Sex, Age, and Risk Group Variations among Individuals Infected with HIV, HTLV-1, and HTLV-2: Review of Data Records (1983–2017) from a Public Health Laboratory in São Paulo, Brazil
Abstract
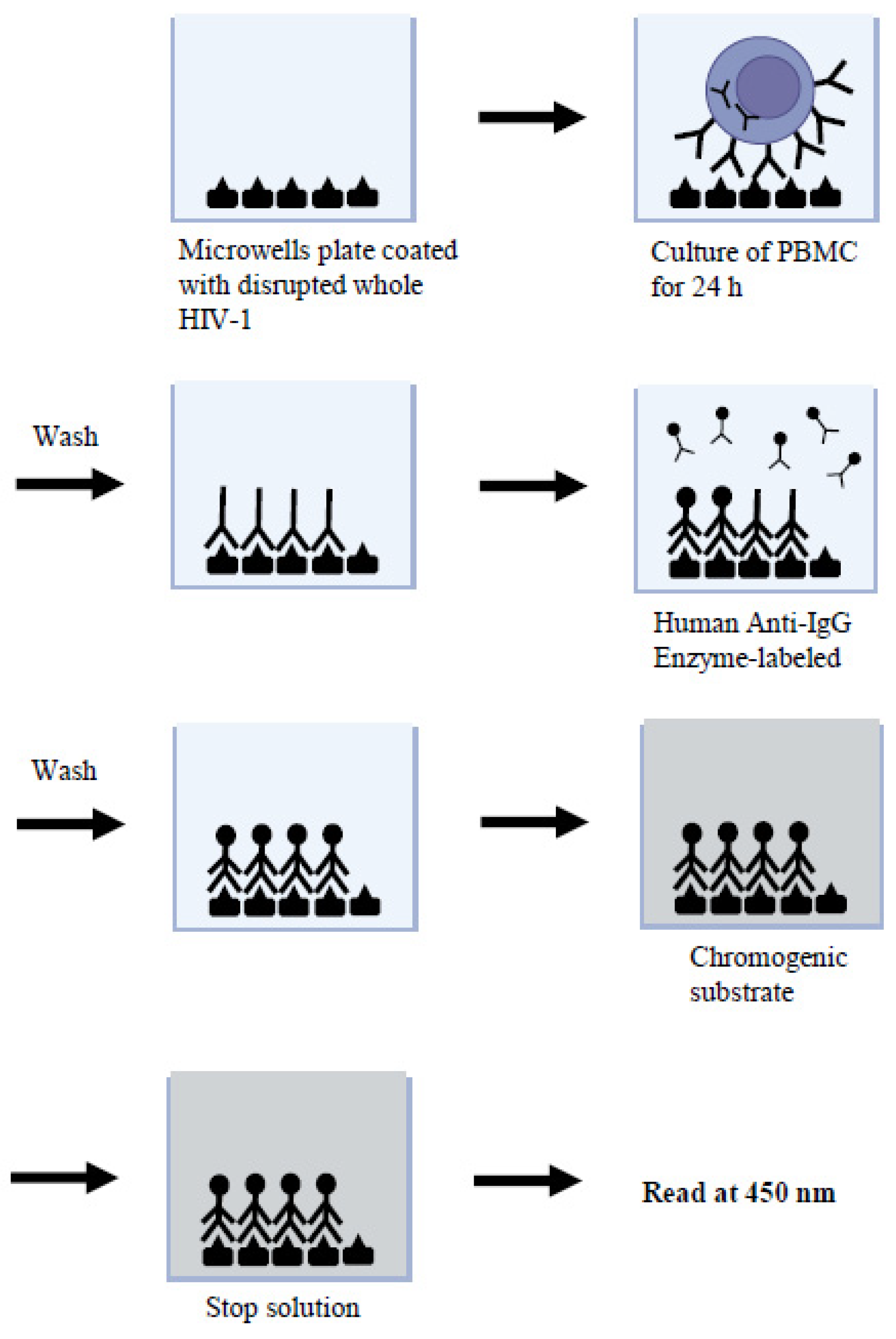
:1. Introduction
2. Materials and Methods
2.1. Study Design and Works Selection Criteria
2.2. Data Presentation and Statistical Analysis
2.3. Ethics Approval
3. Results and Commentaries
3.1. Cellular Immune Profile in AIDS (1983–1985)
3.2. HIV Diagnosis in Children (1989–1993)
3.3. HIV/HTLV Co-Infections in Patients Attended by One Hospital of São Paulo (1991–1994)
3.4. HIV/HTLV Co-Infections in Londrina, Paraná (2001–2002)
3.5. HIV/HTLV Co-Infections in Patients of Several CRTAs and Out-Patient Clinics of São Paulo (1999–2006)
3.6. HIV/HTLV Co-Infections in Patients of the Pioneer CRTA-SP (2014–2015)
3.7. HIV and HIV/HTLV Co-Infection in Patients of Several CRTAs of São Paulo (2010–2016)
3.8. HIV/HTLV Co-Infections in Recent CRTA Settings in São Paulo (2012–2015)
3.9. Variations in HIV/HTLV Co-Infection Prevalence Rates Regarding the Years of Sample Collection (1991–2015)
4. Discussion
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laurindo-Teodorescu, L.; Teixeira, P.R. Histórias da Aids no Brasil: As Respostas Governamentais à Epidemia de Aids; Ministério da Saúde/Secretaria de Vigilância em Saúde/Departamento de DST, Aids e Hepatites Virais: Brasília, Brazil, 2015; Volume 1, pp. 1–464. Available online: https://unesdoc.unesco.org/ark:/48223/pf0000235557?posInSet=2&queryId=f390b88f-74e1-450d-a274-cfb553262cc7 (accessed on 4 July 2023).
