Adolescence and Postpartum: Two Life Periods to Deepen Our Understanding of the Complexity of Female Rat Sexual Behavior
Abstract
1. Introduction
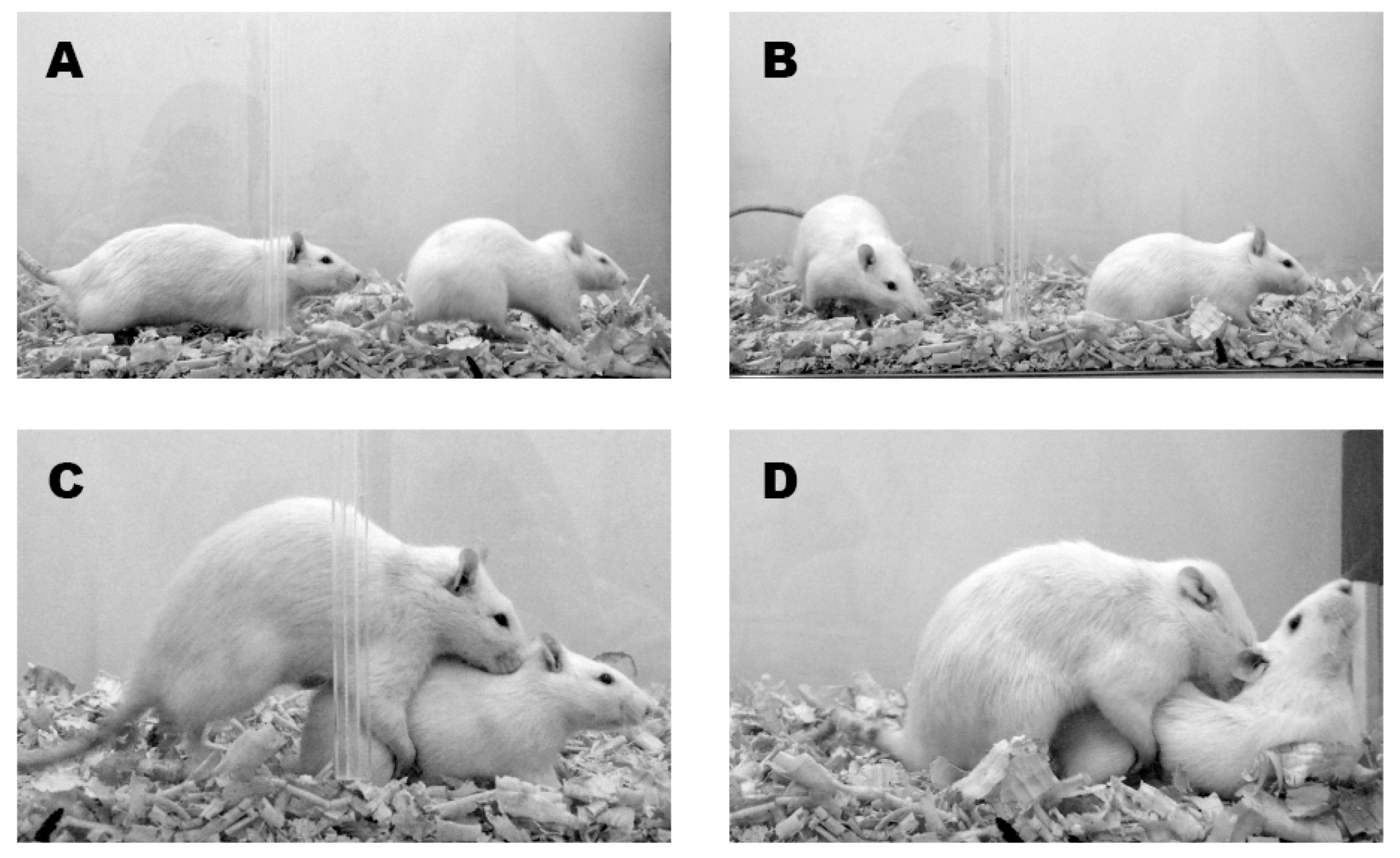
2. Is Female Rat Sexual Behavior a Stereotyped and Passive Response?
Proceptive behavior is functionally as important as other patterns traditionally termed “receptive”, but the female’s tendency to display appetitive responses finds little opportunity for expression in laboratory experiments which focus exclusively upon her receptive behavior, or upon the male’s execution of this coital pattern.
