New Horizons in Male Contraception: Clinical, Cultural and Technological Innovation Aspects
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
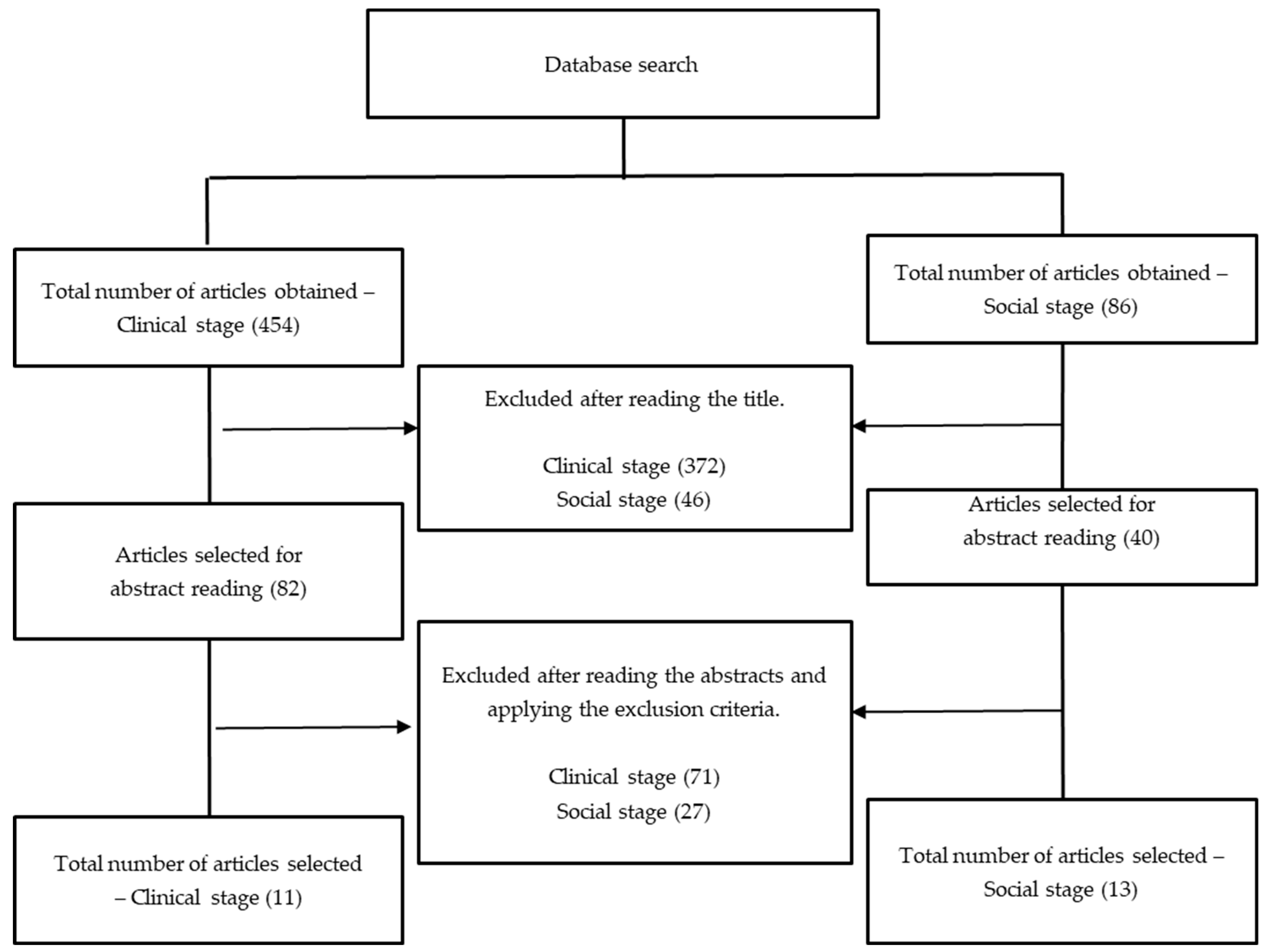
3.1. Study Selection and Clinical Aspects of the Articles Analyzed
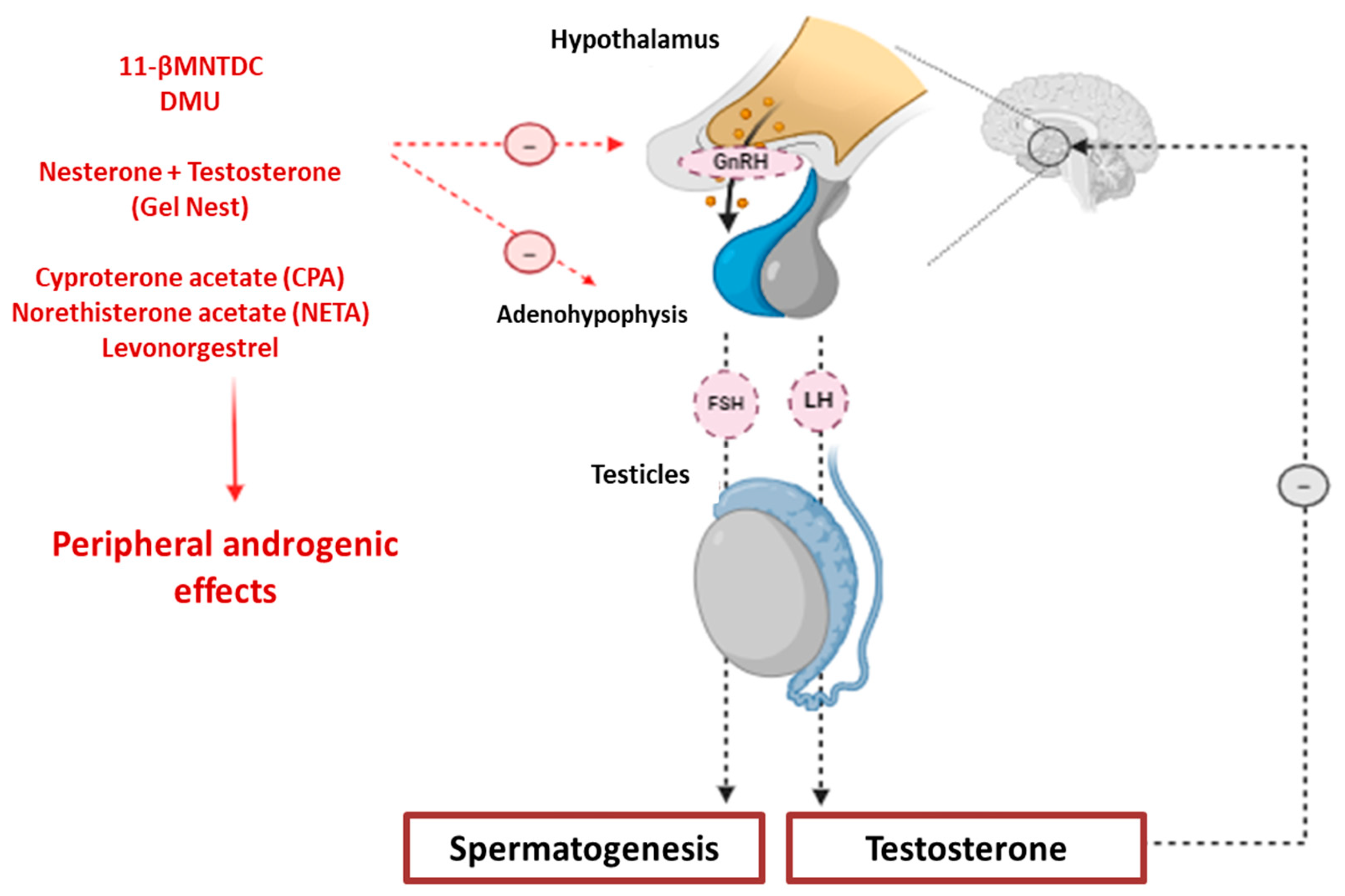
3.2. Pharmacology and Clinical Approach of NMC: Hormonal Contraceptives
3.3. Pharmacology and Clinical Approach of NMC: Non-Hormonal Contraceptives
3.4. Aspects Related to Technological Innovation of NMC from Patent Applications
3.5. Sociocultural Approach to NMC
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NMC | New Male Contraception |
| HPG | Hypothalamic-pituitary-gonadal |
| GnRH | Gonadotropin Releasing Hormone |
| DMU | Methandrolone undecanoate |
| 11-βMNTDC | 11β-methyl-19-nortestosterone-17β-dodecylcarbonate |
| RISUG | Reversible Inhibition of Sperm under Guidance |
| SciELO | Scientific Electronic Library Online |
| VHL | Virtual Health Library |
| CAPES | Coordination for the Improvement of Higher Education Personnel |
| EPO | European Patent Office |
| IVD-A/IVD-B | Intravascular Device |
| CPA | Cyproterone acetate |
| FSH | Follicle-Stimulating Hormone |
| LH | Luteinizing Hormone |
| HDL | High-Density Lipoprotein |
| NHL | Levonorgestrel |