- CDC. Pneumocystis Pneumonia: Los Angeles; Morbidity and Mortality Weekly Report (MMWR); Centers for Disease Control: Atlanta, GA, USA, 5 June 1981; Volume 30, pp. 250–252. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/june_5.htm (accessed on 4 July 2023).
- CDC. Kaposi’s Sarcoma and Pneumocystis Pneumonia among Homosexual Men—New York City and California; Morbidity and Mortality Weekly Report (MMWR); Centers for Disease Control: Atlanta, GA, USA, 3 July 1981; Volume 30, pp. 305–308. Available online: https://www.jstor.org/stable/23300179 (accessed on 4 July 2023).
- CDC. Opportunistic Infections and Kaposi’s Sarcoma among Haitians in the United States; Morbidity and Mortality Weekly Report (MMWR); Centers for Disease Control: Atlanta, GA, USA, 9 July 1982; Volume 31, pp. 353–354, 360–361. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001123.htm (accessed on 4 July 2023).
- CDC. Pneumocystis Carinii Pneumonia among Persons with Hemophilia A; Morbidity and Mortality Weekly Report (MMWR); Centers for Disease Control: Atlanta, GA, USA, 16 July 1982; Volume 31, pp. 365–367. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001126.htm (accessed on 4 July 2023).
- CDC. Current Trends Update on Acquired Immune Deficiency Syndrome (AIDS)—United States; Morbidity and Mortality Weekly Report (MMWR); Centers for Disease Control: Atlanta, GA, USA, 24 September 1982; Volume 31, pp. 507–508, 513–514. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/00001163.htm (accessed on 4 July 2023).
- Poiesz, B.J.; Ruscetti, F.W.; Gazdar, A.F.; Bunn, P.A.; Minna, J.D.; Gallo, R.C. Detection and isolation of type C retrovirus particles from fresh and cultured lymphocytes of a patient with cutaneous T-cell lymphoma. Proc. Natl. Acad. Sci. USA 1980, 77, 7415–7419. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, V.S.; Sarngadharan, M.G.; Robert-Guroff, M.; Miyoshi, I.; Golde, D.; Gallo, R.C. A new subtype of human T-cell leukemia virus (HTLV-II) associated with a T-cell variant of hairy cell leukemia. Science 1982, 218, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vézinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef]
- Popovic, M.; Sarngadharan, M.G.; Read, E.; Gallo, R.C. Detection, isolation, and continuous production of cytopathic retroviruses (HTLV-III) from patients with AIDS and pre-AIDS. Science 1984, 224, 497–500. [Google Scholar] [CrossRef] [PubMed]
- Gallo, R.C.; Salahuddin, S.Z.; Popovic, M.; Shearer, G.M.; Kaplan, M.; Haynes, B.F.; Palker, T.J.; Redfield, R.; Oleske, J.; Safai, B.; et al. Frequent detection and isolation of cytopathic retroviruses (HTLV-III) from patients with AIDS and at risk for AIDS. Science 1984, 224, 500–503. [Google Scholar] [CrossRef]
- Vallinoto, A.C.R.; Rosadas, C.; Machado, L.F.A.; Taylor, G.P.; Ishak, R. HTLV: It is time to reach a consensus on its nomenclature. Front. Microbiol. 2022, 13, 896224. [Google Scholar] [CrossRef]
- Brites, C.; Sampaio, J.; Oliveira, A. HIV/Human T-cell lymphotropic virus coinfection revisited: Impact on AIDS progression. AIDS Rev. 2009, 11, 8–16. Available online: https://www.aidsreviews.com/resumen.php?id=1030&indice=2009111&u=unp (accessed on 4 July 2023).
