Sex Differences in Anxiety and Depression: What Can (and Cannot) Preclinical Studies Tell Us?
Abstract
:1. Introduction
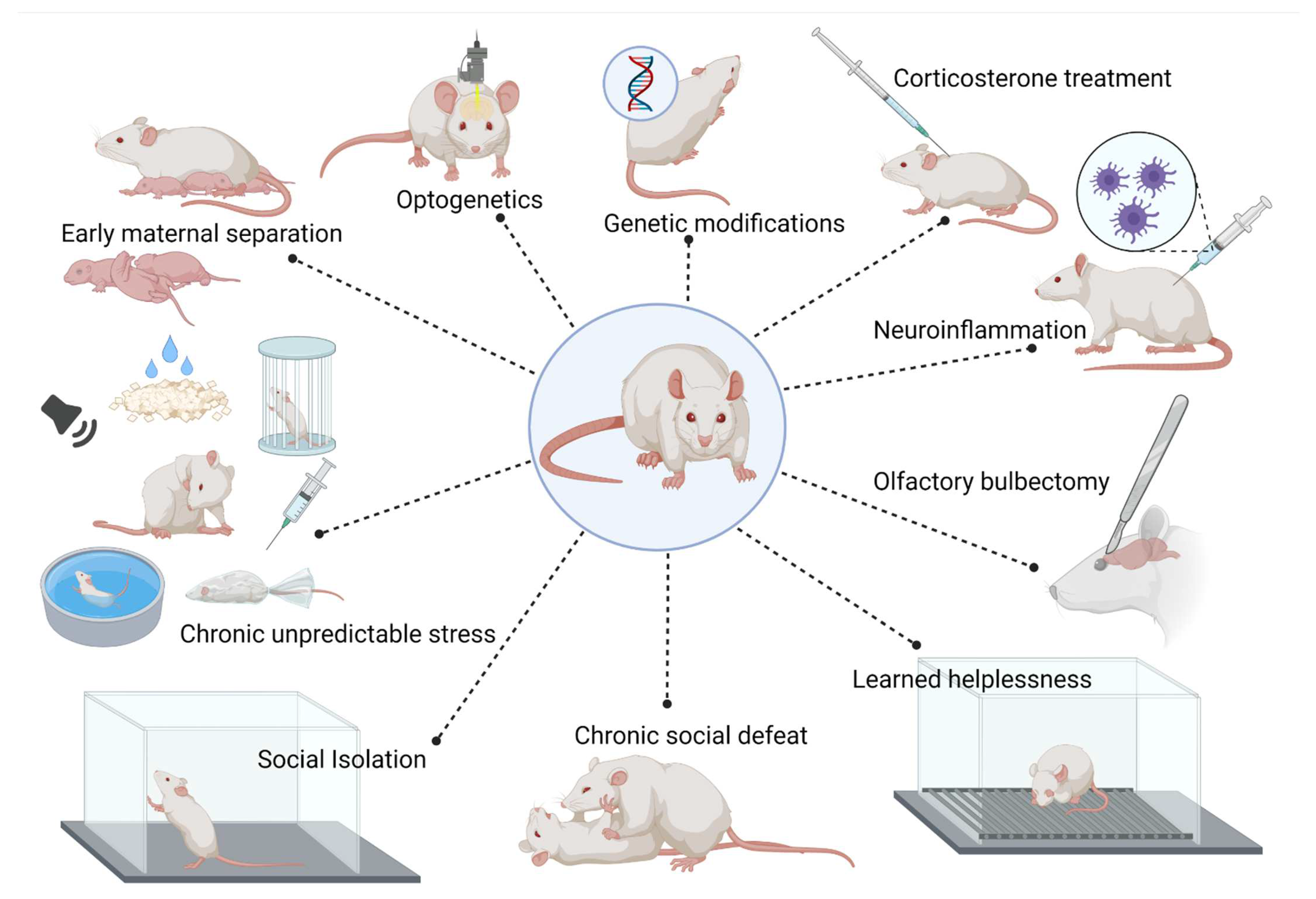
2. Animal Models of Anxiety and Depressive Disorders
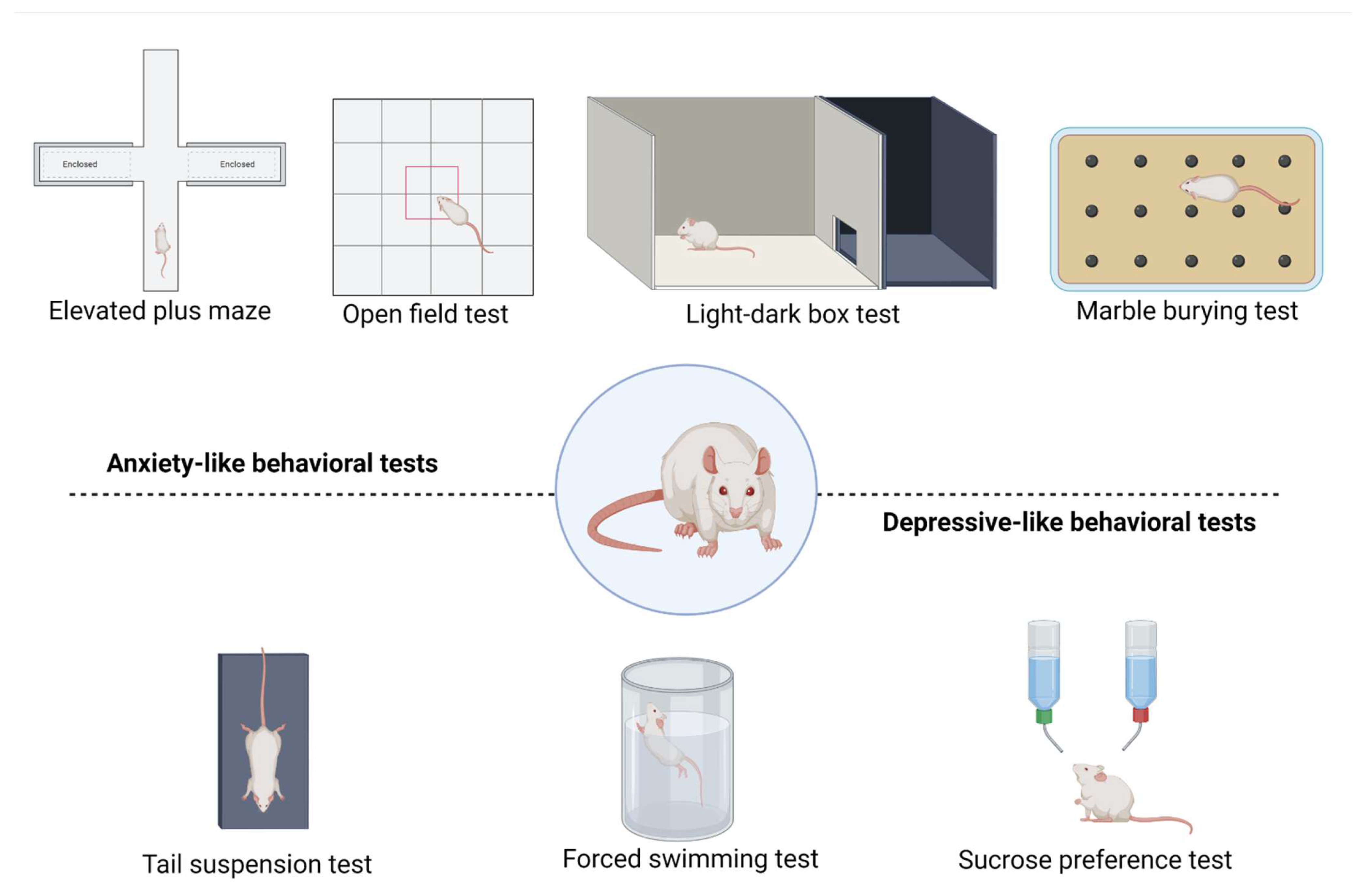
3. Outcomes of Anxiety-like and Depressive-like Behavior Tests
4. Sex Differences under the Scope of the “Unified Model” of Brain Sexual Differentiation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Salk, R.H.; Hyde, J.S.; Abramson, L.Y.; Hyde, J.S.; Abramson, L.Y. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol. Bull. 2017, 143, 783–822. [Google Scholar] [CrossRef]
- Bekker, M.H.J.; van Mens-Verhulst, J. Anxiety Disorders: Sex Differences in Prevalence, Degree, and Background, But Gender-Neutral Treatment. Gend. Med. 2007, 4, S178–S193. [Google Scholar] [CrossRef]
- World Health Organization. Depression and Other Common Mental Disorders: Global Health Estimates; World Health Organization: Geneva, Switzerland, 2017.
- McCarthy, M.M.; Arnold, A.P.; Ball, G.F.; Blaustein, J.D.; de Vries, G.J. Sex differences in the brain: The not so inconvenient truth. J. Neurosci. 2012, 32, 2241–2247. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.R.; Marks, C.; Becker, J.B.; Hurn, P.D.; Chen, W.J.; Woodruff, T.; McCarthy, M.M.; Sohrabji, F.; Schiebinger, L.; Lee Wetherington, C.; et al. Considering sex as a biological variable in preclinical research. FASEB J. 2017, 31, 29–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, M.M.; Arnold, A.P. Reframing sexual differentiation of the brain. Nat. Neurosci. 2011, 14, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Bangasser, D.A.; Valentino, R.J. Sex differences in stress-related psychiatric disorders: Neurobiological perspectives. Front. Neuroendocrinol. 2014, 35, 303–319. [Google Scholar] [CrossRef] [Green Version]
- Kokras, N.; Dalla, C. Sex differences in animal models of psychiatric disorders. Br. J. Pharmacol. 2014, 171, 4595–4619. [Google Scholar] [CrossRef]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Pub: Washington, DC, USA, 2013. [Google Scholar]
- Nestler, E.J.; Hyman, S.E. Animal models of neuropsychiatric disorders. Nat. Neurosci. 2010, 13, 1161–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belzung, C.; Lemoine, M. Criteria of validity for animal models of psychiatric disorders: Focus on anxiety disorders and depression. Biol. Mood Anxiety Disord. 2011, 1, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salgado, J.V.; Sandner, G. A critical overview of animal models of psychiatric disorders: Challenges and perspectives. Rev. Bras. Psiquiatr. 2013, 35 (Suppl. 2), 77–81. [Google Scholar] [CrossRef] [Green Version]
- Eid, R.S.; Gobinath, A.R.; Galea, L.A.M. Sex differences in depression: Insights from clinical and preclinical studies. Prog. Neurobiol. 2019, 176, 86–102. [Google Scholar] [CrossRef] [PubMed]
- Angst, J.; Gamma, A.; Gastpar, M.; Lépine, J.P.; Mendlewicz, J.; Tylee, A. Gender differences in depression: Epidemiological findings from the European DEPRES I and II Studies. Eur. Arch. Psychiatry Clin. Neurosci. 2002, 252, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Goel, N.; Bale, T.L. Examining the intersection of sex and stress in modelling neuropsychiatric disorders. J. Neuroendocrinol. 2009, 21, 415–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vetulani, J. Early maternal separation: A rodent model of depression and a prevailing human condition. Pharmacol. Rep. 2013, 65, 1451–1461. [Google Scholar] [CrossRef]
- Willner, P. The Chronic Mild Stress (CMS) model of depression: History, evaluation and usage. Neurobiol. Stress 2017, 6, 78–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mumtaz, F.; Khan, M.I.; Zubair, M.; Dehpour, A.R. Neurobiology and consequences of social isolation stress in animal model—A comprehensive review. Biomed. Pharmacother. 2018, 105, 1205–1222. [Google Scholar] [CrossRef]
- Wang, L.; Hou, W.; He, Z.; Yuan, W.; Yang, J.; Yang, Y.; Jia, R.; Zhu, Z.; Zhou, Y.; Tai, F. Effects of chronic social defeat on social behaviors in adult female Mandarin Voles (Microtus Mandarinus): Involvement of the oxytocin system in the nucleus accumbens. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2018, 82, 278–288. [Google Scholar] [CrossRef]
- Maier, S.F.; Seligman, M.E.P. Learned helplessness at fifty: Insights from neuroscience. Psychol. Rev. 2016, 123, 349–367. [Google Scholar] [CrossRef]
- Abelaira, H.M.; Reúus, G.Z.; Quevedo, J. Animal models as tools to study the pathophysiology of depression. Rev. Bras. Psiquiatr. 2013, 35 (Suppl. 2), 112–120. [Google Scholar] [CrossRef] [Green Version]
