Author Contributions
Conceptualization, L.J.T. and P.N.; methodology, L.J.T., P.N. and J.I.A.; software, L.J.T., P.N.; validation, E.M. and J.I.A.; investigation, E.M., D.C.; data curation, R.S.; writing—original draft preparation, P.N., D.C.; writing—review and editing, L.J.T., P.N., J.I.A.; visualization, R.S.; All authors have read and agreed to the published version of the manuscript.
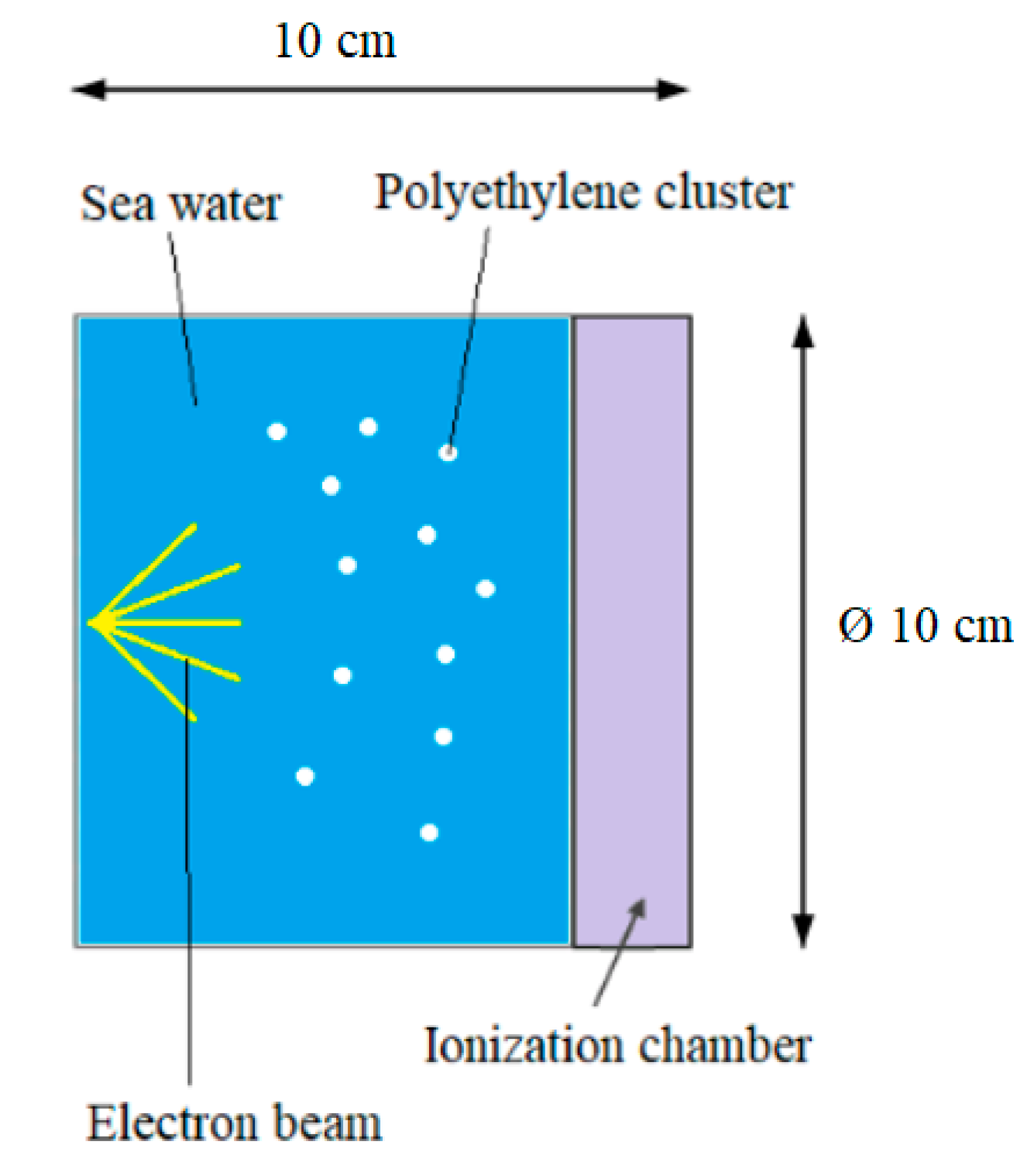
Figure 1.