- Beilke, M.A. Retroviral coinfections: HIV and HTLV: Taking stock of more than a quarter century of research. AIDS Res. Hum. Retroviruses 2012, 28, 139–147. [Google Scholar] [CrossRef]
- Montaño-Castellón, I.; Marconi, C.S.C.; Saffe, C.; Brites, C. Clinical and laboratory outcomes in HIV-1 and HTLV-1/2 coinfection: A systematic review. Front. Public Health 2022, 10, 820727. [Google Scholar] [CrossRef]
- Rosadas, C.; Taylor, G.P. HTLV-1 and co-infections. Front. Med. 2022, 9, 812016. [Google Scholar] [CrossRef]
- Gessain, A.; Cassar, O. Epidemiological aspects and world distribution of HTLV-1 infection. Front. Microbiol. 2012, 3, 388. [Google Scholar] [CrossRef]
- Murphy, E.L.; Cassar, O.; Gessain, A. Estimating the number of HTLV-2 infected persons in the world. Retrovirology 2015, 12 (Suppl. S1), O5. Available online: http://www.retrovirology.com/content/12/S1/O5 (accessed on 22 August 2023). [CrossRef]
- Carneiro-Proietti, A.B.F.; Catalan-Soares, B.C.; Castro-Costa, C.M.; Murphy, E.L.; Sabino, E.C.; Hisada, M.; Galvão-Castro, B.; Alcantara, L.C.J.; Remondegui, C.; Verdonck, K.; et al. HTLV in the Americas: Challenges and perspectives. Rev. Panam. Salud Publica 2006, 19, 44–53. [Google Scholar] [CrossRef]
- Briggs, N.C.; Battjes, R.J.; Cantor, K.P.; Blattner, W.A.; Yellin, F.M.; Wilson, S.; Ritz, A.L.; Weiss, S.H.; Goedert, J.J. Seroprevalence of human T cell lymphotropic virus type II infection, with or without human immunodeficiency virus type 1 coinfection, among US intravenous drug users. J. Infect. Dis. 1995, 172, 51–58. [Google Scholar] [CrossRef]
- Paiva, A.; Casseb, J. Origin and prevalence of human T-lymphotropic virus type 1 (HTLV-1) and type 2 (HTLV-2) among indigenous populations in the Americas. Rev. Inst. Med. Trop. Sao Paulo 2015, 57, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ishak, R.; Ishak, M.O.G.; Azevedo, V.N.; Machado, L.F.A.; Vallinoto, I.M.C.; Queiroz, M.A.F.; Costa, G.L.C.; Guerreiro, J.F.; Vallinoto, A.C.R. HTLV in South America: Origins of a silent ancient human infection. Virus Evol. 2020, 6, veaa053. [Google Scholar] [CrossRef]
- De-Araujo, A.C. Cell-mediated immunity in the Acquired Immunodeficiency Syndrome. Braz. J. Med. Biol. Res. 1987, 20, 579–582. Available online: https://www.researchgate.net/publication/372156658_de-Araujo_AC_Cell-mediated_immunity_response_in_AIDS_Brazilian_J_Med_Biol_Res_1987?_tp=eyJjb250ZXh0Ijp7ImZpcnN0UGFnZSI6InByb2ZpbGUiLCJwYWdlIjoicHJvZmlsZSJ9fQ (accessed on 6 July 2023). [PubMed]
- Amadori, A.; Zamarchi, R.; Ciminale, V.; Del Mistro, A.; Siervo, A.; Alberti, A.; Colombatti, M.; Chieco-Bianchi, L. HIV-1-specific B cell activation. A major constituent of spontaneous B cell activation during HIV-1 infection. J. Immunol. 1989, 143, 2146–2152. [Google Scholar] [CrossRef] [PubMed]
- Amadori, A.; De Rossi, A.; Faulkner-Valle, G.P.; Chieco-Bianchi, L. Spontaneous in vitro production of virus-specific antibody by lymphocytes from HIV-infected subjects. Clin. Immunol. Immunopathol. 1988, 46, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Caterino-de-Araujo, A.; de-los-Santos-Fortuna, E.; Grumach, A.S. An alternative method for in vitro production of HIV-1-specific antibodies. Braz. J. Med. Biol. Res. 1991, 24, 797–799. Available online: https://www.researchgate.net/publication/21359230_An_alternative_method_for_in_vitro_production_of_HIV-1-specific_antibodies. (accessed on 6 July 2023).