- Krishnadas, R.; Cavanagh, J. Depression: An inflammatory illness? J. Neurol. Neurosurg. Psychiatry 2012, 83, 495–502. [Google Scholar] [CrossRef] [Green Version]
- Planchez, B.; Surget, A.; Belzung, C. Animal models of major depression: Drawbacks and challenges. J. Neural. Transm. 2019, 126, 1383–1408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and major depressive disorder—Translating findings from humans to animal models and back. Front. Psychiatry 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- El Yacoubi, M.; Vaugeois, J.M. Genetic rodent models of depression. Curr. Opin. Pharmacol. 2007, 7, 3–7. [Google Scholar] [CrossRef]
- Urani, A.; Chourbaji, S.; Gass, P. Mutant mouse models of depression: Candidate genes and current mouse lines. Neurosci. Biobehav. Rev. 2005, 29, 805–828. [Google Scholar] [CrossRef]
- Renoir, T.; Pang, T.; Hannan, A. Effects of environmental manipulations in genetically targeted animal models of affective disorders. Neurobiol. Dis. 2013, 57, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Czéh, B.; Fuchs, E.; Wiborg, O.; Simon, M. Animal models of major depression and their clinical implications. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 293–310. [Google Scholar] [CrossRef]
- Biselli, T.; Lange, S.S.; Sablottny, L.; Steffen, J.; Walther, A. Optogenetic and chemogenetic insights into the neurocircuitry of depression-like behaviour: A systematic review. Eur. J. Neurosci. 2021, 53, 9–38. [Google Scholar] [CrossRef]
- Muir, J.; Lopez, J.; Bagot, R.C. Wiring the depressed brain: Optogenetic and chemogenetic circuit interrogation in animal models of depression. Neuropsychopharmacology 2019, 44, 1013–1026. [Google Scholar] [CrossRef]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The elevated plus maze test for measuring anxiety-like behavior in rodents. In Pre-Clinical Models. Methods in Molecular Biology; Guest, P., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1916, pp. 56–74. [Google Scholar]
- Knight, P.; Chellian, R.; Wilson, R.; Behnood-Rod, A.; Panunzio, S.; Bruijnzeel, A.W. Sex differences in the elevated plus-maze test and large open field test in adult wistar rats. Pharmacol. Biochem. Behav. 2021, 204, 173168. [Google Scholar] [CrossRef]
- Belviranli, M.; Atalik, K.E.N.; Okudan, N.; Gökbel, H. Age and sex affect spatial and emotional behaviors in rats: The role of repeated elevated plus maze test. Neuroscience 2012, 227, 1–9. [Google Scholar] [CrossRef]
- Xiang, X.; Huang, W.; Haile, C.N.; Kosten, T.A. Hippocampal GluR1 associates with behavior in the elevated plus maze and shows sex differences. Behav. Brain Res. 2011, 222, 326–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renard, G.M.; Suárez, M.M.; Levin, G.M.; Rivarola, M.A. Sex differences in rats: Effects of chronic stress on sympathetic system and anxiety. Physiol. Behav. 2005, 85, 363–369. [Google Scholar] [CrossRef] [PubMed]
- Albrechet-Souza, L.; Schratz, C.L.; Gilpin, N.W. Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol. Sex Differ. 2020, 11, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Scholl, J.L.; Afzal, A.; Fox, L.C.; Watt, M.J.; Forster, G.L. Sex differences in anxiety-like behaviors in rats. Physiol. Behav. 2019, 211, 112670. [Google Scholar] [CrossRef] [PubMed]
- Marcondes, F.K.; Miguel, K.J.; Melo, L.L.; Spadari-Bratfisch, R.C. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiol. Behav. 2001, 74, 435–440. [Google Scholar] [CrossRef]
- Domonkos, E.; Borbélyová, V.; Csongová, M.; Bosý, M.; Kačmárová, M.; Ostatníková, D.; Hodosy, J.; Celec, P. Sex differences and sex hormones in anxiety-like behavior of aging rats. Horm. Behav. 2017, 93, 159–165. [Google Scholar] [CrossRef]
- Kulkarni, S.; Singh, K.; Bishnoi, M. Elevated Zero-Maze: A paradigm to evaluate anti-anxiety effects of drugs. Methods Find Exp. Clin. Pharm. 2007, 29, 343–348. [Google Scholar]
- Tucker, L.B.; McCabe, J.T. Behavior of male and female C57Bl/6J mice is more consistent with repeated trials in the elevated zero maze than in the elevated plus maze. Front. Behav. Neurosci. 2017, 11, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kraeuter, A.-K.; Guest, P.C.; Sarnyai, Z. The open field test for measuring locomotor activity and anxiety-like behavior. In Pre-Clinical Models. Methods in Molecular Biology; Guest, P.C., Ed.; Humana Press: New York, NY, USA, 2019; Volume 1916, pp. 99–103. [Google Scholar]
- Sturman, O.; Germain, P.L.; Bohacek, J. Exploratory rearing: A context-and stress-sensitive behavior recorded in the open-field test. Stress 2018, 21, 443–452. [Google Scholar] [CrossRef]