| T | Testosterone |
| SEDDS | Self-Emulsifying Drug Delivery Systems |
| PO | Oral Route |
| NES | Nestorone |
| NETA | Norethisterone Acetate |
| VSB | No-scalpel Vasectomy |
References
- Garnett, C.; Pollack, L.; Rodriguez, F.; Renteria, R.; Puffer, M.; Tebb, K.P. The association between nonbarrier contraceptive use and condom use among sexually active Latina adolescents. J. Adolesc. Health 2021, 68, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Festin, M.P.R. Overview of modern contraception. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 66, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Jacobstein, R.; Radloff, S.; Khan, F.; Mimno, K.; Pal, M.; Snell, J.; Stafford, R.; Touré, C.; Tripathi, V. Down but not out: Vasectomy is faring poorly almost everywhere-we can do better to make it a true method option. Glob. Health Sci. Pract. 2023, 28, e2200369. [Google Scholar] [CrossRef]
- Simetinger, G. A qualitative study of sexuality and contraceptive choice among women using withdrawal. Int. J. Impot. Res. 2025, 1–5. [Google Scholar] [CrossRef]
- World Health Organization. Selected Practice Recommendations for Contraceptive Use. 2016. Available online: https://www.who.int/publications/i/item/9789241565400 (accessed on 17 September 2025).