- Caterino-de-Araujo, A. Rapid in vitro detection of HIV-1-specific antibody secretion by cells-culture with virus antigens. Mem. Inst. Oswaldo Cruz Rio Janeiro 1992, 87, 239–247. Available online: https://www.researchgate.net/publication/21846277_Rapid_in_vitro_detection_of_HIV-1-specific_antibody_secretion_by_cells-culture_with_virus_antigens (accessed on 6 July 2023). [CrossRef] [PubMed]
- De Rossi, A.; Mammano, F.; Del Mistro, A.; Chieco-Bianchi, L. Serological and molecular evidence of infection by human T-cell lymphotropic virus type II in Italian drug addicts by use of synthetic peptides and polymerase chain reaction. Eur. J. Cancer Clin. Oncol. 1991, 27, 835–838. [Google Scholar] [CrossRef]
- Koech, C.C.; Lwembe, R.M.; Odari, E.O.; Budambula, N.L.M. Prevalence and associated risk factors of HTLV/HIV co-infection among people who inject drugs (PWIDs): A review. J. Hum. Virol. Retrovirol. 2018, 6, 00188. [Google Scholar] [CrossRef]
- Caterino de Araujo, A.; do Rosario Casseb, J.S.; Neitzert, E.; Xavier de Souza, M.L.; Mammano, F.; Del Mistro, A.; De Rossi, A.; Chieco-Bianchi, L. HTLV-I and HTLV-II infections among HIV-1 seropositive patients in Sao Paulo, Brazil. Eur. J. Epidemiol. 1994, 10, 165–171. [Google Scholar] [CrossRef]
- Caterino-de-Araujo, A.; de los Santos-Fortuna, E.; Meleiro, M.C.Z.; Suleiman, J.; Calabrò, M.L.; Favero, A.; De Rossi, A.; Chieco-Bianchi, L. Sensitivity of two enzyme-linked Immunosorbent assay tests in relation to Western blot in detecting human T-cell lymphotropic virus types I and II infection among HIV-1 infected patients from São Paulo, Brazil. Diagn. Microbiol. Infect. Dis. 1998, 30, 173–182. [Google Scholar] [CrossRef]
- Morimoto, H.K.; Caterino-De-Araujo, A.; Morimoto, A.A.; Reiche, E.M.V.; Ueda, L.T.; Matsuo, T.; Stegmann, J.W.; Reiche, F.V. Seroprevalence and risk factors for Human T cell lymphotropic virus type 1 and 2 infection in human immunodeficiency virus-infected patients attending AIDS referral center health units in Londrina and other communities in Paraná, Brazil. AIDS Res. Hum. Retroviruses 2005, 21, 256–262. [Google Scholar] [CrossRef]
- Morimoto, H.K.; Morimoto, A.A.; Reiche, E.M.V.; Ueda, L.T.; Matsuo, T.; Reiche, F.V.; Caterino-de-Araujo, A. Difficulties in the diagnosis of HTLV-2 infection in HIV/AIDS patients from Brazil: Comparative performances of serologic and molecular assays, and detection of HTLV-2b subtype. Rev. Inst. Med. Trop. Sao Paulo 2007, 49, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Santos-Fortuna, E.; Azevedo, R.S.; Caterino-de-Araujo, A. Serological patterns for HTLV-I/II and its temporal trend in high-risk populations attended at Public Health Units of São Paulo, Brazil. J. Clin. Virol. 2008, 42, 149–155. [Google Scholar] [CrossRef]
- Caterino-de-Araujo, A.; Sacchi, C.T.; Gonçalves, M.G.; Campos, K.R.; Magri, M.C.; Alencar, W.K.; Group of Surveillance and Diagnosis of HTLV of São Paulo (GSuDiHTLV-SP). Current prevalence and risk factors associated with HTLV-1 and HTLV-2 infections among HIV/AIDS patients in São Paulo, Brazil. AIDS Res. Hum. Retroviruses 2015, 31, 543–549. [Google Scholar] [CrossRef]
- Campos, K.R.; Gonçalves, M.G.; Caterino-de-Araujo, A. Failures in detecting HTLV-1 and HTLV-2 in patients infected with HIV-1. AIDS Res. Hum. Retroviruses 2017, 33, 382–385. [Google Scholar] [CrossRef]
- Campos, K.R.; Gonçalves, M.G.; Costa, N.A.; Caterino-de-Araujo, A. Comparative performances of serologic and molecular assays for detecting HTLV-1 and HTLV-2 in patients infected with HIV-1. Braz. J. Infect. Dis. 2017, 21, 297–305. [Google Scholar] [CrossRef] [PubMed]
- Casseb, J.; Souza, T.; Pierre-Lima, M.T.; Yeh, E.; Hendry, M.; Gallo, D. Testing problems in diagnosing HTLV infection among intravenous drug users with AIDS in São Paulo city, Brazil. AIDS Res. Hum. Retroviruses 1997, 13, 1639–1641. [Google Scholar] [CrossRef] [PubMed]
- Zunt, J.R.; Tapia, K.; Thiede, H.; Lee, R.; Hagan, H. HTLV-2 infection in injection drug users in King County, Washington. Scand. J. Infect. Dis. 2006, 38, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, F.; Kral, A.; Reingold, A.; Bueno, R.; Trigueiros, D.; Araujo, P.J. Santos Metropolitan Region Collaborative Study Group. Trends of HIV infection among injection drug users in Brazil in the 1990s: The impact of changes in patterns of drug use. J. Acquir. Immune Defic. Syndr. 2001, 28, 298–302. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, F.; Doneda, D.; Gandolfi, D.; Nemes, M.I.B.; Andrade, T.; Bueno, R.; Trigueiros, D.P. Brazilian response to the human immunodeficiency virus/acquired immunodeficiency syndrome epidemic among injection drug users. Clin. Infect. Dis. 2003, 37 (Suppl. 5), S382–S385. [Google Scholar] [CrossRef] [PubMed]
- Caterino-de-Araujo, A.; Campos, K.R. Spread of human retrovirus infections in individuals at the second and third decades of life in São Paulo, Brazil. Austin J. HIV/AIDS Res. 2017, 4, 1036. Available online: https://austinpublishinggroup.com/hiv-aids-research/fulltext/ajhr-v4-id1036.pdf (accessed on 11 July 2023).