- An, X.L.; Zou, J.X.; Wu, R.Y.; Ying, Y.; Tai, F.D.; Zeng, S.Y.; Rui, J.; Xia, Z.; Liu, E.Q.; Hugh, B. Strain and sex differences in anxiety-like and social behaviors in C57Bl/6J and BALB/CJ mice. Exp. Anim. 2011, 60, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Carreira, M.B.; Cossio, R.; Britton, G.B. Individual and sex differences in high and low responder phenotypes. Behav. Processes 2017, 136, 20–27. [Google Scholar] [CrossRef]
- Palumbo, M.C.; Dominguez, S.; Dong, H. Sex differences in hypothalamic–pituitary–adrenal axis regulation after chronic unpredictable stress. Brain Behav. 2020, 10, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bishnoi, I.R.; Ossenkopp, K.P.; Kavaliers, M. Sex and age differences in locomotor and anxiety-like behaviors in rats: From adolescence to adulthood. Dev. Psychobiol. 2021, 63, 496–511. [Google Scholar] [CrossRef]
- Burke, N.N.; Coppinger, J.; Deaver, D.R.; Roche, M.; Finn, D.P.; Kelly, J. Sex differences and similarities in depressive- and anxiety-like behaviour in the Wistar-Kyoto Rat. Physiol. Behav. 2016, 167, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourin, M.; Hascoët, M. The Mouse Light/Dark Box Test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- De Oliveira Sergio, T.; Wetherill, L.; Kwok, C.; Khoyloo, F.; Hopf, F.W. Sex differences in specific aspects of two animal tests of anxiety-like behavior. Psychopharmacology 2021, 238, 2775–2787. [Google Scholar] [CrossRef]
- Deacon, R.M.J. Digging and marble burying in mice: Simple methods for in vivo identification of biological impacts. Nat. Protoc. 2006, 1, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Schneider, T.; Popik, P. Attenuation of estrous cycle-dependent marble burying in female rats by acute treatment with progesterone and antidepressants. Psychoneuroendocrinology 2007, 32, 651–659. [Google Scholar] [CrossRef]
- Llaneza, D.C.; Frye, C.A. Progestogens and estrogen influence impulsive burying and avoidant freezing behavior of naturally cycling and ovariectomized rats. Pharmacol. Biochem. Behav. 2009, 93, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Goel, N.; Bale, T.L. Organizational and activational effects of testosterone on masculinization of female physiological and behavioral stress responses. Endocrinology 2008, 149, 6399–6405. [Google Scholar] [CrossRef] [Green Version]
- Taylor, G.T.; Lerch, S.; Chourbaji, S. Marble burying as compulsive behaviors in male and female mice. Acta Neurobiol. Exp. 2017, 77, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Slattery, D.A.; Cryan, J.F. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat. Protoc. 2012, 7, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Kokras, N.; Antoniou, K.; Mikail, H.G.; Kafetzopoulos, V.; Papadopoulou-Daifoti, Z.; Dalla, C. Forced Swim Test: What about females? Neuropharmacology 2015, 99, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Mota, L.; Ulloa, R.E.; Herrera-Pérez, J.; Chavira, R.; Fernández-Guasti, A. Sex and age differences in the impact of the forced swimming test on the levels of steroid hormones. Physiol. Behav. 2011, 104, 900–905. [Google Scholar] [CrossRef]
- Kokras, N.; Polissidis, A.; Antoniou, K.; Dalla, C. Head Shaking in the Forced Swim Test: A robust but unexplored sex difference. Pharmacol. Biochem. Behav. 2017, 152, 90–96. [Google Scholar] [CrossRef]
- Can, A.; Dao, D.T.; Terrillion, C.E.; Piantadosi, S.C.; Bhat, S.; Gould, T.D. The Tail Suspension Test. J. Vis. Exp. 2011, 58, 2–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Wang, Y.; Zhang, Y.F. Light deprivation produces a sexual dimorphic effect on neural excitability and depression-like behavior in mice. Neurosci. Lett. 2016, 633, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Andreasen, J.T.; Redrobe, J.P. Antidepressant-like effects of nicotine and mecamylamine in the mouse forced swim and tail suspension tests: Role of strain, test and sex. Behav. Pharmacol. 2009, 20, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.D.; Lucki, I. Sex differences in the regulation of serotonergic transmission and behavior in 5-HT receptor knockout mice. Neuropsychopharmacology 2005, 30, 1039–1047. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.; An, A.Y.; Banker, C.; Qian, Y.H.; O’Donnell, J.M. Single housing-induced effects on cognitive impairment and depression-like behavior in male and female mice involve neuroplasticity-related signaling. Eur. J. Neurosci. 2020, 52, 2694–2704. [Google Scholar] [CrossRef]