- Lethbridge, D.J. Coitus interruptus: Considerations as a method of birth control. J. Obstet. Gynecol. Neonatal Nurs. 1991, 20, 80–85. [Google Scholar] [CrossRef]
- Sharma, R.S.; Mathur, A.K.; Singh, R.; Das, H.C.; Singh, G.J.; Toor, D.P.S.; Guha, S.K. Safety & efficacy of an intravasal, one-time injectable & non-hormonal male contraceptive (RISUG): A clinical experience. Indian J. Med. Res. 2019, 150, 81–86. [Google Scholar]
- Yuen, F.; Nguyen, B.T.; Swerdloff, R.S.; Wang, C. Continuing the search for a hormonal male contraceptive. Best Pract. Res. Clin. Obstet. Gynaecol. 2020, 66, 83–94. [Google Scholar] [CrossRef]
- Long, J.E.; Lee, M.S.; Blithe, D.L. Update on novel hormonal and nonhormonal male contraceptive development. J. Clin. Endocrinol. Metab. 2021, 106, e2381–e2392. [Google Scholar] [CrossRef]
- Pyo, Y.; Kwon, K.H. A review of various types of male contraception. J. Men’s Health 2024, 20, 1–8. [Google Scholar]
- Heise, L.; Greene, M.E.; Opper, N.; Stavropoulou, M.; Harper, C.; Nascimento, M.; Zewdie, D. Gender Equality, Norms, and Health Steering Committee. Gender inequality and restrictive gender norms: Framing the challenges to health. Lancet 2019, 393, 2440–2454. [Google Scholar] [CrossRef] [PubMed]
- Connell, R.W.; Messerschmidt, J.W. Hegemonic masculinity: Rethinking the concept. Gend. Soc. 2005, 19, 829–859. [Google Scholar] [CrossRef]
- Brazil Ministry of Health. Sistema de Informações Hospitalares do SUS (SIH/SUS). 2023. Available online: http://tabnet.datasus.gov.br/cgi/tabcgi.exe?sih/cnv/qiuf.def (accessed on 10 March 2025).
- Vahdat, H.L.; Shane, K.; Nickels, L.M. The role of team science in the future of male contraception. Biol. Reprod. 2020, 103, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Abbe, C.R.; Page, S.T.; Thirumalai, A. Male Contraception. Yale J. Biol. Med. 2020, 93, 603–613. [Google Scholar] [PubMed]
- Kent, K.; Johnston, M.; Strump, N.; Garcia, T.X. Toward Development of the Male Pill: A decade of potential non-hormonal contraceptive targets. Front. Cell Dev. Biol. 2020, 26, 61. [Google Scholar] [CrossRef] [PubMed]
- Nickels, L.; Yan, W. Nonhormonal male contraceptive development-strategies for progress. Pharmacol. Rev. 2023, 76, 37–48. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Yuen, F.; Farrant, M.; Thirumalai, A.; Fernando, F.; Amory, J.K.; Swerdloff, R.S.; Anawalt, B.D.; Blithe, D.L.; Long, J.E.; et al. Acceptability of the oral hormonal male contraceptive prototype, 11β-methyl-19-nortestosterone dodecylcarbonate (11β-MNTDC), in a 28-day placebo-controlled trial. Contraception 2021, 104, 531–537. [Google Scholar] [CrossRef]
- Wu, S.; Yuen, F.; Swerdloff, R.S.; Pak, Y.; Thirumalai, A.; Liu, P.Y.; Amory, J.K.; Bai, F.; Hull, L.; Blithe, D.L.; et al. Safety and pharmacokinetics of single-dose novel oral androgen 11β-methyl-19-nortestosterone-17β-dodecylcarbonate in men. J. Clin. Endocrinol. Metab. 2019, 104, 629–638. [Google Scholar] [CrossRef]
- Yuen, F.; Thirumalai, A.; Fernando, F.A.; Swerdloff, R.S.; Liu, P.Y.; Pak, Y.; Hull, L.; Bross, R.; Blithe, D.L.; Long, J.E.; et al. Comparison of metabolic effects of the progestational androgens dimethandrolone undecanoate and 11β-MNTDC in healthy men. Andrology 2021, 9, 1526–1539. [Google Scholar] [CrossRef]
- Ayoub, R.; Page, S.T.; Swerdloff, R.S.; Liu, P.Y.; Amory, J.K.; Leung, A.; Hull, L.; Blithe, D.; Christy, A.; Chao, J.H.; et al. Comparison of the single dose pharmacokinetics, pharmacodynamics, and safety of two novel oral formulations of dimethandrolone undecanoate (DMAU): A potential oral, male contraceptive. Andrology 2017, 5, 278–285. [Google Scholar] [CrossRef]
- Thirumalai, A.; Ceponis, J.; Amory, J.K.; Swerdloff, R.; Surampudi, V.; Liu, P.Y.; Bremner, W.J.; Harvey, E.; Blithe, D.L.; Lee, M.S.; et al. Effects of 28 days of oral dimethandrolone undecanoate in healthy men: A prototype male pill. J. Clin. Endocrinol. Metab. 2019, 104, 423–432. [Google Scholar] [CrossRef]
- Surampudi, P.; Page, S.T.; Swerdloff, R.S.; Nya-Ngatchou, J.J.; Liu, P.Y.; Amory, J.K.; Hull, L.; Blithe, D.L.; Woo, J.; Bremner, W.J.; et al. Single, escalating dose pharmacokinetics, safety and food effects of a new oral androgen dimethandrolone undecanoate in man: A prototype oral male hormonal contraceptive. Andrology 2014, 2, 579–587. [Google Scholar] [CrossRef]
- Anawalt, B.D.; Roth, M.Y.; Ceponis, J.; Surampudi, V.; Amory, J.K.; Swerdloff, R.S.; Liu, P.Y.; Dart, C.; Bremner, W.J.; Sitruk-Ware, R.; et al. Combined nestorone-testosterone gel suppresses serum gonadotropins to concentrations associated with effective hormonal con-traception in men. Andrology 2019, 7, 878–887. [Google Scholar] [CrossRef]