- Paiva, A.; Casseb, J. Sexual transmission of human T-cell lymphotropic virus type 1. Rev. Soc. Bras. Med. Trop. 2014, 47, 265–274. [Google Scholar] [CrossRef]
- Ano V, No 1 da 27a a 53a Semanas Epidemiológicas—Julho a Dezembro de 2015 e da 01a a 26a Semanas Epidemiológicas—Janeiro a Junho de 2016. Available online: http://antigo.aids.gov.br/pt-br/pub/2016/boletim-epidemiologico-de-aids-2016 (accessed on 12 July 2023).
- Kuramitsu, M.; Sekizuka, T.; Yamochi, T.; Firouzi, S.; Sato, T.; Umeki, K.; Sasaki, D.; Hasegawa, H.; Kubota, R.; Sobata, R.; et al. Proviral features of human T cell leukemia virus type 1 in carriers with indeterminate Western blot analysis results. J. Clin. Microbiol. 2017, 55, 2838–2849. [Google Scholar] [CrossRef]
- Okuma, K.; Kuramitsu, M.; Niwa, T.; Taniguchi, T.; Masaki, Y.; Ueda, G.; Matsumoto, C.; Sobata, R.; Sagara, Y.; Nakamura, H.; et al. Establishment of a novel diagnostic test algorithm for human T-cell leukemia virus type 1 infection with line immunoassay replacement of western blotting: A collaborative study for performance evaluation of diagnostic assays in Japan. Retrovirology 2020, 17, 26. [Google Scholar] [CrossRef]
- Brasil Ministério da Saúde. Guia de Manejo Clínico da Infecção pelo HTLV/Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Doenças de Condições Crônicas e Infecções Sexualmente Transmissíveis; Ministério da Saúde: Brasília, Brazil, 2021; 104p, ISBN 978-65-5993-116-3. Available online: http://antigo.aids.gov.br/pt-br/pub/2022/guia-de-manejo-clinico-da-infeccao-pelo-htlv (accessed on 14 July 2023).
- Vallinoto, A.C.R.; Azevedo, V.N.; Santos, D.E.M.; Caniceiro, S.; Mesquita, F.C.L.; Hall, W.W.; Ishak, M.O.G.; Ishak, R. Serological evidence of HTLV-I and HTLV-II coinfections in HIV-1 positive patients in Belém, state of Pará, Brazil. Mem. Inst. Oswaldo Cruz Rio Janeiro 1998, 93, 407–409. [Google Scholar] [CrossRef]
- Laurentino, R.V.; Lopes, I.G.L.; Azevedo, V.N.; Machado, L.F.A.; Moreira, M.R.C.; Lobato, L.; Ishak, M.O.G.; Ishak, R.; Vallinoto, A.C.R. Molecular characterization of human T-cell lymphotropic virus coinfecting human immunodeficiency virus 1 infected patients in the Amazon region of Brazil. Mem. Inst. Oswaldo Cruz 2005, 100, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Alencar, S.P.; Souza, M.C.; Fonseca, R.R.S.; Menezes, C.R.; Azevedo, V.N.; Ribeiro, A.L.R.; Lima, S.S.; Laurentino, R.V.; Barbosa, M.A.A.P.; Freitas, F.B.; et al. Prevalence and molecular epidemiology of human T-lymphotropic virus (HTLV) infection in people living with HIV/AIDS in the Pará state, Amazon region of Brazil. Front. Microbiol. 2020, 11, 572381. [Google Scholar] [CrossRef] [PubMed]
- Brites, C.; Goyanna, F.; França, L.G.; Pedroso, C.; Netto, E.M.; Adriano, S.; Sampaio, J.; Harrington, W., Jr. Coinfection by HTLV-I/II is associated with an increased risk of strongyloidiasis and delay in starting antiretroviral therapy for AIDS patients. Braz. J. Infect. Dis. 2011, 15, 6–11. [Google Scholar] [CrossRef]