- Liu, M.Y.; Yin, C.Y.; Zhu, L.J.; Zhu, X.H.; Xu, C.; Luo, C.X.; Chen, H.; Zhu, D.Y.; Zhou, Q.G. Sucrose preference test for measurement of stress-induced anhedonia in mice. Nat. Protoc. 2018, 13, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Sclafani, A.; Hertwig, H.; Vigorito, M.; Feigin, M.B. Sex differences in polysaccharide and sugar preferences in rats. Neurosci. Biobehav. Rev. 1987, 11, 241–251. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, X.; Zhou, Y.; Zheng, Q.; Chen, Z.; Zhang, H.; Sun, Z.; Xu, G.; Hu, G. Hypothalamus-pituitary-adrenal axis imbalance and inflammation contribute to sex differences in separation- and restraint-induced depression. Horm. Behav. 2020, 122, 104741. [Google Scholar] [CrossRef] [PubMed]
- Oliver, D.K.; Intson, K.; Sargin, D.; Power, S.K.; McNabb, J.; Ramsey, A.J.; Lambe, E.K. Chronic social isolation exerts opposing sex-specific consequences on serotonin neuronal excitability and behaviour. Neuropharmacology 2020, 168, 108015. [Google Scholar] [CrossRef]
- Pooley, A.E.; Benjamin, R.C.; Sreedhar, S.; Eagle, A.L.; Robison, A.J.; Mazei-Robison, M.S.; Breedlove, S.M.; Jordan, C.L. Sex differences in the traumatic stress response: The role of adult gonadal hormones. Biol. Sex Differ. 2018, 9, 1–13. [Google Scholar] [CrossRef]
- Fester, L.; Rune, G.M. Sex neurosteroids: Hormones made by the brain for the brain. Neurosci. Lett. 2021, 753, 135849. [Google Scholar] [CrossRef]
- Roselli, C.E.; Liu, M.; Hurn, P.D. Brain aromatization: Classic roles and new perspectives. Semin. Reprod. Med. 2009, 27, 207–217. [Google Scholar] [CrossRef] [Green Version]
- Schulz, K.M.; Molenda-Figueira, H.A.; Sisk, C.L. Back to the future: The organizational-activational hypothesis adapted to puberty and adolescence. Horm. Behav. 2009, 55, 597–604. [Google Scholar] [CrossRef] [Green Version]
- Crow, T.J. The XY gene hypothesis of psychosis: Origins and current status. Am. J. Med. Genet. Part B Neuropsychiatr. Genet. 2013, 162, 800–824. [Google Scholar] [CrossRef] [Green Version]
- Seney, M.L.; Logan, R.W. Critical roles for developmental hormones and genetic sex in stress-induced transcriptional changes associated with depression. Neuropsychopharmacology 2021, 46, 221–222. [Google Scholar] [CrossRef]
- Mankiw, C.; Park, M.T.M.; Reardon, P.K.; Fish, A.M.; Clasen, L.S.; Greenstein, D.; Giedd, J.N.; Blumenthal, J.D.; Lerch, J.P.; Chakravarty, M.M.; et al. Allometric analysis detects brain size-independent effects of sex and sex chromosome complement on human cerebellar organization. J. Neurosci. 2017, 37, 5221–5231. [Google Scholar] [CrossRef] [PubMed]
- Fish, A.M.; Cachia, A.; Fischer, C.; Mankiw, C.; Reardon, P.K.; Clasen, L.S.; Blumenthal, J.D.; Greenstein, D.; Giedd, J.N.; Mangin, J.F.; et al. Influences of brain size, sex, and sex chromosome complement on the architecture of human cortical folding. Cereb. Cortex 2017, 27, 5557–5567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nadig, A.; Reardon, P.K.; Seidlitz, J.; McDermott, C.L.; Blumenthal, J.D.; Clasen, L.S.; Lalonde, F.; Lerch, J.P.; Mallar Chakravarty, M.; Raznahan, A. Carriage of supernumerary sex chromosomes decreases the volume and alters the shape of limbic structures. eNeuro 2018, 5, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Cox, K.H.; Bonthuis, P.J.; Rissman, E.F. Mouse model systems to study sex chromosome genes and behavior: Relevance to humans. Front. Neuroendocrinol. 2014, 35, 405–419. [Google Scholar] [CrossRef] [Green Version]
- Arnold, A.P.; Chen, X. What does the “four core genotypes” mouse model tell us about sex differences in the brain and other tissues? Front. Neuroendocrinol. 2009, 30, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puralewski, R.; Vasilakis, G.; Seney, M.L. Sex-related factors influence expression of mood-related genes in the basolateral amygdala differentially depending on age and stress exposure. Biol. Sex Differ. 2016, 7, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Kopsida, E.; Lynn, P.M.; Humby, T.; Wilkinson, L.S.; Davies, W. Dissociable effects of sry and sex chromosome complement on activity, feeding and anxiety-related behaviours in mice. PLoS ONE 2013, 8, 1–15. [Google Scholar]
- McPhie-Lalmansingh, A.A.; Tejada, L.D.; Weaver, J.L.; Rissman, E.F. Sex chromosome complement affects social interactions in mice. Horm. Behav. 2008, 54, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Seu, E.; Groman, S.M.; Arnold, A.P.; Jentsch, J.D. Sex chromosome complement influences operant responding for a palatable food in mice. Genes Brain Behav. 2014, 13, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Schuch, J.J.J.; Roest, A.M.; Nolen, W.A.; Penninx, B.W.J.H.; de Jonge, P. Gender differences in major depressive disorder: Results from the Netherlands Study of depression and anxiety. J. Affect. Disord. 2014, 156, 156–163. [Google Scholar] [CrossRef]