- Zitzmann, M.; Rohayem, J.; Raidt, J.; Kliesch, S.; Kumar, N.; Sitruk-Ware, R.; Nieschlag, E. Impact of various progestins with or without transdermal testosterone on gonadotropin levels for non-invasive hormonal male contraception: A randomized clinical trial. Andrology 2017, 5, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Bhat, G.S.; Shastry, A. A prospective double-blind, randomized, placebo-controlled study to evaluate the efficacy of silodosin 8 mg as an on-demand, reversible, nonhormonal oral contraceptive for males: A pilot study. World J. Urol. 2020, 38, 747–751. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.H.; Liang, X.W.; Gu, Y.Q.; Wu, W.X.; Bo, L.W.; Zheng, T.G.; Chen, Z.W. A randomized, controlled, multicenter contraceptive efficacy clinical trial of the intravas device, a nonocclusive surgical male sterilization. Asian J. Androl. 2014, 6, 432–436. [Google Scholar] [CrossRef] [PubMed]
- Newmann, S.J.; Zakaras, J.M.; Rocca, C.H.; Gorrindo, P.; Ndunyu, L.; Gitome, S.; Withers, M.; Bukusi, E.A.; Dworkin, S.L. Transforming masculine norms to improve men’s contraceptive acceptance: Results from a pilot intervention with men in western Kenya. Sex. Reprod. Health Matters. 2023, 31, 2170084. [Google Scholar] [CrossRef]
- Attardi, B.J.; Hild, S.A.; Reel, J.R. Dimethandrolone undecanoate: A new potent orally active androgen with progestational activity get access arrow. Endocrinology 2006, 147, 3016–3026. [Google Scholar] [CrossRef]
- Nigro, E.; Scudiero, O.; Monaco, M.L.; Palmieri, A.; Mazzarella, G.; Costagliola, C.; Bianco, A.; Daniele, A. New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res. Int. 2014, 2014, 658913. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T. Adiponectin and adiponectin receptors. Endocr. Rev. 2005, 26, 439–451. [Google Scholar] [CrossRef]
- Faludi, A.A.; Izar, M.C.O.; Saraiva, J.F.K.; Chacra, A.P.M.; Bianco, H.T.; Afiune Neto, A.; Bertolami, A.; Pereira, A.C.; Lottenberg, A.M.; Sposito, A.C.; et al. Update of the Brazilian guideline on dyslipidemia and prevention of atherosclerosis. Arq. Bras. Cardiol. 2017, 109, 1–76. [Google Scholar]
- Sitruk-Ware, R.; Blithe, D.L.; Page, S.T.; Wang, C. Development of a transdermal gel for reversible male contraception. Contraception 2025, 145, 110830. [Google Scholar] [CrossRef]
- Nguyen, B.T. Male contraceptive acceptability versus male acceptance of contraceptive responsibility. Andrology 2024, 12, 1585–1589. [Google Scholar] [CrossRef] [PubMed]
- Amory, J.K. Male contraception. Fertil. Steril. 2016, 106, 1303–1309. [Google Scholar] [CrossRef] [PubMed]
- Bennink, H.J.T.C. New endocrine method of oral male contraception. Contraception 2025, 145, 110782. [Google Scholar] [CrossRef] [PubMed]
- Newmann, S.J.; Zakaras, J.M.; Dwoekin, S.L.; Withers, M.; Ndunyu, L.; Gitome, S.; Gorrindo, P.; Bukusi, E.A.; Rocca, C.H. Measuring men’s gender norm beliefs related to contraception: Development of the masculine norms and family planning acceptance scale. Arch. Sex. Behav. 2021, 50, 2691–2702. [Google Scholar] [CrossRef]
- Yuen, F.; Wu, S.; Thirumalai, A.; Swerdloff, R.S.; Page, S.T.; Liu, P.Y.; Dart, C.; Wu, H.; Blithe, D.L.; Sitruk-Ware, R.; et al. Preventing secondary exposure to women from men applying a novel nestorone/testosterone contraceptive gel. Andrology 2019, 7, 235–243. [Google Scholar] [CrossRef]
- Mariani, N.A.P.; Silva, J.V.; Fardilha, M.; Silva, E.J.R. Advances in non-hormonal male contraception targeting sperm motility. Hum. Reprod. Update. 2023, 29, 545–569. [Google Scholar] [CrossRef]
- Yan, W.; Amory, J.K. Emerging approaches to male contraception. Andrology 2024, 12, 1568–1573. [Google Scholar] [CrossRef]
- Page, S.T. Synthetic androgens for male contraception. Contraception 2025, 145, 110735. [Google Scholar] [CrossRef]
- Rossi, M.; Roumeguère, T. Silodosin in the treatment of benign prostatic hyperplasia. Drug Des. Devel. Ther. 2010, 4, 291–297. [Google Scholar]
- Yoshida, M.; Kudoh, J.; Homma, Y.; Kawabe, K. Safety and efficacy of silodosin for the treatment of benign prostatic hyperplasia. Clin. Interv. Aging. 2011, 6, 161–172. [Google Scholar] [CrossRef]
- Akkari, A.C.S.; Munhoz, I.P.; Tomioka, J.; Santos, N.M.B.F.; Santos, R.F. Technological innovation in the pharmaceutical industry: Differences between Europe, the USA and emerging pharmaceutical countries. Gest. Prod. 2019, 23, 365–380. [Google Scholar] [CrossRef]
- Amory, J.K.; Blithe, D.L.; Sitruk-Ware, R.; Swerdloff, R.S.; Bremner, W.J.; Dart, C.; Liu, P.Y.; Thirumalai, A.; Nguyen, B.T.; Anawalt, B.D.; et al. Design of an international male contraceptive efficacy trial using a self-administered daily transdermal gel containing testosterone and segesterone acetate (Nestorone). Contraception 2023, 124, 110064. [Google Scholar] [CrossRef] [PubMed]
- Burke, H.M.; Ridgeway, K.; Murray, K.; Mickler, A.; Thomas, R.; Williams, K. Reproductive empowerment and contraceptive self-care: A systematic review. Sex. Reprod. Health Matters. 2021, 29, 2090057. [Google Scholar] [CrossRef] [PubMed]