- Pereira, F.M.; Santos, F.L.N.; Silva, Â.A.O.; Nascimento, N.M.; Almeida, M.C.C.; Carreiro, R.P.; Galvão-Castro, B.; Grassi, M.F.R. Distribution of human immunodeficiency virus and human T-leukemia virus co-infection in Bahia, Brazil. Front. Med. 2022, 8, 788176. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, E.H.; Oliveira-Filho, A.B.; Souza, L.A.; da Silva, L.V.; Ishak, M.O.G.; Ishak, R.; Vallinoto, A.C.R. Human T-cell lymphotropic virus in patients infected with HIV-1: Molecular epidemiology and risk factors for transmission in Piaui, Northeastern Brazil. Curr. HIV Res. 2012, 10, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Santos de Souza, M.; Prado Gonçales, J.; Santos de Morais, V.M.; Silva Júnior, J.V.J.; Lopes, T.R.R.; Costa, J.E.F.D.; Côelho, M.R.C.D. Prevalence and risk factor analysis for HIV/HTLV 1/2 coinfection in Paraíba state, Brazil. J. Infect. Dev. Ctries. 2021, 15, 1551–1554. [Google Scholar] [CrossRef] [PubMed]
- Etzel, A.; Shibata, G.; Rozman, M.; Jorge, M.L.S.G.; Damas, C.D.; Segurado, A.A.C. HTLV-1 and HTLV-2 infections in HIV-infected individuals from Santos, Brazil: Seroprevalence and risk factors. JAIDS 2001, 26, 185–190. Available online: https://journals.lww.com/jaids/abstract/2001/02010/htlv_1_and_htlv_2_infections_in_hiv_infected.13.aspx (accessed on 24 August 2023).
- Kleine-Neto, W.; SanabaniI, S.S.; Jamal, L.F.; Sabino, E.C. Prevalence, risk factors and genetic characterization of human T-cell lymphotropic virus types 1 and 2 in patients infected with human immunodeficiency virus type 1 in the cities of Ribeirão Preto and São Paulo. Rev. Soc. Bras. Med. Trop. 2009, 42, 264–270. [Google Scholar] [CrossRef]
- Barcellos, N.T.; Fuchs, S.C.; Mondini, L.G.; Murphy, E.L. Human T lymphotropic virus type I/II infection: Prevalence and risk factors in individuals testing for HIV in counseling centers from Southern Brazil. Sex. Transm. Dis. 2006, 33, 302–306. [Google Scholar] [CrossRef]
- Galetto, L.R.; Lunge, V.R.; Béria, J.U.; Tietzmann, D.C.; Stein, A.T.; Simon, D. Prevalence and risk factors for human T cell lymphotropic virus infection in Southern Brazilian HIV-positive patients. AIDS Res. Hum. Retroviruses 2014, 30, 907–911. [Google Scholar] [CrossRef]
- Chequer, P.; Marins, J.R.P.; Possas, C.; Valero, J.D.A.; Bastos, F.I.; Castilho, E.; Hearst, N. AIDS research in Brazil. AIDS 2005, 19 (Suppl. S4), S1–S3. [Google Scholar] [CrossRef] [PubMed]
- Okie, S. Fighting HIV—Lessons from Brazil. N. Engl. J. Med. 2006, 354, 1977–1981. [Google Scholar] [CrossRef] [PubMed]
- Boletim Epidemiológico. HIV—AIDS 2022. Secretaria de Vigilância em Saúde, Ministério da Saúde, Número Especial. Dez. Brasília. 2022. Available online: https://www.gov.br/aids/pt-br/centrais-de-conteudo/boletins-epidemiologicos/2022/hiv-aids/boletim_hiv_aids_-2022_internet_31-01-23.pdf/view (accessed on 17 July 2023).
- Colón-López, V.; Miranda-De León, S.; Machin-Rivera, M.; Soto-Abreu, R.; Marrero-Cajigas, E.L.; Rolón-Colón, Y.; Valencia-Torres, I.M.; Suárez-Pérez, E.L. New diagnoses among HIV+ men and women in Puerto Rico: Data from the HIV surveillance system 2003–2014. Puerto Rico Health Sci. J. 2019, 38, 33–39. Available online: https://prhsj.rcm.upr.edu/index.php/prhsj/article/viewFile/1772/1171 (accessed on 22 August 2023).