Physical model x-z section of ocean water and polyethylene.
Figure 1.
Physical model x-z section of ocean water and polyethylene.
Figure 2.
Geometrical model of x-z section.
Figure 2.
Geometrical model of x-z section.
Figure 3.
Volumetric cluster cells in 3D.
Figure 3.
Volumetric cluster cells in 3D.
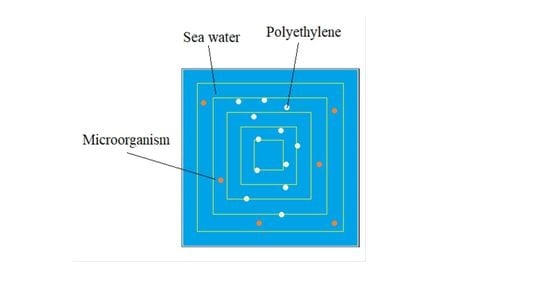
Figure 4.
Ocean water polyethylene plus microorganisms, x-z section model.
Figure 4.
Ocean water polyethylene plus microorganisms, x-z section model.
Figure 5.
Carbon total photon cross section as a function of energy.
Figure 5.
Carbon total photon cross section as a function of energy.
Figure 6.
Carbon incoherent photon cross section as a function of energy.
Figure 6.
Carbon incoherent photon cross section as a function of energy.
Figure 7.
Carbon coherent photon cross section as a function of energy.
Figure 7.
Carbon coherent photon cross section as a function of energy.
Figure 8.
Carbon photoelectric photon cross section as a function of energy.
Figure 8.
Carbon photoelectric photon cross section as a function of energy.
Figure 9.
Carbon pair production photon cross section as a function of energy.
Figure 9.
Carbon pair production photon cross section as a function of energy.
Figure 10.
Oxygen total photon cross section as a function of energy.
Figure 10.
Oxygen total photon cross section as a function of energy.
Figure 11.
Oxygen incoherent photon cross section as a function of energy.
Figure 11.
Oxygen incoherent photon cross section as a function of energy.
Figure 12.
Oxygen coherent photon cross section as a function of energy.
Figure 12.
Oxygen coherent photon cross section as a function of energy.
Figure 13.
Oxygen photoelectric photon cross section as a function of energy.
Figure 13.
Oxygen photoelectric photon cross section as a function of energy.
Figure 14.
Oxygen pair production photon cross section as a function of energy.
Figure 14.
Oxygen pair production photon cross section as a function of energy.
Figure 15.
Phosphorus total photon cross section as a function of energy.
Figure 15.
Phosphorus total photon cross section as a function of energy.
Figure 16.
Phosphorus incoherent photon cross section as a function of energy.
Figure 16.
Phosphorus incoherent photon cross section as a function of energy.
Figure 17.
Phosphorus coherent photon cross section as a function of energy.
Figure 17.
Phosphorus coherent photon cross section as a function of energy.
Figure 18.
Phosphorus photoelectric photon cross section as a function of energy.
Figure 18.
Phosphorus photoelectric photon cross section as a function of energy.
Figure 19.
Phosphorus pair production photon cross section as a function of energy.
Figure 19.
Phosphorus pair production photon cross section as a function of energy.
Figure 20.
Ocean water total electron stopping power as a function of energy.
Figure 20.
Ocean water total electron stopping power as a function of energy.
Figure 21.
Ocean water total photon cross section as a function of energy.
Figure 21.
Ocean water total photon cross section as a function of energy.
Figure 22.
Ocean water incoherent photon cross section as a function of energy.
Figure 22.
Ocean water incoherent photon cross section as a function of energy.
Figure 23.
Ocean water coherent photon cross section as a function of energy.
Figure 23.
Ocean water coherent photon cross section as a function of energy.
Figure 24.
Ocean water photoelectric photon cross section as a function of energy.
Figure 24.
Ocean water photoelectric photon cross section as a function of energy.
Figure 25.
Photon flux—ocean water vs contamination.
Figure 25.
Photon flux—ocean water vs contamination.