- Glasier, A. Acceptability of contraception for men: A review. Contraception 2010, 82, 453–456. [Google Scholar] [CrossRef]
- Fuentes, P.F. La moral, la ética y la bioética como limitantes sociales a la protección de las invenciones por la vía de las patentes. Frónesis 2006, 13, 9–31. [Google Scholar]
- Hamm, M.; Evans, M.; Miller, E.; Browne, M.; Bell, M.; Bell, D.; Borrero, S. “It’s her body”: Low-income men’s perceptions of limited reproductive agency. Contraception 2019, 99, 111–117. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Jacobsohn, T.L. Men’s willingness to use novel male contraception is linked to gender-equitable attitudes: Results from an exploratory online survey. Contraception 2023, 123, 110001. [Google Scholar] [CrossRef]
- Brewer, C.; Nguyen, B.T. Web traffic and Google Trends data show increased interest in novel male contraception following the Supreme Court’s Dobbs v. Jackson ruling. Contraception 2025, 145, 110835. [Google Scholar] [CrossRef]
- Gipson, J.D.; Bornstein, M.; Duong, A.; Nguyen, B.T. Motivations to use a novel hormonal male contraceptive: Perspectives from male contraceptive clinical trial participants. Contraception 2025, 148, 110932. [Google Scholar] [CrossRef]
- Jacobsohn, T.; Nguyen, B.T.; Brown, J.E.; Thirumalai, A.; Massone, M.; Page, S.T.; Wang, C.; Kroopnick, J.; Blithe, D.L. Male contraception is coming: Who do men want to prescribe their birth control? Contraception 2022, 115, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.T. The demand for male contraception: Estimating the potential market for users of novel male contraceptive methods using United States National Survey of Family Growth data. Contraception 2024, 135, 110438. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Torres, P.; Lucha-López, A.C.; Martínez-Pérez, G.Z.; Sheridan, T.; Vera Cruz, G. Acceptability and determinants of using male hormonal contraceptives: A systematic review from a gender perspective. Psychol Sex. 2023, 14, 689–719. [Google Scholar] [CrossRef]
- Bitzer, J. Male contraception—Part of gender medicine and reproductive rights of men. Contraception 2025, 145, 110734. [Google Scholar] [CrossRef]
- Kidula, N.; Nguyen, B.T.; Habib, N.; Kiarie, J. The impact of male contraception on global sexual and reproductive health and rights. Contraception 2025, 145, 110811. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Soule, L.; Jarow, J.P.; Odlind, V. Regulatory challenges of new male contraceptive methods. Andrology 2024, 7, 1590–1599. [Google Scholar] [CrossRef]
- Thirumalai, A.; Amory, J.K. Emerging approaches to male contraception. Fertil. Steril. 2021, 115, 1369–1376. [Google Scholar] [CrossRef]
- Shi, R.; Wolgemuth, D.J.; Georg, G.I. Development of the retinoic acid receptor alpha-specific antagonist YCT-529 for male contraception: A brief review. Contraception 2025, 145, 110809. [Google Scholar] [CrossRef]
- Matsumoto, N.M.; Chiartas, T.G.; Paysour, B.R.; Barry, T.J.; Ott, L.E.; Tropsha, Y.; Eisenfrats, K.S. Preclinical development of a novel injectable hydrogel for vas-occlusion. Contraception 2025, 145, 110839. [Google Scholar] [CrossRef]
| Authors/Year | Substance(s) | Clinical Trials Stage | Dosage | Laboratory Parameters | Undesirable Effects | Drug Interactions |
|---|---|---|---|---|---|---|
| Nguyen et al. (2021) [18] | 11β-MNTDC | Phase Ib (n = 40) | 200 mg (group 1) and 400 mg (group 2) in the morning, orally, 30 min after a high-fat meal, for 28 days. | - | Decreased libido, mood swings, weight gain and fatigue. | Fat intake increases drug bioavailability |
| Wu et al. (2019) [19] | 11β-MNTDC | Preclinical phase and Phase I (n = 12) | 100; 200; 400; or 800 mg, orally, on an empty stomach for at least 8 h and, on another occasion, after a meal rich in fat (>50%). Single dose. | No statistically significant suppression of FSH. | Weight gain, decreased hematocrit, without serious adverse effects | Fatty foods increase drug bioavailability |
| Yuen et al. (2021) [20] | 11β-MNTDC and DMU | (n = 78) [38 DMU; 40 11β-MNTDC] | 200 and 400 mg once a day, orally, for 28 days, 30 min after a high-fat meal | Adiponectin: DMU (placebo, 1.4 μg/mL; active, −1.7 μg/mL; p < 0.01)/11β-MNTDC (placebo, −1.5 μg/mL; active, −0.4 μg/mL) HDL-C: DMU (200 mg–13 mg/dL and 400 mg–11 mg/dL)/11β-MNTDC (200 mg–11 mg/dL and 400 mg–16 mg/dL) Hematocrit: variations of 1% for 11β-MNTDC 200 mg and 400 mg and 2% for DMU 400 mg | Significant increase in weight and hematocrit; decrease in HDL-C not related to dose | - |
| Ayoub et al. (2017) [21] | DMU | Phase Ib (n = 44) | 100; 200 and 400 mg formulated in castor oil, SEDDS and powder (in capsule). After a fatty meal. Single dose. | Ranges for the two doses (200 and 400 mg): LH: 1.5–2 UI/L FSH: 2–2.5 UI/L T: 200–250 ng/dL Values with 400 mg were lower. | - | Fat administration increases drug absorption and serum concentration. |