- Agwu, A. Sexuality, sexual health, and sexually transmitted infections in adolescents and young adults. Top. Antivir. Med. 2020, 28, 459–462. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7482983/ (accessed on 22 August 2023). [PubMed]
- The Path That Ends AIDS: UNAIDS Global AIDS Update 2023; Joint United Nations Programme on HIV/AIDS: Geneva, Switzerland, 2023; Available online: https://thepath.unaids.org/wp-content/themes/unaids2023/assets/files/2023_report.pdf (accessed on 22 August 2023).
- Martin, F.; Tagaya, Y.; Gallo, R. Time to eradicate HTLV-1: An open letter to WHO. Lancet 2018, 391, 1893–1894. [Google Scholar] [CrossRef]
- WHO. Human T-lymphotropic Virus Type 1: Technical Report; WHO: Geneva, Switzerland, 2021. Available online: https://www.who.int/publications/i/item/9789240020221 (accessed on 24 August 2023).
- Rosadas, C.; Menezes, M.L.B.; Galvão-Castro, B.; Assone, T.; Miranda, A.E.; Aragon, M.; Caterino-de-Araujo, A.; Taylor, G.P.; Ishak, R. Blocking HTLV-1/2 silent transmission in Brazil: Current public health policies and proposal of additional strategies. PLoS Negl. Trop. Dis. 2021, 15, e0009717. [Google Scholar] [CrossRef]
- Miranda, A.E.; Rosadas, C.; Assone, T.; Pereira, G.F.M.; Vallinoto, A.C.R.; Ishak, R. Strengths, Weaknesses, Opportunities and Threats (SWOT) analysis of the implementation of public health policies on HTLV-1 in Brazil. Front. Med. 2022, 9, 859115. [Google Scholar] [CrossRef]
- Sagara, Y.; Nakamura, H.; Satake, M.; Watanabe, T.; Hamaguchi, I. Increasing horizontal transmission of human T-cell leukemia virus type 1 in adolescents and young adults in Japan. J. Clin. Virol. 2022, 157, 105324. [Google Scholar] [CrossRef]


| Groups | ||||
|---|---|---|---|---|
| CONTROL (n = 11) | RISK (n = 27) | LAS/ARC (n = 37) | AIDS (n = 47) | |
| Age (years) | 30.4 | 30.7 | 31.9 | 30.8 |
| Leucocytes/mm3 | 5263.6 | 5955.6 | 5689.2 | 4763.8 * |
| Lymphocytes/mm3 | 1967.6 | 2316.7 | 2264.2 | 1218.2 * |
| T lymphocyte (%) | 64.0 | 64.8 | 62.2 | 52.1 * |
| T lymphocytes/mm3 | 1247 | 1509.8 | 1403.9 | 662.3 * |
| B lymphocytes (%) | 18.2 | 16.6 | 15.0 | 18.6 |
| B lymphocytes/mm3 | 397.3 | 362.7 | 307.6 | 214.4 * |
| T4 (%) | 44.4 | 37.8 | 26.3 | 13.5 * |
| T4/mm3 | 863 | 833.9 | 591.4 | 191.2 * |
| T8 (%) | 21.6 | 26.6 | 37.3 * | 39.0 * |
| T8/mm3 | 451.9 | 620.2 | 797.6 * | 550.7 |
| T4/T8 | 2.3 | 1.6 | 0.74 * | 0.53 * |
| PHA 20 µg/mL | 255,979.7 | 233,381.8 | 175,852.9 | 118,450.1 * |
| Con-A 20 µg/mL | 172,269.5 | 136,493.3 | 111,128.2 | 54,795.5 * |
| PWM 20 µg/mL | 75,942.8 | 71,469.8 | 27,760.5 | 17,930.1 * |
| PPD 10 µg/mL | 59,989.1 | 16,696.0 | 15,505.3 * | 7098.8 * |
| Year of Collection | Local/ Group | Number of Cases | Mean Age (Years) | Sex (Number) | HTLV-1/-2 (%) | HTLV-1 (%) | HTLV-2 (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| 1991–1992 | IIER, SP | 471 | M (406) F (65) | 62 (13.2) | 37 (7.9) | 25 (5.3) | [30] | |
| IDU | 216 | 29 | M (155) F (61) | 57 (26.4) | 33 (15.3) | 24 (11.1) | ||
| Homo/Bis | 229 | 34 | M (229) | 3 (1.3) | 2 (0.9) | 1 (0.4) | ||
| Others a | 26 | 30 | M (22) F (4) | 2 (7.7) | 2 (7.7) | |||
| 1994 | IIER, SP | 553 | 32 | M (358) F (195) | 56 (10.1) | 22 (4.0) | 34 (6.1) | [31] |
| IDU | 89 | 29 | M (65) F (24) | 25 (28.0) | 10 (11.2) | 15 (16.8) | ||