- Gatewood, J.D.; Wills, A.; Shetty, S.; Xu, J.; Arnold, A.P.; Burgoyne, P.S.; Rissman, E.F. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J. Neurosci. 2006, 26, 2335–2342. [Google Scholar] [CrossRef] [Green Version]
- Barker, J.M.; Torregrossa, M.M.; Arnold, A.P.; Taylor, J.R. Dissociation of genetic and hormonal influences on sex differences in alcoholism-related behaviors. J. Neurosci. 2010, 30, 9140–9144. [Google Scholar] [CrossRef] [PubMed]
- Saunders, P.A.; Franco, T.; Sottas, C.; Maurice, T.; Ganem, G.; Veyrunes, F. Masculinised behaviour of XY females in a mammal with naturally occuring sex reversal. Sci. Rep. 2016, 6, 1–9. [Google Scholar]
- Isles, A.R.; Davies, W.; Burrmann, D.; Burgoyne, P.S.; Wilkinson, L.S. Effects on fear reactivity in XO mice are due to haploinsufficiency of a Non-PAR X gene: Implications for emotional function in turner’s syndrome. Hum. Mol. Genet. 2004, 13, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, M.M. How it’s made: Organisational effects of hormones on the developing brain. J. Neuroendocrinol. 2010, 22, 736–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, M.M.; Wright, C.L.; Schwarz, J.M. New tricks by an old dogma: Mechanisms of the organizational/activational hypothesis of steroid-mediated sexual differentiation of brain and behavior. Horm. Behav. 2009, 55, 655–665. [Google Scholar] [CrossRef] [Green Version]
- Amiel Castro, R.T.; Ehlert, U.; Fischer, S. Variation in genes and hormones of the hypothalamic-pituitary-ovarian axis in female mood disorders—A Systematic Review and Meta-analysis. Front. Neuroendocrinol. 2021, 62, 100929. [Google Scholar] [CrossRef]
- McHenry, J.; Carrier, N.; Hull, E.; Kabbaj, M. Sex differences in anxiety and depression: Role of testosterone. Front. Neuroendocrinol. 2014, 35, 42–57. [Google Scholar] [CrossRef] [Green Version]
- Seiffe, A.; Ramirez, M.F.; Barrios, C.D.; Albarrán, M.M.; Depino, A.M. Early estradiol exposure masculinizes disease-relevant behaviors in female mice. Eur. J. Neurosci. 2021, 53, 2483–2499. [Google Scholar] [CrossRef]
- Carrier, N.; Kabbaj, M. Testosterone and imipramine have antidepressant effects in socially isolated male but not female rats. Horm. Behav. 2012, 61, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Altemus, M.; Sarvaija, N.; Epperson, N. Sex differences in anxiety and depression clinical perspectives margaret. Front. Neuroendocrinol. 2014, 35, 320–330. [Google Scholar] [CrossRef] [Green Version]
- Boivin, J.R.; Piekarski, D.J.; Wahlberg, J.K.; Wilbrecht, L. Age, sex, and gonadal hormones differently influence anxiety- and depression-related behavior during puberty in mice. Psychoneuroendocrinology 2017, 85, 78–87. [Google Scholar] [CrossRef]
- Leussis, M.P.; Andersen, S.L. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse 2008, 62, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Maeng, L.Y.; Milad, M.R. Sex differences in anxiety disorders: Interactions between fear, stress, and gonadal hormones. Horm. Behav. 2015, 76, 106–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rubinow, D.R.; Schmidt, P.J. Is there a role for reproductive steroids in the etiology and treatment of affective disorders? Dialogues Clin. Neurosci. 2018, 20, 187–196. [Google Scholar] [PubMed]
- Mueller, B.R.; Bale, T.L. Sex-specific programming of offspring emotionality after stress early in pregnancy. J. Neurosci. 2008, 28, 9055–9065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, E.P.; Pfaff, D. Sexually dimorphic responses to early adversity: Implications for affective problems and autism spectrum disorder. Psychoneuroendocrinology 2014, 49, 11–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iturra-Mena, A.; Arriagada-Solimano, M.; Luttecke-Anders, A.; Dagnino-Subiabre, A. Effects of prenatal stress on anxiety- and depressive-like behaviours are sex-specific in prepubertal rats. J. Neuroendocrinol. 2018, 30, e12609. [Google Scholar] [CrossRef]
- van den Hove, D.; Leibold, N.; Strackx, E.; Martinez-Claros, M.; Lesch, K.; Steinbusch, H.; Schruers, K.; Prickaerts, J. Prenatal stress and subsequent exposure to chronic mild stress in rats; interdependent effects on emotional behavior and the serotonergic system. Eur. Neuropsychopharmacol. 2014, 24, 595–607. [Google Scholar] [CrossRef]
- Sickmann, H.M.; Arentzen, T.S.; Dyrby, T.B.; Plath, N.; Kristensen, M.P. Prenatal stress produces sex-specific changes in depression-like behavior in rats: Implications for increased vulnerability in females. J. Dev. Orig. Health Dis. 2015, 6, 462–474. [Google Scholar] [CrossRef]