| Thirumalai et al. (2019) [22] | DMAU | Phase Ib (n = 100) | Group C: 100, 200 or 400 mg of 70% castor oil/30% benzyl benzoate. Group P: 200 or 400 mg of powder in capsule form, daily, for 28 days. After breakfast containing 25 to 30 g of fat. | Group C100: 54% LH and FSH < 1 IU/L 46.2% serum T < 50 ng/dL Group C200: 64% LH and FSH < 1 IU/L 64.3% serum T < 50 ng/dL Group C400: 92% LH and FSH < 1 IU/L 92.3% serum T < 50 ng/dL Group P200: 69% LH and FSH < 1 IU/L 76.9% serum T < 50 ng/dL Group P400: 100% LH and FSH < 1 IU/L 100% serum T < 50 ng/dL There was no significant decrease in sperm levels | Headache (13 patients, “mild”); decreased libido (9 patients, “moderate”); erectile dysfunction (3 patients, “mild”); acne (8 patients, “moderate”); Weight gain, elevated hematocrit levels, and decreased HDL-C | Fat administration increases drug absorption |
| Surampudi et al. (2014) [23] | DMU | Phase I (n = 12) | 25; 50; 100; 200; 400 and 800 mg fasting; 200, 400 and 800 mg after high-fat diet (>50%). Single dose. | LH (200 mg): 3.5–4.0 IU/L (fasting) 2.5–3.0 IU/L (post meal) LH (400 and 800 mg): 3.0–3.5 IU/L (fasting) ~2 IU/L (post meal) FSH (200 mg): 3.5–4.0 IU/L (fasting) 3.0–3.5 IU/L (post meal) FSH (400 and 800 mg): ~3.0 (fasting) 3.5–4.0 IU/L (800 mg fasting) 2.5–3.0 IU/L (post meal) T (200 mg): 15–18 nmol/L (fasting) 15–18 nmol/L (post meal) T (400 mg and 800 mg): 15–18 nmol/L (fasting) ~10 mmol/L (after meal) | “Mild” acne | Fat intake increases drug bioavailability |
| Anwalt et al. (2019) [24] | Segesterone acetate (Nesterone) and testosterone. | Phase 1 (n = 44) | Nes/T Group: Nes (8.3 mg) and 1.62% T (62.5 mg) in 5 mL of gel. T Group: 1.62% T (AndroGel® III T 62.7 mg) in 4.4 mL of gel. Daily application of the gel in the abdominal region for 28 days. | Nes/T Group: (21–28 days) LH and FSH ≤ 1 UI/L. (Day 28) No significant differences in free T value. (Day 28) Sperm concentration decreased significantly (40 ± 43 million/mL). T Group: LH and FSH values ≤ 1 UI/L were not reached. (Day 28) Free T value was significantly higher. (Day 28) Sperm concentration was unchanged (85 ± 72 million/mL) | Rash (dry and scaly) at the application site (1 patient), sunburn at the application site (2 patients), and decreased libido (1 patient) | - |
| Zitzmann et al. (2017) [25] | Cyproterone acetate (CPA), nesterone (NES), norethisterone acetate (NETA), levonorgestrel (LNG) and testosterone. | Phase I (n = 56) | 1st phase (2 weeks, 8 groups): Cyproterone acetate (CPA)—10 mg/day or 20 mg/day; Norethisterone acetate (NETA)—5 mg/day or 10 mg/day; Levonorgestrel (LNG)—120 µg/day or 240 µg/day and Nesterone (transdermal)—1 g of gel per day, 2 mg/g or 3 mg/g gel. 2nd phase (4 weeks, 8 groups): 50 mg of testosterone gel (10 mg/g) in combination with each of the progestins. | FSH: CPA 10 mg/day and 20 mg/day: >0.5 IU/L CPA combined: <0.5 IU/L NES 2 mg/g: >0.5 IU/L NES 3 mg/g: >0.5 IU/L NES combined: >0.5 IU/L (2 mg/g); =0.5 IU/L (3 mg/g) NETA 10 mg/day and 20 mg/day: >0.5 IU/L NETA combined: <0.5 IU/L LNG 10 mg/day and 20 mg/day: >0.5 IU/L LNG combined: <0.5 IU/L LH: CPA 10 mg/day and 20 mg/day: >0.5 IU/L CPA combined: <0.5 IU/L NES 2 mg/g: >0.5 IU/L NES 3 mg/g: >0.5 IU/L NES combined: >0.5 IU/L (2 mg/g); <0.5 IU/L (3 mg/g). NETA 10 mg/day and 20 mg/day: >0.5 IU/L NETA combined: <0.5 IU/L. LNG 10 mg/day and 20 mg/day: >0.5 IU/L LNG combined: <0.5 IU/L | NETA (with T): night sweats (possible), axillary eczema (possible), laryngitis (possible). NES (with T): increased aggression, increased anger, decreased libido (all possible). | - |
| Bhat et al. (2019) [26] | Silodosin | Phase I (n = 63) | 8 mg, 3 h before masturbation or sexual intercourse. | Number of sperm (millions/ejaculate): 1–7th day Group A (silodosin): 0 Group B (placebo): 78.41 ± 1.95 8th–14th Group A (placebo): 73.06 ± 3.13 Group B (silodosin): 0 million/ejaculate 15th–30th Group A (placebo): 75.42 ± 1.37 Group B (placebo): 77.14 ± 1.78 | Nasal congestion: >35% Vertigo: >16% Weakness: >15% Scarce ejaculation: >88% | - |
| Lu et al. (2014) [27] | Non-obstructive polyurethane and barium sulfate intravascular device (IVD-A and IVD-B). | Phase III (n = 1459) | 3 groups: IVD-A, IVD-B and no-scalpel vasectomy (VSB). Followed from the 3rd to the 6th month postoperatively and 12 months postoperatively. | 3–6 months Sperm concentration = 0 mL−1 IVD-A: 60.22% IVD-B—56.54% VSB—86.50% Sperm without progressive motility IVD-A: 32.96% IVD-B: 35.20% VSB: 60.66% 12 months Sperm concentration = 0 mL−1 IVD-A: 79.96% IVD-B—84.15% VSB—95.98% Sperm without progressive motility IVD-A: 45.56% IVD-B: 50.70% VSB: 68.42% | Congestive epididymitis and granuloma. After 12 months: 0.89% for the IVD-B group and 1.70% for the VSB group. No serious adverse events were observed. | - |