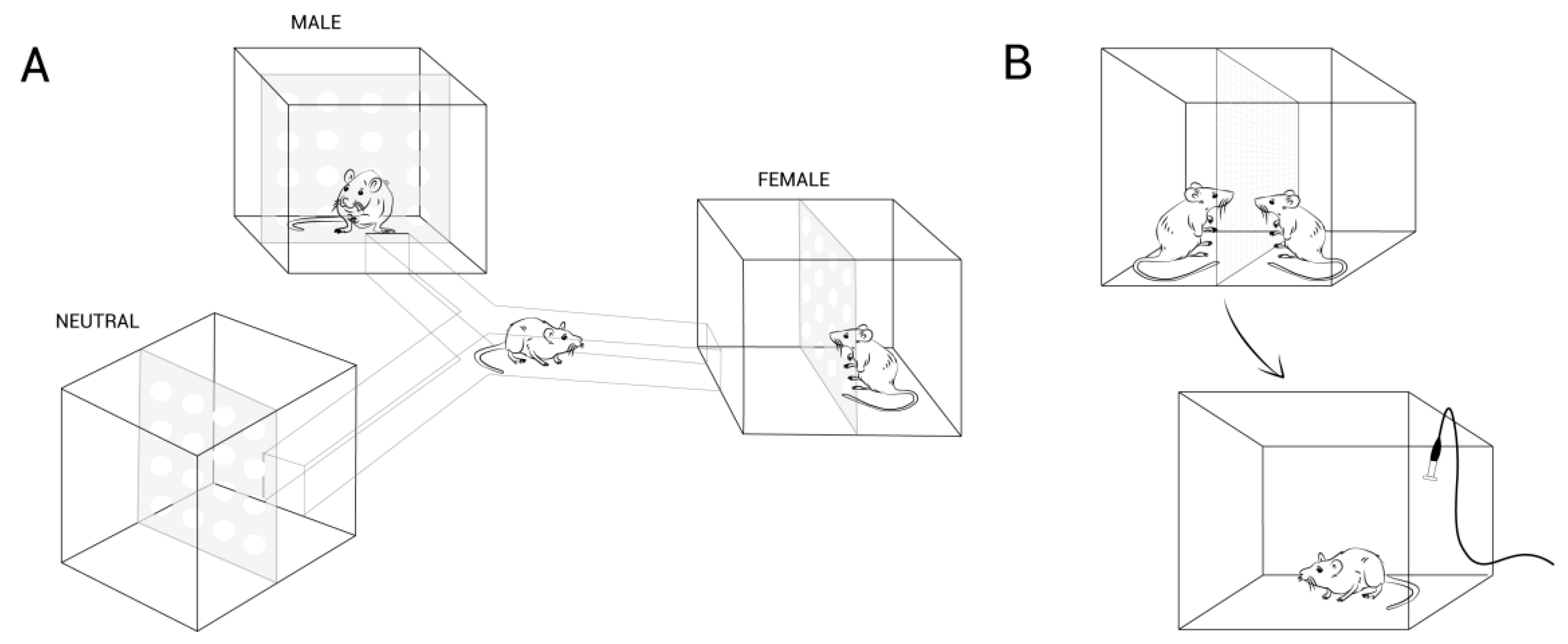
3. Adding Complexity to the Sexual Behavior of Females: The Role of Sexual Motivation
As studies of brain and behavior become more sophisticated, it has become clear that our understanding of the neurobiological mechanisms controlling behavior will be significantly deepened if more naturalistic aspects of the behavior are incorporated into our studies.
| Testing Conditions | Female Behavior | Contributions |
|---|---|---|
| Sexual interaction tests | ||
| Standard laboratory conditions | ||
| - One female and one male in small enclosure | Lordosis and paracopulatory behaviors | Endocrine regulation. Lordosis behavior circuitry. for revision see [5,6,15,16,17] |
| Adding complexity to physical environment | ||
| - Paced-mating chamber - Bilevel chamber - Semi-natural enclosure | Lordosis and other paracopulatory displays Pacing the rhythm of mating | Active role of the female. Importance of the stimulation received. Significance of the context. Broadening of endocrine and contextual modulation. Limbic-hypothalamic control. for revision see [1,4,6,17,19,28] |
| Adding complexity to social environment | ||
| - Mate-choice test with two or more males - Group mating in semi-natural enclosure | Mating preference Female competition | |
| Sexual motivation tests | ||
| Unconditioned tests | ||
| - Sexual preference tests - USV emission test * | Approach behavior | Male sexual incentive value. Endocrine and experiential regulation. Importance of genitosensory stimulation. Sexual motivation circuitry. for revision see [1,4,44,46,54,64,65] |
| Conditioned tests | ||
| - Conditioned place preference - Operant conditioning | Reinforced behaviors | |
4. Adolescence: The Developmental Period When the Female’s Sexual Behavior Emerges
5. Postpartum Estrus: A Unique Reproductive Period When Sexual and Maternal Motivations Converge
6. Conclusions and Final Considerations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ventura-Aquino, E.; Paredes, R.G. Sexual behavior in rodents: Where do we go from here? Horm. Behav. 2020, 118, 104678. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Kippin, T.E.; Coria-Avila, G. What Can Animal Models Tell Us about Human Sexual Response? Annu. Rev. Sex Res. 2003, 14, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Le Moëne, O.; Ågmo, A. Modeling Human Sexual Motivation in Rodents: Some Caveats. Front. Behav. Neurosci. 2019, 13, 187. [Google Scholar] [CrossRef] [PubMed]
- Guarraci, F.A.; Frohardt, R.J. “What a Girl Wants”: What Can We Learn From Animal Models of Female Sexual Motivation? Front. Behav. Neurosci. 2019, 13, 216. [Google Scholar] [CrossRef]
- Beach, F.A. Sexual attractivity, proceptivity, and receptivity in female mammals. Horm. Behav. 1976, 7, 105–138. [Google Scholar] [CrossRef]
- Blaustein, J.D.; Erskine, M.S. 2-Feminine Sexual Behavior: Cellular Integration of Hormonal and Afferent Information in the Rodent Forebrain. In Hormones, Brain and Behavior; Pfaff, D.W., Arnold, A.P., Fahrbach, S.E., Etgen, A.M., Rubin, R.T., Eds.; Academic Press: San Diego, CA, USA, 2002; pp. 139–214. [Google Scholar]
- Pfaff Donald, W. Luteinizing Hormone-Releasing Factor Potentiates Lordosis Behavior in Hypophysectomized Ovariectomized Female Rats. Science 1973, 182, 1148–1149. [Google Scholar] [CrossRef]
- Kow, L.-M.; Pfaff, D.W. Sensory requirements for the lordosis reflex in female rats. Brain Res. 1976, 101, 47–66. [Google Scholar] [CrossRef]
- Pfaff, D.; Montgomery, M.; Lewis, C. Somatosensory determinants of lordosis in female rats: Behavioral definition of the estrogen effect. J. Comp. Physiol. Psychol. 1977, 91, 134–145. [Google Scholar] [CrossRef]
- Pfaff, D.W.; Kow, L.M.; Loose, M.D.; Flanagan-Cato, L.M. Reverse engineering the lordosis behavior circuit. Horm. Behav. 2008, 54, 347–354. [Google Scholar] [CrossRef]
- Kow, L.-M.; Pfaff, D.W. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc. Natl. Acad. Sci. USA 2004, 101, 12354. [Google Scholar] [CrossRef]
- Pfaff, D.W. Estrogens and brain function; Springer: Berlin/Heidelberg, Germany, 1979. [Google Scholar]
- Pfaff, D.W.; Vasudevan, N.; Kia, H.K.; Zhu, Y.-S.; Chan, J.; Garey, J.; Morgan, M.; Ogawa, S. Estrogens, brain and behavior: Studies in fundamental neurobiology and observations related to women’s health. J. Steroid Biochem. Mol. Biol. 2000, 74, 365–373. [Google Scholar] [CrossRef]
- Micevych, P.E.; Meisel, R.L. Integrating neural circuits controlling female sexual behavior. Front. Syst. Neurosci. 2017, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Jones, S.L.; Flanagan-Cato, L.M.; Blaustein, J.D. Female sexual behavior. Knobil Neill’s Physiol. Reprod. 2015, 2, 2287–2370. [Google Scholar]