- Frye, C.A.; Wawrzycki, J. Effect of prenatal stress and gonadal hormone condition on depressive behaviors of female and male rats. Horm. Behav. 2003, 44, 319–326. [Google Scholar] [CrossRef]
- Glover, V.; Hill, J. Sex differences in the programming effects of prenatal stress on psychopathology and stress responses: An evolutionary perspective. Physiol. Behav. 2012, 106, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.; Pearson, J.; Neeley, E.; Berger, R.; Leonard, S.; Adams, C.; Stevens, K. Maternal Stress during pregnancy causes sex-specific alterations in offspring memory performance, social interactions, indices of anxiety, and body mass. Physiol. Behav. 2011, 104, 340–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, S.; Brunwasser, S.M. Sex differences in vulnerability to prenatal stress: A Review of the recent literature. Curr. Psychiatry Rep. 2018, 20, 102. [Google Scholar] [CrossRef]
- Lei, L.; Wu, X.; Gu, H.; Ji, M.; Yang, J. Differences in DNA methylation reprogramming underlie the sexual dimorphism of behavioral disorder caused by prenatal stress in rats. Front. Neurosci. 2020, 14, 1–10. [Google Scholar] [CrossRef]
- Sandman, C.A.; Glynn, L.M.; Davis, E.P. Is There a viability-vulnerability tradeoff? Sex differences in fetal programming. J. Psychosom. Res. 2013, 75, 327–335. [Google Scholar] [CrossRef] [Green Version]
- Buss, C.; Davis, E.P.; Shahbaba, B.; Pruessner, J.C.; Head, K.; Sandman, C.A. Maternal cortisol over the course of pregnancy and subsequent child amygdala and hippocampus volumes and affective problems. Proc. Natl. Acad. Sci. USA 2012, 109, E1312–E1319. [Google Scholar] [CrossRef] [Green Version]
- Juruena, M.F.; Gadelrab, R.; Cleare, A.J.; Young, A.H. Epigenetics: A missing link between early life stress and depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 109, 110231. [Google Scholar] [CrossRef]
- VanTieghem, M.; Tottenham, N. Neurobiological programming of early life stress: Functional development of amygdala-prefrontal circuitry and vulnerability for stress-related psychopathology. Curr. Top. Behav. Neurosci. 2018, 38, 117–136. [Google Scholar]
- Pryce, C.R.; Feldon, J. Long-term neurobehavioural impact of the postnatal environment in rats: Manipulations, effects and mediating mechanisms. Neurosci. Biobehav. Rev. 2003, 27, 57–71. [Google Scholar] [CrossRef]
- Grissom, E.M.; Hawley, W.R.; Bromley-Dulfano, S.S.; Marino, S.E.; Stathopoulos, N.G.; Dohanich, G.P. Learning strategy is influenced by trait anxiety and early rearing conditions in prepubertal male, but not prepubertal female rats. Neurobiol. Learn. Mem. 2012, 98, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.J.; Czéh, B.; Corbach, S.; Wuttke, W.; Schulte-Herbrüggen, O.; Hellweg, R.; Flügge, G.; Fuchs, E. Chronic social instability stress in female rats: A potential animal model for female depression. Neuroscience 2009, 159, 982–992. [Google Scholar] [CrossRef] [PubMed]
- George, E.D.; Bordner, K.A.; Elwafi, H.M.; Simen, A.A. Maternal separation with early weaning: A novel mouse model of early life neglect. BMC Neurosci. 2010, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwill, H.L.; Manzano-Nieves, G.; Gallo, M.; Lee, H.-I.; Oyerinde, E.; Serre, T.; Bath, K.G. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology 2019, 44, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Amiri, S.; Haj-Mirzaian, A.; Rahimi-balaei, M.; Razmi, A.; Kordjazy, N.; Shirzadian, A.; Ejtemaei Mehr, S.; Sianati, H.; Dehpour, A.R. Co-occurrence of anxiety and depressive-like behaviors following adolescent social isolation in male mice; possible role of nitrergic system. Physiol. Behav. 2015, 145, 38–44. [Google Scholar] [CrossRef]
- Bachiller, S.; Paulus, A.; Vázquez-Reyes, S.; García-Domínguez, I.; Deierborg, T. Maternal separation leads to regional hippocampal microglial activation and alters the behavior in the adolescence in a sex-specific manner. Brain Behav. Immun. Heal. 2020, 9, 100142. [Google Scholar] [CrossRef]
- Cui, Y.; Cao, K.; Lin, H.; Cui, S.; Shen, C.; Wen, W.; Mo, H.; Dong, Z.; Bai, S.; Yang, L.; et al. Early-life stress induces depression-like behavior and synaptic-plasticity changes in a maternal separation rat model: Gender difference and metabolomics study. Front. Pharmacol. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, G.; Wang, H.; He, J.; Zhang, N.; Wu, Z.; Xiao, L.; Yang, C. Sex-dependent impact of different degrees of maternal separation experience on OFT behavioral performances after adult chronic unpredictable mild stress exposure in rats. Physiol. Behav. 2018, 194, 153–161. [Google Scholar] [CrossRef]