| Sharma et al. (2019) [7] | Styrene maleic anhydride (SMA) copolymer in dimethyl sulfoxide (DMSO). | Phase III (n = 133) | 60 mg of SMA in chemical complex with 120 μL of DMSO = 120 μL of RISUG. | Azoospermia in all individuals (133) in the period of 1–6 months. Azoospermia: 82.7% in 2 months 17.3% in 3–6 months | Diffuse edema of scrotal tissue and mild scrotal pain (disappeared within one month of use). Scrotal lump at the injection site (disappeared within six months). | - |
| Promising Substances | Quantity | Patents |
|---|---|---|
| Undecanoate dimethandrolone | 1 | US2008167283A1 |
| 118-methyl-19-nortestosterone | 10 | EP1846434A1 RS20050049A PL207582B1 HRP20050172A2 CN1923841A CN1257181C EP1212345A2 WO2021252761A2 CN1481388A CN101945853A |
| Reversible sperm inhibition under guidance | 21 | WO2016205239A1 US6011013A WO2023066923A1 WO2020167789A1 AU2016353345A1 WO0121829A1 US2004161824A1 WO2005019457A2 CN1840544A WO2023225692A1 WO2010014253A2 WO2005019448A2 US6610657B1 WO2005018657A2 WO2005018660A1 US2018028715A1 WO2022232259A1 US2005054576A1 CN1812746A CN115335367A WO0194621A1 |
| Authors/Year | Name/Type of Study | Number of Participants (n) | Main Results |
|---|---|---|---|
| Hamm et al. (2019) [49] | Men’s Fertility Attitudes and Behaviors (MFAB), qualitative study | 58 low-income cisgender men | No influence on the choice of contraceptive methods and reproductive decisions (~50%) Known people who had already suffered some form of deception regarding contraception (46.55%). “It’s her body”: low-income men described feeling a lack of autonomy regarding pregnancy and fatherhood. The main reported factors were the belief that women should control contraception, reluctance to discuss the subject, the lack of acceptable male contraceptive methods, and fatalistic attitudes toward pregnancy. |
| Nguyen et al. (2023) [50] | Online cross-sectional survey with a Gender Equitable Men’s Scale (GEMS) Exploratory online survey on men’s willingness to use NMC and gender-equitable attitudes. | 2066 cisgender men | “Couples should decide together whether they want to have children” (69%; 1466/2066) “Men should be tough” (46%; 944/2066) Willing to use a hormonal NAPCF (75%; 1540/2060) “Men should feel ashamed if they can’t maintain an erection” (>33.33%). Men’s willingness to use NMC is associated with their attitudes toward gender equality. 54% of men were willing to use a male hormonal method, and 65% were willing to use any type of new male contraceptive. Higher scores on the Gender Equity Measure for Men (GEMS) and prior experience with abortion were positively associated with the willingness to use NMC. |
| Newmann et al. (2021) [37] | Measuring men’s beliefs and gender norms related to contraception: development of the Male Norms and Family Planning Acceptance (MNFPA) scale. | 150 men | Development and validation of the MNFPA scale. MNFPA scores were associated with greater self-efficacy and contraceptive intention. This indicates a greater perceived ability to use contraceptives and less difficulty accepting their use by a partner. There was no significant correlation between MNFPA scores and actual contraceptive use. |
| Newmann et al. (2023) [28] | Masculinity norms to improve men’s acceptance of contraceptive methods: results from a pilot intervention with men in western Kenya. | 150 men (75 intervention/75 control) | Participation in the intervention was associated with increased scores on the MNFPA scale for contraceptive acceptance, including knowledge of contraceptive methods and discussions about contraception with partners and others. The intervention was not associated with increased behavioral intention or use of modern contraceptive methods. Men have the final say in contraceptive decisions (~50%) |
| Brewer & Nguyen (2025) [51] | Growing interest in new male contraceptive methods following the U.S. Supreme Court’s Dobbs v. Jackson decision. | Web traffic data and Google searches. | Following the Dobbs v. Jackson decision, there was an accentuated increase in Google search volumes for “male birth control” and traffic to related websites. The average number of searches increased significantly (45.4%) in the two weeks after the decision compared to the two weeks before (19.6%). |
| Gipson et al., 2025 [52] | Clinical trials on male hormonal contraceptives | 30 men | There are many reasons why people use male hormonal contraceptives, including social concerns such as overpopulation and responsible pregnancy, partnership factors such as sharing contraceptive responsibility and concerns about the “trap,” and individual factors such as pleasure, intimacy, and bodily autonomy. Single men viewed male hormonal contraceptives as a form of protection, whereas men in committed relationships wanted to share responsibility and minimize potential side effects for their partners. |