- Pfaff, D. Cellular and molecular mechanisms of female rproductive behaviors. Physiol. Reprod. 1994, 2, 107–220. [Google Scholar]
- Ventura-Aquino, E.; Paredes, R.G. Animal Models in Sexual Medicine: The Need and Importance of Studying Sexual Motivation. Sex. Med. Rev. 2017, 5, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Peirce, J.T.; Nuttall, D.L. Self-paced sexual behavior in the female rat. J. Comp. Physiol. Psychol. 1961, 54, 310–313. [Google Scholar] [CrossRef] [PubMed]
- Erskine, M.S. Solicitation behavior in the estrous female rat: A review. Horm. Behav. 1989, 23, 473–502. [Google Scholar] [CrossRef]
- Coopersmith, C.; Candurra, C.; Erskine, M.S. Effects of paced mating and intromissive stimulation on feminine sexual behavior and estrus termination in the cycling rat. J. Comp. Physiol. Psychol. 1996, 110, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Erskine, M.S. Pelvic and pudendal nerves influence the display of paced mating behavior in response to estrogen and progesterone in the female rat. Behav. Neurosci. 1992, 106, 690–697. [Google Scholar] [CrossRef]
- Martínez, I.; Paredes, R.G. Only Self-Paced Mating Is Rewarding in Rats of Both Sexes. Horm. Behav. 2001, 40, 510–517. [Google Scholar] [CrossRef]
- Paredes, R.G.; Alonso, A. Sexual behavior regulated (paced) by the female induces conditioned place preference. Behav. Neurosci. 1997, 111, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Coopersmith, C.; Erskine, M.S. Influence of paced mating and number of intromissions on fertility in the laboratory rat. Reproduction 1994, 102, 451–458. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McClintock, M.K.; Adler, N.T. The Role of the Female During Copulation in Wild and Domestic Norway Rats (Rattus Norvegicus). Behaviour 1978, 67, 67–95. [Google Scholar] [CrossRef]
- McClintock, M.K.; Anisko, J.J. Group mating among Norway rats I. Sex differences in the pattern and neuroendocrine consequences of copulation. Anim. Behav. 1982, 30, 398–409. [Google Scholar] [CrossRef]
- McClintock, M.K.; Anisko, J.J.; Adler, N.T. Group mating among Norway rats II. The social dynamics of copulation: Competition, cooperation, and mate choice. Anim. Behav. 1982, 30, 410–425. [Google Scholar] [CrossRef]
- McClintock, M.K. Group mating in the domestic rat as a context for sexual selection: Consequences for the analysis of sexual behavior and neuroendocrine responses. In Advances in the Study of Behavior; Elsevier: Amsterdam, The Netherlands, 1984; Volume 14, pp. 1–50. [Google Scholar]
- Bergheim, D.; Chu, X.; Ågmo, A. The function and meaning of female rat paracopulatory (proceptive) behaviors. Behav. Process. 2015, 118, 34–41. [Google Scholar] [CrossRef]
- Chu, X.; Ågmo, A. Sociosexual behaviours in cycling, intact female rats (Rattus norvegicus) housed in a seminatural environment. Behaviour 2014, 151, 1143–1184. [Google Scholar] [CrossRef]
- Chu, X.; Ågmo, A. Sociosexual behaviors during the transition from non-receptivity to receptivity in rats housed in a seminatural environment. Behav. Process. 2015, 113, 24–34. [Google Scholar] [CrossRef]
- Chu, X.; Snoeren, E.; Ågmo, A. Functions of vocalization in sociosexual behaviors in rats (Rattus norvegicus) in a seminatural environment. J. Comp. Psychol. 2017, 131, 10. [Google Scholar] [CrossRef]
- Le Moëne, O.; Hernández-Arteaga, E.; Chu, X.; Ågmo, A. Rapid changes in sociosexual behaviors around transition to and from behavioral estrus, in female rats housed in a seminatural environment. Behav. Process. 2020, 174, 104101. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Smith, W.J.; Coopersmith, C.B. Appetitive and Consummatory Sexual Behaviors of Female Rats in Bilevel Chambers: I. A Correlational and Factor Analysis and the Effects of Ovarian Hormones. Horm. Behav. 1999, 35, 224–240. [Google Scholar] [CrossRef] [PubMed]
- Pfaus, J.G.; Mendelson, S.D.; Phillips, A.G. A correlational and factor analysis of anticipatory and consummatory measures of sexual behavior in the male rat. Psychoneuroendocrinology 1990, 15, 329–340. [Google Scholar] [CrossRef]
- Mendelson, S.D.; Gorzalka, B.B. An improved chamber for the observation and analysis of the sexual behavior of the female rat. Physiol. Behav. 1987, 39, 67–71. [Google Scholar] [CrossRef]
- Ferreira-Nuño, A.; Morales-Otal, A.; Paredes, R.G.; Velázquez-Moctezuma, J. Sexual behavior of female rats in a multiple-partner preference test. Horm. Behav. 2005, 47, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Lovell, J.L.; Diehl, A.; Joyce, E.; Cohn, J.; Lopez, J.; Guarraci, F.A. “Some guys have all the luck”: Mate preference influences paced-mating behavior in female rats. Physiol. Behav. 2007, 90, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Winland, C.; Bolton, J.L.; Ford, B.; Jampana, S.; Tinker, J.; Frohardt, R.J.; Guarraci, F.A.; Zewail-Foote, M. “Nice guys finish last”: Influence of mate choice on reproductive success in Long–Evans rats. Physiol. Behav. 2012, 105, 868–876. [Google Scholar] [CrossRef]