Figure 26.
Photon fluxes—spectrum vs contamination.
Figure 26.
Photon fluxes—spectrum vs contamination.
Figure 27.
30 keV—ocean water vs contamination.
Figure 27.
30 keV—ocean water vs contamination.
Figure 28.
40 keV—ocean water vs contamination.
Figure 28.
40 keV—ocean water vs contamination.
Figure 29.
50 keV—ocean water vs contamination.
Figure 29.
50 keV—ocean water vs contamination.
Figure 30.
Photon flux—polyethylene vs microorganisms.
Figure 30.
Photon flux—polyethylene vs microorganisms.
Figure 31.
30 KeV—polyethylene vs microorganisms.
Figure 31.
30 KeV—polyethylene vs microorganisms.
Figure 32.
40 KeV—polyethylene vs microorganisms.
Figure 32.
40 KeV—polyethylene vs microorganisms.
Figure 33.
50 KeV—polyethylene vs microorganisms.
Figure 33.
50 KeV—polyethylene vs microorganisms.
Table 1.
Ocean water weight chemical composition.
Table 1.
Ocean water weight chemical composition.
| Element. | Element (%) | Element | Element (%) |
|---|
| Oxygen | 85.7 | Molybdenum | 0.000001 |
| Hydrogen | 10.8 | Zinc | 0.000001 |
| Chlorine | 1.9 | Nickel | 0.00000054 |
| Sodium | 1.05 | Arsenic | 0.0000003 |
| Magnesium | 0.135 | Copper | 0.0000003 |
| Sulfur | 0.0885 | Tin | 0.0000003 |
| Calcium | 0.04 | Uranium | 0.0000003 |
| Potassium | 0.038 | Chromium | 0.00000003 |
| Bromine | 0.0065 | Krypton | 0.00000025 |
| Carbon | 0.0028 | Manganese | 0.0000002 |
| Strontium | 0.00081 | Vanadium | 0.0000001 |
| Boron | 0.00046 | Titanium | 0.0000001 |
| Silicon | 0.0003 | Cesium | 0.00000005 |
| Fluoride | 0.00013 | Cerium | 0.00000004 |
| Argon | 0.00006 | Antimony | 0.000000033 |
| Nitrogen | 0.00005 | Silver | 0.00000003 |
| Lithium | 0.000018 | Yttrium | 0.00000003 |
| Rubidium | 0.000012 | Cobalt | 0.000000027 |
| Phosphorus | 0.000007 | Neon | 0.000000014 |
| Iodine | 0.000006 | Cadmium | 0.000000011 |
| Barium | 0.000003 | Tungsten | 0.00000001 |
| Aluminum | 0.000001 | Lead | 0.000000005 |
| Iron | 0.000001 | Mercury | 0.000000003 |
| Indium | 0.000001 | Selenium | 0.000000002 |
Table 2.
Photon Creation.
Table 2.
Photon Creation.
| | Ocean Water No Contamination | Polyethylene 10 ppm | Polyethylene 100 ppm | Polyethylene 1000 ppm | Polyethylene 10,000 ppm |
|---|
| Bremsstrahlung | 99.1265% | 99.1237% | 99.1182% | 99.1545% | 99.3538% |
| 1st Fluorescence | 0.8733% | 0.8755% | 0.8812% | 0.8449% | 0.6448% |
| 2nd Fluorescence | 0.0002% | 0.0008% | 0.0006% | 0.0006% | 0.0015% |
| Norm | 100.0000% | 100.0000% | 100.0000% | 100.0000% | 100.0000% |
Table 3.
Nuclide Photon Activity.
Table 3.