| Jacobsohn et al., 2022 [53] | Male contraception is coming: who do men want to prescribe their contraceptive method? | 124 men (participants in clinical trials on male hormonal contraceptives) | Most people chose to obtain male hormonal contraceptives from their regular doctor (43.5%) or community pharmacist (17.7%). Specialists in family planning, men’s health, reproductive health, or hormones were the least preferred. Participants with more education tended to prefer specialists more often. |
| Nguyen, 2024 [54] | Research using secondary data obtained from the U.S. National Survey of Family Growth | 3340 respondents | Estimate the potential market for NMC in the EUA. 23.2% of men did not use any method of contraception during their last sexual encounter. A significant proportion of these men would be “very upset” if they got someone pregnant: 4.3% of those who do not use contraception and 19.7% of condom users. The potential market for NMC is estimated to be between 7 and 15.5 million men. |
| Nguyen, 2024 [34] | Review of more than 30 studies on the acceptability of male contraceptives versus male acceptance of contraceptive responsibility. | Own survey with 2066 men | Despite men’s consistent interest and willingness to use NMC, investments remain limited. Men’s views on their role in preventing pregnancy are shifting toward greater gender equality, which is reflected in their willingness to use NMC. Increase awareness among men about their reproductive freedoms. Men prefer to consult primary care physicians for NMC. |
| Gomez-Torres et al., 2023 [55] | Systematic review on the acceptability and determinants of NMC use from a gender perspective | - | The acceptability of NMC in men and women is high (over 70%). Acceptability is influenced by factors such as side effects, route and frequency of administration, efficacy, and cost. The use of NMC varies by country and culture. The daily NMC pill would be the most acceptable option for men. However, women would prefer a quarterly injectable MHC because they are concerned that men would forget to take the daily pill. |
| Blitzer, 2025 [56] | Theoretical essay on male contraception—Part of gender medicine and men’s reproductive rights | - | Two historical models of contraceptive practice overlap: the patriarchal model, in which men have exclusive control over reproductive decisions and women’s health is disregarded, and the female emancipation model, in which women have access to means of deciding whether to reproduce, such as the pill, which has reduced the cost for women to invest in their careers. The Collaborative Model is a future project in which both individuals decide together on reproduction and the methods to use. This increases choice and awareness of shared responsibilities. |
| Kidula et al. (2025) [57] | Review and synthesis of published literature on the impacts of male contraception on global sexual and reproductive health and rights | - | Global policies and structures neglect men’s sexual and reproductive health, and data on the topic is scarce. Failing to address men’s sexual and reproductive health perpetuates the sexual and reproductive burdens faced by women. Advances in male contraception can raise awareness of and investment in men’s sexual and reproductive health. This promotes gender equality and shared responsibility in preventing pregnancy, sexually transmitted infections, and human immunodeficiency virus (HIV) infection. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, L.M.S.; Rieper, R.L.A.; Lima, V.C.F.; Aragón Novoa, D.M.; de Araújo, I.B.; Alves, I.A.; de Freitas Santos Júnior, A. New Horizons in Male Contraception: Clinical, Cultural and Technological Innovation Aspects. Sexes 2025, 6, 60. https://doi.org/10.3390/sexes6040060
Silva LMS, Rieper RLA, Lima VCF, Aragón Novoa DM, de Araújo IB, Alves IA, de Freitas Santos Júnior A. New Horizons in Male Contraception: Clinical, Cultural and Technological Innovation Aspects. Sexes. 2025; 6(4):60. https://doi.org/10.3390/sexes6040060
Chicago/Turabian StyleSilva, Lucca Moisés Santiago, Ryan Lago Araujo Rieper, Vanessa Castro Felix Lima, Diana Marcela Aragón Novoa, Igor Brasil de Araújo, Izabel Almeida Alves, and Aníbal de Freitas Santos Júnior. 2025. "New Horizons in Male Contraception: Clinical, Cultural and Technological Innovation Aspects" Sexes 6, no. 4: 60. https://doi.org/10.3390/sexes6040060
APA StyleSilva, L. M. S., Rieper, R. L. A., Lima, V. C. F., Aragón Novoa, D. M., de Araújo, I. B., Alves, I. A., & de Freitas Santos Júnior, A. (2025). New Horizons in Male Contraception: Clinical, Cultural and Technological Innovation Aspects. Sexes, 6(4), 60. https://doi.org/10.3390/sexes6040060