- Zewail-Foote, M.; Diehl, A.; Benson, A.; Lee, K.H.; Guarraci, F.A. Reproductive success and mate choice in Long–Evans rats. Physiol. Behav. 2009, 96, 98–103. [Google Scholar] [CrossRef]
- Berridge, K.C. Motivation concepts in behavioral neuroscience. Physiol. Behav. 2004, 81, 179–209. [Google Scholar] [CrossRef]
- Bermant, G. Response Latencies of Female Rats during Sexual Intercourse. Science 1961, 133, 1771–1773. [Google Scholar] [CrossRef]
- Uphouse, L.; Pinkston, J.; Baade, D.; Solano, C.; Onaiwu, B. Use of an operant paradigm for the study of antidepressant-induced sexual dysfunction. Behav. Pharmacol. 2015, 26, 697–705. [Google Scholar] [CrossRef]
- Ågmo, A. Unconditioned sexual incentive motivation in the male Norway Rat (Rattus norvegicus). J. Comp. Psychol. 2003, 117, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Agrati, D.; Machado, L.; Delgado, H.; Uriarte, N.; Zuluaga, M.J.; Ferreira, A. Sexual behaviour of the female rat during late adolescence: Effect of chronic cocaine treatment. Behav. Pharmacol. 2019, 30, 396–404. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.G. Evaluating the neurobiology of sexual reward. ILAR J. 2009, 50, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Matuszczyk, J.V.; Larsson, K. Role of androgen, estrogen and sexual experience on the female rat’s partner preference. Physiol. Behav. 1991, 50, 139–142. [Google Scholar] [CrossRef]
- Clark, A.S.; Kelton, M.C.; Guarraci, F.A.; Clyons, E.Q. Hormonal status and test condition, but not sexual experience, modulate partner preference in female rats. Horm. Behav. 2004, 45, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Agrati, D.; Fernández-Guasti, A.; Ferreira, A. The reproductive stage and experience of sexually receptive mothers alter their preference for pups or males. Behav. Neurosci. 2008, 122, 998. [Google Scholar] [CrossRef]
- Guarraci, F.A.; Gonzalez, C.M.F.; Lucero, D.; Davis, L.K.; Meerts, S.H. Sexual Behavior is Enhanced by Regular, Repeated Mating from Young Adulthood to Middle Age in Female Long-Evans Rats. Curr. Aging Sci. 2020, 13, 169–177. [Google Scholar] [CrossRef]
- Paredes-Ramos, P.; Miquel, M.; Manzo, J.; Pfaus, J.G.; López-Meraz, M.L.; Coria-Avila, G.A. Tickling in juvenile but not adult female rats conditions sexual partner preference. Physiol. Behav. 2012, 107, 17–25. [Google Scholar] [CrossRef]
- Meerts, S.H.; Clark, A.S. Artificial vaginocervical stimulation induces a conditioned place preference in female rats. Horm. Behav. 2009, 55, 128–132. [Google Scholar] [CrossRef]
- Parada, M.; Chamas, L.; Censi, S.; Coria-Avila, G.; Pfaus, J.G. Clitoral stimulation induces conditioned place preference and Fos activation in the rat. Horm. Behav. 2010, 57, 112–118. [Google Scholar] [CrossRef]
- Pfaus, J.G.; Scardochio, T.; Parada, M.; Gerson, C.; Quintana, G.R.; Coria-Avila, G.A. Do rats have orgasms? Socioaffect. Neurosci. Psychol. 2016, 6, 31883. [Google Scholar] [CrossRef] [PubMed]
- Clemens, L.G.; Yang, L.-Y. MPOA lesions affect female pacing of copulation in rats. Behav. Neurosci. 2000, 114, 1191. [Google Scholar] [CrossRef]
- Xiao, K.; Kondo, Y.; Sakuma, Y. Differential regulation of female rat olfactory preference and copulatory pacing by the lateral septum and medial preoptic area. Neuroendocrinology 2005, 81, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Kondo, Y.; Sakuma, Y. The Medial Amygdala Controls Coital Access of Female Rats: A Possible Involvement of Emotional Responsiveness. Jpn. J. Physiol. 2005, 55, 0601160021. [Google Scholar] [CrossRef] [PubMed]
- Guarraci, F.A.; Megroz, A.B.; Clark, A.S. Paced mating behavior in the female rat following lesions of three regions responsive to vaginocervical stimulation. Brain Res. 2004, 999, 40–52. [Google Scholar] [CrossRef]
- Clark, A.S.; Pfeifle, J.K.; Edwards, D.A. Ventromedial hypothalamic damage and sexual proceptivity in female rats. Physiol. Behav. 1981, 27, 597–602. [Google Scholar] [CrossRef]
- Emery, D.E.; Moss, R.L. Lesions confined to the ventromedial hypothalamus decrease the frequency of coital contacts in female rats. Horm. Behav. 1984, 18, 313–329. [Google Scholar] [CrossRef]
- García-Horsman, S.P.; Ǻgmo, A.; Paredes, R.G. Infusions of naloxone into the medial preoptic area, ventromedial nucleus of the hypothalamus, and amygdala block conditioned place preference induced by paced mating behavior. Horm. Behav. 2008, 54, 709–716. [Google Scholar] [CrossRef]