- Huang, J.; Shen, C.; Ye, R.; Shi, Y.; Li, W. He effect of early maternal separation combined with adolescent chronic unpredictable mild stress on behavior and synaptic plasticity in adult female rats. Front. Psychiatry 2021, 5, 539299. [Google Scholar] [CrossRef]
- Jaric, I.; Rocks, D.; Cham, H.; Herchek, A.; Kundakovic, M. Sex and estrous cycle effects on anxiety- and depression-related phenotypes in a two-hit developmental stress model. Front. Mol. Neurosci. 2019, 12, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Zhang, X.; Kong, Y.; Gou, L.; Lian, B.; Wang, Y.; Jiang, L.; Li, Q.; Sun, H.; Sun, L. Maternal separation-induced histone acetylation correlates with BDNF-programmed synaptic changes in an animal model of PTSD with sex differences. Mol. Neurobiol. 2021, 58, 1738–1754. [Google Scholar] [CrossRef] [PubMed]
- Beery, A.K.; Zucker, I. Sex bias in neuroscience and biomedical research. Neurosci. Biobehav. Rev. 2011, 35, 565–572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Will, T.R.; Proaño, S.B.; Thomas, A.M.; Kunz, L.M.; Thompson, K.C.; Ginnari, L.A.; Jones, C.H.; Lucas, S.C.; Reavis, E.M.; Dorris, D.M.; et al. Problems and progress regarding sex bias and omission in neuroscience research. eNeuro 2017, 4, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mateus-Pinheiro, A.; Patrício, P.; Alves, N.D.; Machado-Santos, A.R.; Morais, M.; Bessa, J.M.; Sousa, N.; Pinto, L. The Sweet Drive Test: Refining phenotypic characterization of anhedonic behavior in rodents. Front. Behav. Neurosci. 2014, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dalla, C.; Pitychoutis, P.; Kokras, N.; Papadopoulou-Daifoti, Z. Sex differences in response to stress and expression of depressive-like behaviours in the rat. In Biological Basis of Sex Differences in Psychopharmacology. Current Topics in Behavioral Neurosciences; Neill, J.C., Kulkarni, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 289–320. [Google Scholar]
- Dalla, C.; Edgecomb, C.; Whetstone, A.S.; Shors, T.J. Females do not express learned helplessness like males do. Neuropsychopharmacology 2008, 33, 1559–1569. [Google Scholar] [CrossRef] [Green Version]
- Kamper, E.F.; Chatzigeorgiou, A.; Tsimpoukidi, O.; Kamper, M.; Dalla, C.; Pitychoutis, P.; Papadopoulou-Daifoti, Z. Sex differences in oxidant/antioxidant balance under a chronic mild stress regime. Physiol. Behav. 2009, 98, 215–222. [Google Scholar] [CrossRef]
- Karisetty, B.C.; Joshi, P.C.; Kumar, A.; Chakravarty, S. Sex differences in the effect of chronic mild stress on mouse prefrontal cortical BDNF levels: A role of major ovarian hormones. Neuroscience 2017, 356, 89–101. [Google Scholar] [CrossRef]
- Konkle, A.T.M.; Baker, S.L.; Kentner, A.C.; Barbagallo, L.S.M.; Merali, Z.; Bielajew, C. Evaluation of the effects of chronic mild stressors on hedonic and physiological responses: Sex and strain compared. Brain Res. 2003, 992, 227–238. [Google Scholar] [CrossRef]
- Xing, Y.; He, J.; Hou, J.; Lin, F.; Tian, J.; Kurihara, H. Gender differences in CMS and the effects of antidepressant venlafaxine in rats. Neurochem. Int. 2013, 63, 570–575. [Google Scholar] [CrossRef]
- Sachs, B.; Ni, J.; Caron, M. Sex differences in response to chronic mild stress and congenital serotonin deficiency. Psychoneuroendocrinology 2014, 40, 123–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschelli, A.; Herchick, S.; Thelen, C.; Papadopoulou-Daifoti, Z.; Pitychoutis, P.M. Sex differences in the chronic mild stress model of depression. Behav. Pharmacol. 2014, 25, 372–383. [Google Scholar] [CrossRef] [PubMed]
- Caldarone, B.J.; George, T.P.; Zachariou, V.; Picciotto, M.R. Gender differences in learned helplessness behavior are influenced by genetic background. Pharmacol. Biochem. Behav. 2000, 66, 811–817. [Google Scholar] [CrossRef]
- Padilla, E.; Barrett, D.; Shumake, J.; Gonzalez-Lima, F. Strain, sex, and open-field behavior: Factors underlying the genetic susceptibility to helplessness. Behav. Brain Res. 2009, 201, 257–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, J.A.; Williams, P.; Kramer, G.L.; Davis, L.L.; Petty, F. The influence of gender and the estrous cycle on learned helplessness in the rat. Biol. Psychol. 2001, 58, 147–158. [Google Scholar] [CrossRef]
- Setnik, B.; De Souza, F.G.; D’Almeida, V.; Nobrega, J.N. Increased homocysteine levels associated with sex and stress in the learned helplessness model of depression. Pharmacol. Biochem. Behav. 2004, 77, 155–161. [Google Scholar] [CrossRef]
- Bland, S.T.; Schmid, M.J.; Der-Avakian, A.; Watkins, L.R.; Spencer, R.L.; Maier, S.F. Expression of C-Fos and BDNF MRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain Res. 2005, 1051, 90–99. [Google Scholar] [CrossRef]