Nuclide Photon Activity.
| Element | Ocean Water No Contamination | Polyethylene 10 ppm | Polyethylene 100 ppm | Polyethylene 1000 ppm | Polyethylene 10,000 ppm |
|---|
| Oxygen | 76.210% | 76.273% | 76.387% | 73.211% | 52.813% |
| Hydrogen | 7.585% | 7.405% | 6.998% | 6.686% | 4.259% |
| Chlorine | 12.357% | 12.107% | 12.179% | 11.938% | 8.902% |
| Sodium | 1.924% | 1.912% | 1.873% | 1.912% | 1.384% |
| Magnesium | 0.306% | 0.325% | 0.316% | 0.370% | 0.244% |
| Sulfur | 0.490% | 0.573% | 0.536% | 0.448% | 0.372% |
| Calcium | 0.429% | 0.512% | 0.434% | 0.409% | 0.277% |
| Potassium | 0.316% | 0.360% | 0.337% | 0.384% | 0.330% |
| Bromine | 0.322% | 0.294% | 0.281% | 0.340% | 0.198% |
| Carbon | 0.000% | 0.193% | 0.628% | 4.257% | 31.188% |
| Strontium | 0.056% | 0.046% | 0.031% | 0.044% | 0.029% |
| Silicon | 0.005% | 0.000% | 0.000% | 0.000% | 0.000% |
| Argon | 0.000% | 0.000% | 0.000% | 0.000% | 0.004% |
Table 4.
Parts per million contamination in cluster configuration.
Table 4.
Parts per million contamination in cluster configuration.
| Cluster N | (10 ppm) | (100 ppm) | (1000 ppm) | (10,000 ppm) |
|---|
| ppm perCluster | ppm perCluster | ppm perCluster | ppm perCluster |
|---|
| 1 | 1 | 10 | 100 | 1000 |
| 2 | 0.5 | 5 | 50 | 500 |
| 3 | 2 | 20 | 200 | 2000 |
| 4 | 1.3 | 13 | 130 | 1300 |
| 5 | 1.9 | 19 | 190 | 1900 |
| 6 | 0.3 | 3 | 30 | 300 |
| 7 | 0.8 | 8 | 80 | 800 |
| 8 | 0.4 | 4 | 40 | 400 |
| 9 | 0.2 | 2 | 20 | 200 |
| 10 | 0.9 | 9 | 90 | 900 |
| 11 | 0.7 | 7 | 70 | 700 |
| Norm | 10 | 100 | 1000 | 10,000 |
Table 5.
Particles and volume in 10 ppm.
Table 5.
Particles and volume in 10 ppm.
| Cluster N | (10 ppm) | (10 ppm) | Particles N | Volume (mm3) |
|---|
| ppm per Cluster | % ppm Cluster | per Cluster | per Cluster |
|---|
| 1 | 1 | 10% | 262 | 1 |
| 2 | 0.5 | 5% | 131 | 1 |
| 3 | 2 | 20% | 525 | 2 |
| 4 | 1.3 | 13% | 341 | 1 |
| 5 | 1.9 | 19% | 498 | 2 |
| 6 | 0.3 | 3% | 79 | 0.3 |
| 7 | 0.8 | 8% | 210 | 1 |
| 8 | 0.4 | 4% | 105 | 0.4 |
| 9 | 0.2 | 2% | 52 | 0.2 |
| 10 | 0.9 | 9% | 236 | 1 |
| 11 | 0.7 | 7% | 184 | 1 |
| Norm | 10 | 100.00% | 2623 | 11 |
Table 6.
Particles and volume in 100 ppm.
Table 6.
Particles and volume in 100 ppm.
| Cluster N | (100 ppm) | (100 ppm) | Particles N | Volume (mm3) |
|---|
| ppm per Cluster | % ppm Cluster | per Cluster | per Cluster |
|---|
| 1 | 10 | 10% | 2623 | 11 |
| 2 | 5 | 5% | 1311 | 5 |
| 3 | 20 | 20% | 5245 | 22 |
| 4 | 13 | 13% | 3409 | 14 |
| 5 | 19 | 19% | 4983 | 21 |
| 6 | 3 | 3% | 787 | 3 |
| 7 | 8 | 8% | 2098 | 9 |
| 8 | 4 | 4% | 1049 | 4 |
| 9 | 2 | 2% | 525 | 2 |
| 10 | 9 | 9% | 2360 | 10 |
| 11 | 7 | 7% | 1836 | 8 |
| Norm | 100 | 100.00% | 26,227 | 110 |
Table 7.
Particles and volume in 1000 ppm.
Table 7.