- Coria-Avila, G.A.; Manzo, J.; Garcia, L.I.; Carrillo, P.; Miquel, M.; Pfaus, J.G. Neurobiology of social attachments. Neurosci. Biobehav. Rev. 2014, 43, 173–182. [Google Scholar] [CrossRef]
- Stolzenberg, D.S.; Numan, M. Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci. Biobehav. Rev. 2011, 35, 826–847. [Google Scholar] [CrossRef]
- Ventura-Aquino, E.; Portillo, W.; Paredes, R.G. Sexual motivation: A comparative approach in vertebrate species. Curr. Sex. Health Rep. 2018, 10, 114–123. [Google Scholar] [CrossRef]
- Pfaus, J.G. REVIEWS: Pathways of Sexual Desire. J. Sex. Med. 2009, 6, 1506–1533. [Google Scholar] [CrossRef] [PubMed]
- Paredes, R.G.; Ågmo, A. Has dopamine a physiological role in the control of sexual behavior?: A critical review of the evidence. Prog. Neurobiol. 2004, 73, 179–225. [Google Scholar] [CrossRef] [PubMed]
- Holder, M.K.; Blaustein, J.D. Puberty and adolescence as a time of vulnerability to stressors that alter neurobehavioral processes. Front. Neuroendocrinol. 2014, 35, 89–110. [Google Scholar] [CrossRef]
- Södersten, P. Receptive behavior in developing female rats. Horm. Behav. 1975, 6, 307–317. [Google Scholar] [CrossRef]
- Brenhouse, H.C.; Andersen, S.L. Developmental trajectories during adolescence in males and females: A cross-species understanding of underlying brain changes. Neurosci. Biobehav. Rev. 2011, 35, 1687–1703. [Google Scholar] [CrossRef]
- Spear, L.P. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Connor, J.R.; Davis, H.N. Postpartum Estrus in Norway Rats. I. Behavior. Biol. Reprod. 1980, 23, 994–999. [Google Scholar] [CrossRef]
- Gilbert, A.N.; Pelchat, R.J.; Adler, N.T. Postpartum copulatory and maternal behaviour in Norway rats under seminatural conditions. Anim. Behav. 1980, 28, 989–995. [Google Scholar] [CrossRef]
- Gilbert, A.N.; Pelchat, R.J.; Adler, N.T. Sexual and maternal behaviour at the postpartum oestrus: The role of experience in time-sharing. Anim. Behav. 1984, 32, 1045–1053. [Google Scholar] [CrossRef]
- McGinnis, M.Y.; Vakulenko, M. Characterization of 50-kHz ultrasonic vocalizations in male and female rats. Physiol. Behav. 2003, 80, 81–88. [Google Scholar] [CrossRef]
- Schulz, K.M.; Sisk, C.L. The organizing actions of adolescent gonadal steroid hormones on brain and behavioral development. Neurosci. Biobehav. Rev. 2016, 70, 148–158. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.; Romeo, R.D.; Andersen, S.L. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacol. Biochem. Behav. 2009, 93, 199–211. [Google Scholar] [CrossRef] [PubMed]
- Panksepp, J. The ontogeny of play in rats. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 1981, 14, 327–332. [Google Scholar] [CrossRef]
- Doremus-Fitzwater, T.L.; Varlinskaya, E.I.; Spear, L.P. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain Cogn. 2010, 72, 114–123. [Google Scholar] [CrossRef]
- Simon, N.W.; Moghaddam, B. Neural processing of reward in adolescent rodents. Dev. Cogn. Neurosci. 2015, 11, 145–154. [Google Scholar] [CrossRef]
- Teicher, M.H.; Andersen, S.L.; Polcari, A.; Anderson, C.M.; Navalta, C.P. Developmental neurobiology of childhood stress and trauma. Psychiatr. Clin. 2002, 25, 397–426. [Google Scholar] [CrossRef]
- Sisk, C.L.; Foster, D.L. The neural basis of puberty and adolescence. Nat. Neurosci. 2004, 7, 1040–1047. [Google Scholar] [CrossRef]
- Hashizume, K.; Ōhashi, K. Timing of sexual receptivity and the release of gonadotrophins during puberty in female rats. Reproduction 1984, 72, 87–91. [Google Scholar] [CrossRef]
- Hansen, S. Mounting behavior and receptive behavior in developing female rats and the effect of social isolation. Physiol. Behav. 1977, 19, 749–752. [Google Scholar] [CrossRef]
- Meaney, M.J.; Stewart, J. A descriptive study of social development in the rat (Rattus norvegicus). Anim. Behav. 1981, 29, 34–45. [Google Scholar] [CrossRef]
- Hashizume, K.; Ohashi, K.; Hamajima, F. Adolescent pregnancy and growth of progeny in rats. Physiol. Behav. 1991, 49, 367–371. [Google Scholar] [CrossRef]
- Hliňák, Z. Estradiol plus progesterone treatment and precopulatory behavior in prepubertally ovariectomized female rats: Dose-response relationships. Horm. Behav. 1986, 20, 263–269. [Google Scholar] [CrossRef]
- Hlinák, Z.; Madlafousek, J. Importance of Female’s Precopulatory Behaviour for the Primary Initiation of Male’s Copulatory Behaviour in the Laboratory Rat. Behaviour 1983, 86, 237–248. [Google Scholar] [CrossRef]
- Armas, M.; Marín, G.; Uriarte, N.; Agrati, D. Increase in sexual motivation throughout adolescence in the cycling female rat. Dev. Psychobiol. 2021, 63, e22162. [Google Scholar] [CrossRef]