- Haller, J.; Fuchs, E.; Halász, J.; Makara, G.B. Defeat is a major stressor in males while social instability is stressful mainly in females: Towards the development of a social stress model in female rats. Brain Res. Bull. 1999, 50, 33–39. [Google Scholar] [CrossRef]
- Donner, N.C.; Lowry, C.A. Sex differences in anxiety and emotional behavior. Pflug. Arch. 2013, 465, 601–626. [Google Scholar] [CrossRef]
- Koert, A.; Ploeger, A.; Bockting, C.L.H.; Schmidt, M.V.; Lucassen, P.J.; Schrantee, A.; Mul, J.D. The social instability stress paradigm in rat and mouse: A systematic review of protocols, limitations, and recommendations. Neurobiol. Stress 2021, 15, 100410. [Google Scholar] [CrossRef]
- Goñi-Balentziaga, O.; Perez-Tejada, J.; Renteria-Dominguez, A.; Lebeña, A.; Labaka, A. Social instability in female rodents as a model of stress related disorders: A systematic review. Physiol. Behav. 2018, 196, 190–199. [Google Scholar] [CrossRef]
- Jarcho, M.R.; Massner, K.J.; Eggert, A.R.; Wichelt, E.L. Behavioral and physiological response to onset and termination of social instability in female mice. Horm. Behav. 2016, 78, 135–140. [Google Scholar] [CrossRef]
- Labaka, A.; Gómez-Lázaro, E.; Vegas, O.; Pérez-Tejada, J.; Arregi, A.; Garmendia, L. Reduced hippocampal IL-10 expression, altered monoaminergic activity and anxiety and depressive-like behavior in female mice subjected to chronic social instability stress. Behav. Brain Res. 2017, 335, 8–18. [Google Scholar] [CrossRef]
- Yohn, C.N.; Ashamalla, S.A.; Bokka, L.; Gergues, M.M.; Garino, A.; Samuels, B.A. Social instability is an effective chronic stress paradigm for both male and female mice. Neuropharmacology 2019, 160, 107780. [Google Scholar] [CrossRef] [PubMed]
- Djordjevic, J.; Djordjevic, A.; Adzic, M.; Radojcic, M.B. Effects of chronic social isolation on wistar rat behavior and brain plasticity markers. Neuropsychobiology 2012, 66, 112–119. [Google Scholar] [CrossRef]
- Pisu, M.G.; Garau, A.; Boero, G.; Biggio, F.; Pibiri, V.; Dore, R.; Locci, V.; Paci, E.; Porcu, P.; Serra, M. Sex differences in the outcome of juvenile social isolation on HPA axis function in rats. Neuroscience 2016, 320, 172–182. [Google Scholar] [CrossRef]
- Hong, S.; Flashner, B.; Chiu, M.; ver Hoeve, E.; Luz, S.; Bhatnagar, S. Social isolation in adolescence alters behaviors in the forced swim and sucrose preference tests in female but not in male rats. Physiol. Behav. 2012, 105, 269–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, I.C.; Pryce, C.R.; Jongen-Rêlo, A.L.; Nanz-Bahr, N.I.; Feldon, J. Effect of social isolation on stress-related behavioural and neuroendocrine state in the rat. Behav. Brain Res. 2004, 152, 279–295. [Google Scholar] [CrossRef] [PubMed]
- Weintraub, A.; Singaravelu, J.; Bhatnagar, S. Enduring and sex-specific effects of adolescent social isolation in rats on adult stress reactivity. Brain Res. 2010, 1343, 83–92. [Google Scholar] [CrossRef]
- Bale, T.L. Stress sensitivity and the development of affective disorders. Horm. Behav. 2006, 50, 529–533. [Google Scholar] [CrossRef]
- Ennaceur, A.; Chazot, P.L. Preclinical animal anxiety research—Flaws and prejudices. Pharmacol. Res. Perspect. 2016, 4, 1–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sramek, J.J.; Murphy, M.F.; Cutler, N.R. Sex differences in the psychopharmacological treatment of depression. Dialogues Clin. Neurosci. 2016, 447–457. [Google Scholar]
- Kokras, N.; Dalla, C. Preclinical sex differences in depression and antidepressant response: Implications for clinical research. J. Neurosci. Res. 2017, 95, 731–736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, A.V.; Trainor, B.C. The impact of sex as a biological variable in the search for novel antidepressants. Front. Neuroendocrinol. 2018, 50, 107–117. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mir, F.R.; Rivarola, M.A. Sex Differences in Anxiety and Depression: What Can (and Cannot) Preclinical Studies Tell Us? Sexes 2022, 3, 141-163. https://doi.org/10.3390/sexes3010012
Mir FR, Rivarola MA. Sex Differences in Anxiety and Depression: What Can (and Cannot) Preclinical Studies Tell Us? Sexes. 2022; 3(1):141-163. https://doi.org/10.3390/sexes3010012
Chicago/Turabian StyleMir, Franco Rafael, and María Angélica Rivarola. 2022. "Sex Differences in Anxiety and Depression: What Can (and Cannot) Preclinical Studies Tell Us?" Sexes 3, no. 1: 141-163. https://doi.org/10.3390/sexes3010012
APA StyleMir, F. R., & Rivarola, M. A. (2022). Sex Differences in Anxiety and Depression: What Can (and Cannot) Preclinical Studies Tell Us? Sexes, 3(1), 141-163. https://doi.org/10.3390/sexes3010012





