. Appl. Energy 2018, 228, 535–545. [Google Scholar] [CrossRef]
- Collard, F.-X.; Blin, J. A review on pyrolysis of biomass constituents: Mechanisms and composition of the products obtained from the conversion of cellulose, hemicelluloses and lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Salema, A.A.; Yeow, Y.K.; Ishaque, K.; Ani, F.N.; Afzal, M.T.; Hassan, A. Dielectric properties and microwave heating of oil palm biomass and biochar. Ind. Crops Prod. 2013, 50, 366–374. [Google Scholar] [CrossRef]
- Shukla, A.; Mondal, A.; Upadhyaya, A. Numerical modeling of microwave heating. Sci. Sinter. 2010, 42, 99–124. [Google Scholar] [CrossRef]
- Hill, J.M.; Marchant, T.R. Modelling microwave heating. Appl. Math. Model. 1996, 20, 3–15. [Google Scholar] [CrossRef]
- Velásquez, H.J.C.; Rhenals, J.E.M.; Rhenals, J.E.M. Modelación numérica de un proceso térmico por microondas con énfasis en alimentos. Dyna 2006, 73, 155–166. [Google Scholar]
- Yang, R.; Chen, J. Mechanistic and Machine Learning Modeling of Microwave Heating Process in Domestic Ovens: A Review. Foods 2021, 10, 2029. [Google Scholar] [CrossRef]
- Das, A.; Banik, B.K. Microwave equipment for chemistry. In Microwaves in Chemistry Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 27–59. [Google Scholar] [CrossRef]
- Hauck, H.S. Design Considerations for Microwave Oven Cavities. IEEE Trans. Ind. Gen. Appl. 1970, IGA-6, 74–80. [Google Scholar] [CrossRef]



| Application | Description | Sources |
|---|---|---|
| Plastic waste | Conversion of waste plastics into hydrogen-rich gases and other value-added products. The use of catalysts in conjunction with microwave heating has been shown to significantly improve hydrogen production. | [14] |
| Biomass conversion | Various types of biomasses, including agricultural waste and municipal solid waste, have been successfully treated using MAP. It generates biofuels and helps with efficient management by converting waste into a usable form of energy. | [15,16] |
| Co-pyrolysis | Combining feedstocks (such as biomass and plastics) to further optimize product performance. This co-pyrolysis approach improves the overall efficiency and economic viability of waste-to-energy processes. | [16] |
| Method | Principle | Advantages | Disadvantages | Catalysts |
|---|---|---|---|---|
| Dry media synthesis | Chemical reaction performed in the absence of a solvent, typically using solid supports or reagents | Environmentally friendly (solvent-free) Economical (saves money on solvents) Ease of purification (no solvent removal) High reaction rate Often higher yields | Limited to reactions that can occur in solid state Reactants should mix into a homogeneous system Potential for hotspot formation Problems with dissipating heat safely Side reactions may be accelerated | Solid supports like silica gel or alumina may act as catalysts |
| Neat reaction | Chemical reaction performed without solvents or catalysts, using only reactants | Extremely environmentally friendly Simplifies purification processes Often leads to very high yields Reduced reaction times Maximizes atom economy | Limited to reactions where reactants are liquid or low-melting solids High viscosity in reactant system Potential for overheating and decomposition May be challenging to scale up | Generally no catalysts used, but reactants may have catalytic properties |
| Solvent-mediated synthesis | Chemical reaction performed in the presence of solvents | Broader applicability than solvent-free methods Better heat distribution Easier temperature control Suitable for a wide range of organic syntheses | Requires solvent disposal or recycling Potential for pressure build-up in sealed vessels Lower environmental friendliness compared to solvent-free methods Additional purification steps may be needed | Various catalysts can be used, including homogeneous and heterogeneous catalysts |