- Hardy, D.F.; DeBold, J.F. Effects of coital stimulation upon behavior of the female rat. J. Comp. Physiol. Psychol. 1972, 78, 400–408. [Google Scholar] [CrossRef]
- Sánchez Montoya, E.L.; Hernández, L.; Barreto-Estrada, J.L.; Ortiz, J.G.; Jorge, J.C. The Testosterone Metabolite 3α-Diol Enhances Female Rat Sexual Motivation When Infused in the Nucleus Accumbens Shell. J. Sex. Med. 2010, 7, 3598–3609. [Google Scholar] [CrossRef]
- Hardy, D.F.; DeBold, J.F. Effects of repeated testing on sexual behavior of the female rat. J. Comp. Physiol. Psychol. 1973, 85, 195. [Google Scholar] [CrossRef]
- Holder, M.K.; Mong, J.A. Methamphetamine enhances paced mating behaviors and neuroplasticity in the medial amygdala of female rats. Horm. Behav. 2010, 58, 519–525. [Google Scholar] [CrossRef]
- Douglas, L.A.; Varlinskaya, E.I.; Spear, L.P. Rewarding properties of social interactions in adolescent and adult male and female rats: Impact of social versus isolate housing of subjects and partners. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2004, 45, 153–162. [Google Scholar] [CrossRef]
- Beckmeyer, J.J.; Herbenick, D.; Fu, T.-C.; Dodge, B.; Fortenberry, J.D. Pleasure During Adolescents’ Most Recent Partnered Sexual Experience: Findings from a U.S. Probability Survey. Arch. Sex. Behav. 2021, 50, 2423–2434. [Google Scholar] [CrossRef] [PubMed]
- Olster, D.H.; Blaustein, J.D. Development of progesterone-facilitated lordosis in female guinea pigs: Relationship to neural estrogen and progestin receptors. Brain Res. 1989, 484, 168–176. [Google Scholar] [CrossRef]
- Bakker, J.; Honda, S.-I.; Harada, N.; Balthazart, J. The Aromatase Knock-Out Mouse Provides New Evidence That Estradiol Is Required during Development in the Female for the Expression of Sociosexual Behaviors in Adulthood. J. Neurosci. 2002, 22, 9104. [Google Scholar] [CrossRef]
- Schulz, K.M.; Sisk, C.L. Pubertal hormones, the adolescent brain, and the maturation of social behaviors: Lessons from the Syrian hamster. Mol. Cell. Endocrinol. 2006, 254–255, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Snoeren, E.; Södersten, P.; Ågmo, A. Sexual incentive motivation and male and female copulatory behavior in female rats given androgen from postnatal day 20. Physiol. Behav. 2021, 237, 113460. [Google Scholar] [CrossRef] [PubMed]
- de Jonge, F.H.; Muntjewerff, J.-W.; Louwerse, A.L.; van de Poll, N.E. Sexual behavior and sexual orientation of the female rat after hormonal treatment during various stages of development. Horm. Behav. 1988, 22, 100–115. [Google Scholar] [CrossRef]
- Hlinák, Z. Oestradiol and progesterone treatment and precopulatory behaviour in female rats ovariectomized at different ages. Physiol. Bohemoslov. 1985, 34, 373–380. [Google Scholar]
- Andersen, S.L.; Thompson, A.P.; Krenzel, E.; Teicher, M.H. Pubertal changes in gonadal hormones do not underlie adolescent dopamine receptor overproduction. Psychoneuroendocrinology 2002, 27, 683–691. [Google Scholar] [CrossRef]
- Dewsbury, D.A. Modes of estrus induction as a factor in studies of the reproductive behavior of rodents. Neurosci. Biobehav. Rev. 1990, 14, 147–155. [Google Scholar] [CrossRef]
- Blandau, R.J.; Soderwall, A.L. Post-parturitional heat and the time of ovulation in the albino rat. Data on parturition. Anat. Rec. 1941, 81, 419–431. [Google Scholar] [CrossRef]
- Connor, J.R.; Davis, H.N. Postpartum Estrus in Norway Rats. II. Physiology. Biol. Reprod. 1980, 23, 1000–1006. [Google Scholar] [CrossRef] [PubMed]
- Ferreño, M.; Pose, S.; Agrati, D.; Zuluaga, M.J.; Ferreira, A.; Uriarte, N. Incentive value of newborn pups relative to juveniles for mother rats raising overlapping litters. Behav. Process. 2018, 157, 333–336. [Google Scholar] [CrossRef] [PubMed]
- Pose, S.; Zuluaga, M.J.; Ferreño, M.; Agrati, D.; Bedó, G.; Uriarte, N. Raising overlapping litters: Differential activation of rat maternal neural circuitry after interacting with newborn or juvenile pups. J. Neuroendocrinol. 2019, 31, e12701. [Google Scholar] [CrossRef]
- Uriarte, N.; Ferreira, A.; Rosa, X.F.; Lucion, A.B. Effects of litter-overlapping on emotionality, stress response, and reproductive functions in male and female rats. Dev. Psychobiol. J. Int. Soc. Dev. Psychobiol. 2009, 51, 259–267. [Google Scholar] [CrossRef] [PubMed]
- Uriarte, N.; Ferreira, A.; Rosa, X.F.; Sebben, V.; Lucion, A.B. Overlapping litters in rats: Effects on maternal behavior and offspring emotionality. Physiol. Behav. 2008, 93, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- HAszuME, K.; SUGAwARA, S. Studies on the Reproductive Phenomena in the Post-partum Rat II. Ovulating Hormone Release in the Post-partum Ovulation in Rat. Tohoku J. Agric. Res. 1974, 24, 123–127. [Google Scholar]