| Hetero | 236 | 33 | M (96) F (140) | 21 (8.9) | 8 (3.4) | 13 (5.5) | ||
| Homo/Bis | 139 | 33 | M (139) | 5 (3.6) | 2 (1.4) | 3 (2.2) | ||
| Others/Unk b | 89 | 32 | M (58) F (31) | 5 (5.6) | 2 (2.2) | 3 (3.4) | ||
| 2001–2002 | CRTA-PR | 758 | 36 | M (424) F (334) | 43 (5.7) | 6 (0.8) | 37 (4.9) | [32,33] |
| Sexual | 633 | 16 (2.5) | 2 (0.3) | 14 (2.2) | ||||
| IDU | 57 | 17 (29.8) | 2 (3.5) | 15 (26.3) | ||||
| Sexual + IDU | 33 | 7 (21.2) | 1 (3.0) | 6 (18.2) | ||||
| Other c | 35 | 3 (8.6) | 1 (2.9) | 2 (5.7) | ||||
| 1999–2006 | CRTAs, SP | 1393 | M (982) F (411) | 81 (5.8) | 46 (3.3) | 35 (2.5) | [34] | |
| Clinics, SP | 919 | M (538) F (381) | 121 (13.2) | 88 (9.6) | 33 (3.6) | |||
| 2014–2015 | CRTA-SP | 1608 | 44 | M (1237) F (371) | 50 (3.1) | 26 (1.6) | 22 (1.4) | [35] |
| 2012–2015 | CRTAs, SP | 1383 | 36 | M (930) F (453) | 58 (4.2) | 29 (2.1) | 24 (1.7) | [36,37] |
| Location | Year of Collection | Number of Samples | HIV/HTLV (%) | HIV/HTLV-1 (%) | HIV/HTLV-2 (%) | Risk Factors/ Associations | Ref. |
|---|---|---|---|---|---|---|---|
| North | |||||||
| Belém, Pará | 1994–1996 | 149 | 7.4 | 2.7 | 4.7 | Homosexual/bisexual men, IDU | [48] |
| Belém, Pará | 2005 | 117 | 5.1 | 1.7 | 3.4 | Unknown | [49] |
| Belém, Pará | 2016–2017 | 368 | 1.4 | 1.4 | 0 | Female, sexual contact, sporadic condom use | [50] |
| Northeast | |||||||
| Salvador, Bahia | 1994–1995 | 123 | 20.3 | 17.1 | 3.2 | IDU | [51] |
| State of Bahia | 2004–2013 | 1733 | 2.4 | 2.1 | 0.3 | Female, sexual contact, from Salvador | [52] |
| State of Piauí | 2012 | 805 | 1.6 | 1.1 | 0.5 | Blood transfusion, surgeries, >40 years | [53] |
| João Pessoa, Paraíba | 2015 | 401 | 1.5 | 1.5 | 0 | None | [54] |
| Southeast | |||||||
| Santos, São Paulo | 1997–1998 | 499 | 13.4 | 6.0 | 7.4 | Male, IDU, HCV, no condom use | [55] |
| Ribeirão Preto and São Paulo, São Paulo | 2001 | 319 | 4.7 | 0.6 | 4.1 | IDU, HCV | [56] |
| South | |||||||
| Porto Alegre, Rio Grande do Sul | 1996 | 2985 | 2.4 | 1.4 | 1.0 | IDU, >30 years | [57] |
| Canoas, Rio Grande do Sul | 2008–2009 | 580 | 2.9 | 1.9 | 1.0 | Blood transfusion, tattoo, alcohol abuse | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caterino-de-Araujo, A. Sex, Age, and Risk Group Variations among Individuals Infected with HIV, HTLV-1, and HTLV-2: Review of Data Records (1983–2017) from a Public Health Laboratory in São Paulo, Brazil. Sexes 2023, 4, 638-655. https://doi.org/10.3390/sexes4040041
Caterino-de-Araujo A. Sex, Age, and Risk Group Variations among Individuals Infected with HIV, HTLV-1, and HTLV-2: Review of Data Records (1983–2017) from a Public Health Laboratory in São Paulo, Brazil. Sexes. 2023; 4(4):638-655. https://doi.org/10.3390/sexes4040041
Chicago/Turabian StyleCaterino-de-Araujo, Adele. 2023. "Sex, Age, and Risk Group Variations among Individuals Infected with HIV, HTLV-1, and HTLV-2: Review of Data Records (1983–2017) from a Public Health Laboratory in São Paulo, Brazil" Sexes 4, no. 4: 638-655. https://doi.org/10.3390/sexes4040041
APA StyleCaterino-de-Araujo, A. (2023). Sex, Age, and Risk Group Variations among Individuals Infected with HIV, HTLV-1, and HTLV-2: Review of Data Records (1983–2017) from a Public Health Laboratory in São Paulo, Brazil. Sexes, 4(4), 638-655. https://doi.org/10.3390/sexes4040041