Particles and volume in 1000 ppm.
| Cluster N | (1000 ppm) | (1000 ppm) | Particles N | Volume (mm3) |
|---|
| ppm per Cluster | % ppm Cluster | per Cluster | per Cluster |
|---|
| 1 | 100 | 10% | 26,227 | 110 |
| 2 | 50 | 5% | 13,113 | 55 |
| 3 | 200 | 20% | 52,454 | 220 |
| 4 | 130 | 13% | 34,095 | 143 |
| 5 | 190 | 19% | 49,831 | 209 |
| 6 | 30 | 3% | 7868 | 33 |
| 7 | 80 | 8% | 20,981 | 88 |
| 8 | 40 | 4% | 10,491 | 44 |
| 9 | 20 | 2% | 5245 | 22 |
| 10 | 90 | 9% | 23,604 | 99 |
| 11 | 70 | 7% | 18,359 | 77 |
| Norm | 1000 | 100.00% | 262,268 | 1099 |
Table 8.
Particles and volume in 10,000 ppm.
Table 8.
Particles and volume in 10,000 ppm.
| Cluster N | (10,000 ppm) | (10,000 ppm) | Particles N | Volume (mm3) |
|---|
| ppm per Cluster | % ppm Cluster | per Cluster | per Cluster |
|---|
| 1 | 1000 | 10% | 262,268 | 1099 |
| 2 | 500 | 5% | 131,134 | 549 |
| 3 | 2000 | 20% | 524,535 | 2198 |
| 4 | 1300 | 13% | 340,948 | 1429 |
| 5 | 1900 | 19% | 498,308 | 2088 |
| 6 | 300 | 3% | 78,680 | 330 |
| 7 | 800 | 8% | 209,814 | 879 |
| 8 | 400 | 4% | 104,907 | 440 |
| 9 | 200 | 2% | 52,454 | 220 |
| 10 | 900 | 9% | 236,041 | 989 |
| 11 | 700 | 7% | 183,587 | 769 |
| Norm | 10,000 | 100.00% | 2,622,676 | 10,989 |
Table 9.
Polyethylene ppm.
Table 9.
Polyethylene ppm.
| | C | H |
|---|
| ppm | (mg/L) | (mg/L) |
|---|
| 10 | 8.57142857 | 1.42857143 |
| 100 | 85.7142857 | 14.2857143 |
| 1000 | 857.142857 | 142.857143 |
| 10,000 | 8571.42857 | 1428.57143 |
Table 10.
Ocean Water Vs Polyethylene ppm composition.
Table 10.
Ocean Water Vs Polyethylene ppm composition.
| Element | Origin Element (%) | Element (ppm) | 10 ppm Polyethylene (ppm) | 100 ppm Polyethylene (ppm) | 1000 ppm Polyethylene (ppm) | 10,000 ppm Polyethylene (ppm) |
|---|
| Oxygen | 85.70 | 8.57 × 105 | 8.570 × 105 | 8.569 × 105 | 8.561 × 105 | 8.484 × 105 |
| Hydrogen | 10.80 | 1.08 × 105 | 1.080 × 105 | 1.080 × 105 | 1.081 × 105 | 1.094 × 105 |
| Chlorine | 1.90 | 19,000 | 1.900 × 104 | 1.900 × 104 | 1.898 × 104 | 1.881 × 104 |
| Sodium | 1.05 | 10,500 | 1.050 × 104 | 1.050 × 104 | 1.049 × 104 | 1.040 × 104 |
| Magnesium | 0.14 | 1350 | 1.350 × 103 | 1.350 × 103 | 1.349 × 103 | 1.337 × 103 |
| Sulfur | 0.09 | 885 | 8.850 × 102 | 8.849 × 102 | 8.841 × 102 | 8.762 × 102 |
| Calcium | 0.04 | 400 | 4.000 × 102 | 4.000 × 102 | 3.996 × 102 | 3.960 × 102 |
| Potassium | 0.04 | 380 | 3.800 × 102 | 3.800 × 102 | 3.796 × 102 | 3.762 × 102 |
| Bromine | 0.01 | 65 | 6.500 × 101 | 6.499 × 101 | 6.494 × 101 | 6.435 × 101 |
| Carbon | 0.00 | 28 | 3.657 × 101 | 1.137 × 102 | 8.851 × 102 | 8.599 × 103 |