- Ying, S.-Y.; Gove, S.; Fang, V.S.; Greep, R.O. Ovulation in Postpartum Rats1. Endocrinology 1973, 92, 108–116. [Google Scholar] [CrossRef]
- Takiguchi, S.; Sugino, N.; Esato, K.; Karube-Harada, A.; Sakata, A.; Nakamura, Y.; Ishikawa, H.; Kato, H. Differential regulation of apoptosis in the corpus luteum of pregnancy and newly formed corpus luteum after parturition in rats. Biol. Reprod. 2004, 70, 313–318. [Google Scholar] [CrossRef][Green Version]
- Hoffmann, J.C.; Schwartz, N.B. Timing of Post-partum Ovulation in the Rat1. Endocrinology 1965, 76, 620–625. [Google Scholar] [CrossRef]
- Fox, S.R.; Susan Smith, M. Postpartum Preovulatory Surge of Gonadotropin Secretion in the Rat May Be Initiated by the Labor Process1. Biol. Reprod. 1984, 31, 619–626. [Google Scholar] [CrossRef]
- de la Iglesia, H.O.; Schwartz, W.J. Minireview: Timely ovulation: Circadian regulation of the female hypothalamo-pituitary-gonadal axis. Endocrinology 2006, 147, 1148–1153. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Martínez, G.E.; Gómora-Arrati, P.; González-Arenas, A.; Morimoto, S.; Camacho-Arroyo, I.; González-Flores, O. Role of progesterone receptors during postpartum estrus in rats. Horm. Behav. 2011, 59, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Hedricks, C.; McClintock, M.K. The timing of mating by postpartum estrous rats. Z. Tierpsychol. 1985, 67, 1–16. [Google Scholar] [CrossRef]
- Yoshida, T.; SUZUKI, H.; HATTORI, Y.; NODA, K. Hormonal changes around the parturition in rats. Tohoku J. Exp. Med. 1981, 135, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Sodersten, P.; Eneroth, P. Serum levels of oestradiol-17β and progesterone in relation to sexual receptivity in intact and ovariectomized rats. J. Endocrinol. 1981, 89, 45–54. [Google Scholar] [CrossRef]
- Carrillo-Martínez, G.E.; Gómora-Arrati, P.; González-Arenas, A.; Roldán-Roldán, G.; González-Flores, O.; Camacho-Arroyo, I. Effects of RU486 in the expression of progesterone receptor isoforms in the hypothalamus and the preoptic area of the rat during postpartum estrus. Neurosci. Lett. 2011, 504, 127–130. [Google Scholar] [CrossRef]
- Telleria, C.M.; Mezzadri, M.R.; Deis, R.P. Fertility impairment after mifepristone treatment to rats at proestrus: Actions on the hypothalamic-hypophyseal-ovarian axis. Contraception 1997, 56, 267–274. [Google Scholar] [CrossRef]
- Ferreira, A.; Agrati, D.; Uriarte, N.; Pereira, M.; Zuluaga, M.J. The rat as a model for studying maternal behavior. In Behavioral Animal Models; Research Signpost: Kerala, India, 2012; pp. 73–88. [Google Scholar]
- Pereira, M.; Ferreira, A. Neuroanatomical and neurochemical basis of parenting: Dynamic coordination of motivational, affective and cognitive processes. Horm. Behav. 2016, 77, 72–85. [Google Scholar] [CrossRef]
- Fleming, A.S.; Korsmit, M.; Deller, M. Rat pups are potent reinforcers to the maternal animal: Effects of experience, parity, hormones, and dopamine function. Psychobiology 1994, 22, 44–53. [Google Scholar] [CrossRef]
- Lee, A.; Clancy, S.; Fleming, A.S. Mother rats bar-press for pups: Effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav. Brain Res. 1999, 100, 15–31. [Google Scholar] [CrossRef]
- Paredes, R.G.; Vazquez, B. What do female rats like about sex? Paced mating. Behav. Brain Res. 1999, 105, 117–127. [Google Scholar] [CrossRef]
- Erskine, M.S.; Barfield, R.J.; Goldman, B.D. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav. Biol. 1978, 23, 206–218. [Google Scholar] [CrossRef]
- Ferreira, A.; Hansen, S. Sensory control of maternal aggression in Rattus norvegicus. J. Comp. Psychol. 1986, 100, 173. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, R.M.M.; Ferreira, A.; Agrati, D. Sensory, hormonal, and neural basis of maternal aggression in rodents. Neurosci. Aggress. 2014, 17, 111–130. [Google Scholar] [CrossRef]
- Agrati, D.; Fernández-Guasti, A.; Ferreno, M.; Ferreira, A. Coexpression of sexual behavior and maternal aggression: The ambivalence of sexually active mother rats toward male intruders. Behav. Neurosci. 2011, 125, 446. [Google Scholar] [CrossRef]
- Pereira, M.; Ferreira, A. Demanding pups improve maternal behavioral impairments in sensitized and haloperidol-treated lactating female rats. Behav. Brain Res. 2006, 175, 139–148. [Google Scholar] [CrossRef]
- Bridges, R.S. Long-term alterations in neural and endocrine processes induced by motherhood in mammals. Horm. Behav. 2016, 77, 193–203. [Google Scholar] [CrossRef]