| Microwave-assisted extraction | Method using microwave energy to extract compounds from plant tissues or other matrices | Rapid extraction times Often higher yields than conventional methods Can preserve heat-sensitive compounds Reduced solvent consumption | May require optimization for each plant material Potential for thermal degradation of some compounds Initial equipment costs can be high | Generally no catalysts used, but solvents may enhance extraction efficiency |
| Waste | Conditions | Valorized Product | Sources |
|---|---|---|---|
| Sewage sludge | 400 W to 600 W, 6 min | Bio-oil yield of 49.8%, biogas yield of 60.21% | [60] |
| Waste oil | 5 kW, particle carbon, 250 °C and 700 °C, and nitrogen was applied as the carrier gas with a flow rate of 0.1 L/min to 0.75 L/min | Pyrolysis oil yield of 88 wt% | [61] |
| Lignocellulosic biomass | Activated carbon as catalyst, 700 W, 589 K, 8 min | High concentration of phenol (38.9%) and phenolics (66.9%) | [62] |
| Rice straw | 2 kW maximum power and 2.45 GHz frequency | H2 (55 vol%), CO2 (17 vol%), CO (13 vol%), and CH4 (10 vol%) | [63] |
| Chlorella sp | Power of 1.25 kW and 2.45 GHz frequency, a 500 mL quartz flask, and nitrogen was used as the carrier gas | Bio-oil yield of 28.6% | [64] |
| Urea | Graphite, 300 °C, 3 min | Cyanuric acid yield of 61.2% | [65] |
| Chlorella vulgaris | 3750 W, 2.45 GHz microwave | Bio-oil yield of 35.83 wt%, gas yield of 52.37 wt% | [66] |
| Feedstock | MAP Conditions | Valorized Product | Yield | Ref. |
|---|---|---|---|---|
| Sewage sludge | 400 W, 6 min, 500 °C, 2450 MHz | Bio-oil | 49.8 wt% | [103] |
| Plastic waste | 1 kW, m 400 to 450 °C, 2450 MHz | Combustible fuels and carbon nanotubes | 80% | [104] |
| Mixture of plastics | 5 kW, quartz vessel | Bio-oil and biochar | - | [105] |
| Oil palm biomass | 300 W, 2.45 GHz, quartz reactor, 17 min, N2 system | Solid char Liquid Gas | 27.6% | [106] |
| Moringa seed | 800 W, 13 min, 450 °C | Karanja bio-oil | 10.6% | [107] |
| Rice straw | 2.45 GHz, 300 W, N2 system, 600–700 °C, 30 min | Gas fraction | H2 (50.67 vol%), CO2 (22.56 vol%), CO (16.09 vol%), and CH4 (7.42 vol%) | [108] |
| Soapstock | 800 W at a frequency of 2450 MHz, N2 system, 550 °C, 6 g/min | Alkenes, cycloalkenes, alkadienes, alkynes, aromatics | 65% | [109] |
| Characteristic | Value |
|---|---|
| Volume of glass vessel | 40 L |
| Frequency of microwave oven | 2450 MHz |
| Power outlet of microwave oven | 800 W |
| Pump | VOGT N610 |
| Gas | Argon |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramírez Cabrera, P.A.; Lozano Pérez, A.S.; Guerrero Fajardo, C.A. Design of a Semi-Continuous Microwave System for Pretreatment of Microwave-Assisted Pyrolysis Using a Theoretical Method. Inventions 2025, 10, 24. https://doi.org/10.3390/inventions10020024
Ramírez Cabrera PA, Lozano Pérez AS, Guerrero Fajardo CA. Design of a Semi-Continuous Microwave System for Pretreatment of Microwave-Assisted Pyrolysis Using a Theoretical Method. Inventions. 2025; 10(2):24. https://doi.org/10.3390/inventions10020024
Chicago/Turabian StyleRamírez Cabrera, Paula Andrea, Alejandra Sophia Lozano Pérez, and Carlos Alberto Guerrero Fajardo. 2025. "Design of a Semi-Continuous Microwave System for Pretreatment of Microwave-Assisted Pyrolysis Using a Theoretical Method" Inventions 10, no. 2: 24. https://doi.org/10.3390/inventions10020024
APA StyleRamírez Cabrera, P. A., Lozano Pérez, A. S., & Guerrero Fajardo, C. A. (2025). Design of a Semi-Continuous Microwave System for Pretreatment of Microwave-Assisted Pyrolysis Using a Theoretical Method. Inventions, 10(2), 24. https://doi.org/10.3390/inventions10020024