- Agrati, D.; Ferreño, M.; Marin, G.; Uriarte, N.; Zuluaga, M.J.; Fernández-Guasti, A.; Ferreira, A. Previous and recent maternal experiences modulate pups’ incentive value relative to a male without affecting maternal behavior in postpartum estrous rats. J. Physiol. 2016, 110, 140–148. [Google Scholar] [CrossRef]
- Graham, M.D.; Pfaus, J.G. Differential regulation of female sexual behaviour by dopamine agonists in the medial preoptic area. Pharmacol. Biochem. Behav. 2010, 97, 284–292. [Google Scholar] [CrossRef]
- Graham, M.D.; Pfaus, J.G. Differential effects of dopamine antagonists infused to the medial preoptic area on the sexual behavior of female rats primed with estrogen and progesterone. Pharmacol. Biochem. Behav. 2012, 102, 532–539. [Google Scholar] [CrossRef]
- Matuszewich, L.; Lorrain, D.S.; Hull, E.M. Dopamine release in the medial preoptic area of female rats in response to hormonal manipulation and sexual activity. Behav. Neurosci. 2000, 114, 772. [Google Scholar] [CrossRef] [PubMed]
- Numan, M.; Numan, M.J.; Pliakou, N.; Stolzenberg, D.S.; Mullins, O.J.; Murphy, J.M.; Smith, C.D. The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav. Neurosci. 2005, 119, 1588. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.M.; Lonstein, J.S. Dopamine d1 and d2 receptor antagonism in the preoptic area produces different effects on maternal behavior in lactating rats. Behav. Neurosci. 2005, 119, 1072. [Google Scholar] [CrossRef] [PubMed]
- Mileva-Seitz, V.; Afonso, V.M.; Fleming, A.S. Dopamine: Another “magic bullet” for caregiver responsiveness? In Evolution, Early Experience and Human Development: From Research to Practice and Policy; Oxford University Press: New York, NY, USA, 2013; pp. 152–178. [Google Scholar]
- Numan, M.; Stolzenberg, D.S. Medial preoptic area interactions with dopamine neural systems in the control of the onset and maintenance of maternal behavior in rats. Front. Neuroendocrinol. 2009, 30, 46–64. [Google Scholar] [CrossRef]
- Salamone, J.D.; Correa, M. Motivational views of reinforcement: Implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav. Brain Res. 2002, 137, 3–25. [Google Scholar] [CrossRef]
- Hosking, J.G.; Floresco, S.B.; Winstanley, C.A. Dopamine antagonism decreases willingness to expend physical, but not cognitive, effort: A comparison of two rodent cost/benefit decision-making tasks. Neuropsychopharmacology 2015, 40, 1005–1015. [Google Scholar] [CrossRef]
- Correa, M.; Pardo, M.; Bayarri, P.; López-Cruz, L.; San Miguel, N.; Valverde, O.; Ledent, C.; Salamone, J.D. Choosing voluntary exercise over sucrose consumption depends upon dopamine transmission: Effects of haloperidol in wild type and adenosine A2AKO mice. Psychopharmacology 2016, 233, 393–404. [Google Scholar] [CrossRef]
- Ferreño, M.; Uriarte, N.; Zuluaga, M.J.; Ferreira, A.; Agrati, D. Dopaminergic activity mediates pups’ over male preference of postpartum estrous rats. Physiol. Behav. 2018, 188, 134–139. [Google Scholar] [CrossRef]
- Hernández-Munive, A.K.; Rebolledo-Solleiro, D.; Fernández-Guasti, A. Reduced sexual motivation of diabetic female rats: Restoration with insulin. Horm. Behav. 2021, 132, 104992. [Google Scholar] [CrossRef]
- Ventura-Aquino, E.; Fernández-Guasti, A. Reduced proceptivity and sex-motivated behaviors in the female rat after repeated copulation in paced and non-paced mating: Effect of changing the male. Physiol. Behav. 2013, 120, 70–76. [Google Scholar] [CrossRef]
- Guarraci, F.A.; Bolton, J.L. “Sexy stimulants”: The interaction between psychomotor stimulants and sexual behavior in the female brain. Pharmacol. Biochem. Behav. 2014, 121, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.L.; Ismail, N.; Pfaus, J.G. Facilitation of sexual behavior in ovariectomized rats by estradiol and testosterone: A preclinical model of androgen effects on female sexual desire. Psychoneuroendocrinology 2017, 79, 122–133. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrati, D. Adolescence and Postpartum: Two Life Periods to Deepen Our Understanding of the Complexity of Female Rat Sexual Behavior. Sexes 2022, 3, 282-297. https://doi.org/10.3390/sexes3020022
Agrati D. Adolescence and Postpartum: Two Life Periods to Deepen Our Understanding of the Complexity of Female Rat Sexual Behavior. Sexes. 2022; 3(2):282-297. https://doi.org/10.3390/sexes3020022
Chicago/Turabian StyleAgrati, Daniella. 2022. "Adolescence and Postpartum: Two Life Periods to Deepen Our Understanding of the Complexity of Female Rat Sexual Behavior" Sexes 3, no. 2: 282-297. https://doi.org/10.3390/sexes3020022
APA StyleAgrati, D. (2022). Adolescence and Postpartum: Two Life Periods to Deepen Our Understanding of the Complexity of Female Rat Sexual Behavior. Sexes, 3(2), 282-297. https://doi.org/10.3390/sexes3020022






