Design and Validation of a Portable Handheld Device to Produce Fine Fibers Using Centrifugal Forces
Abstract
1. Introduction
2. Materials and Methods
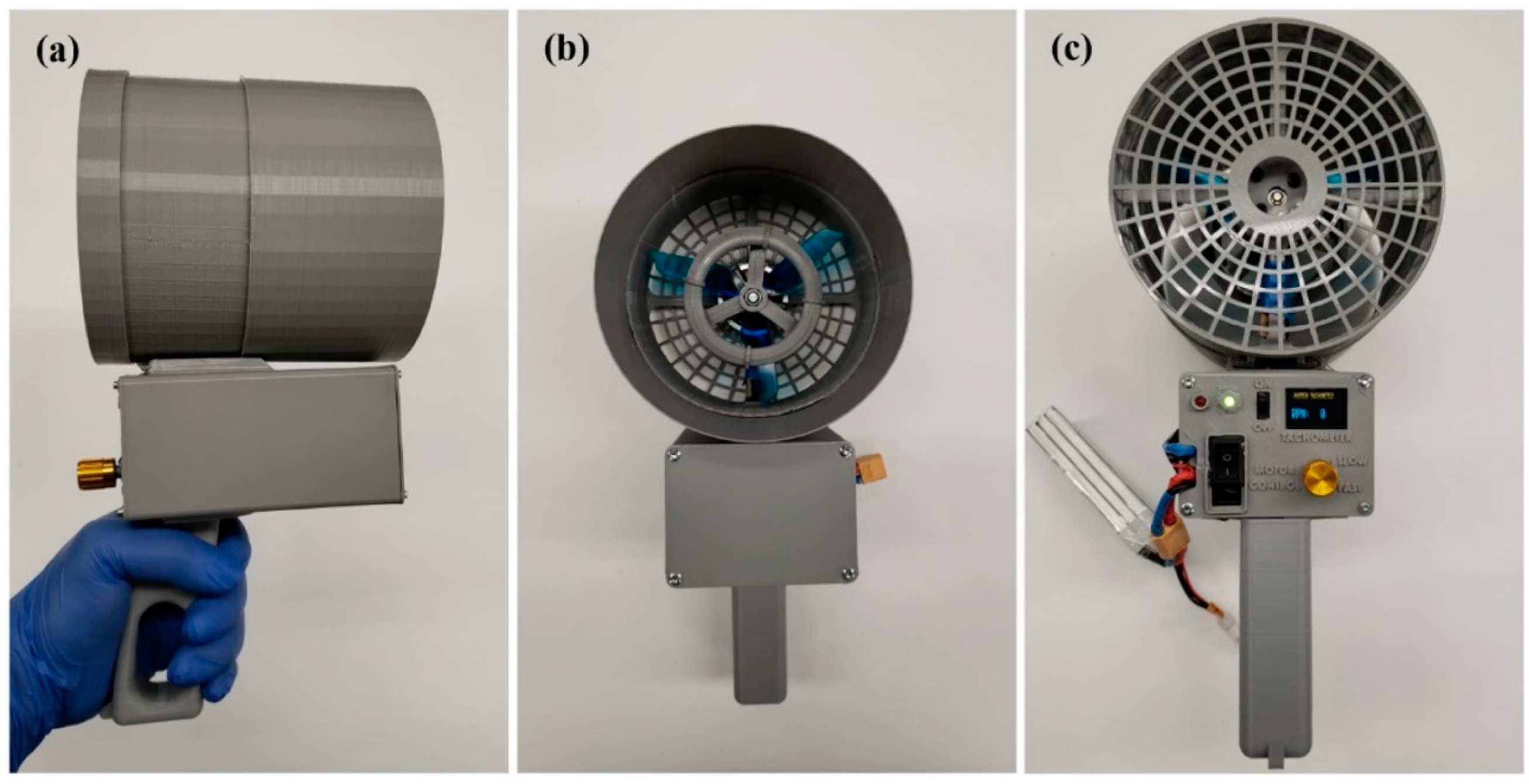
2.1. The Handheld Device (HD)
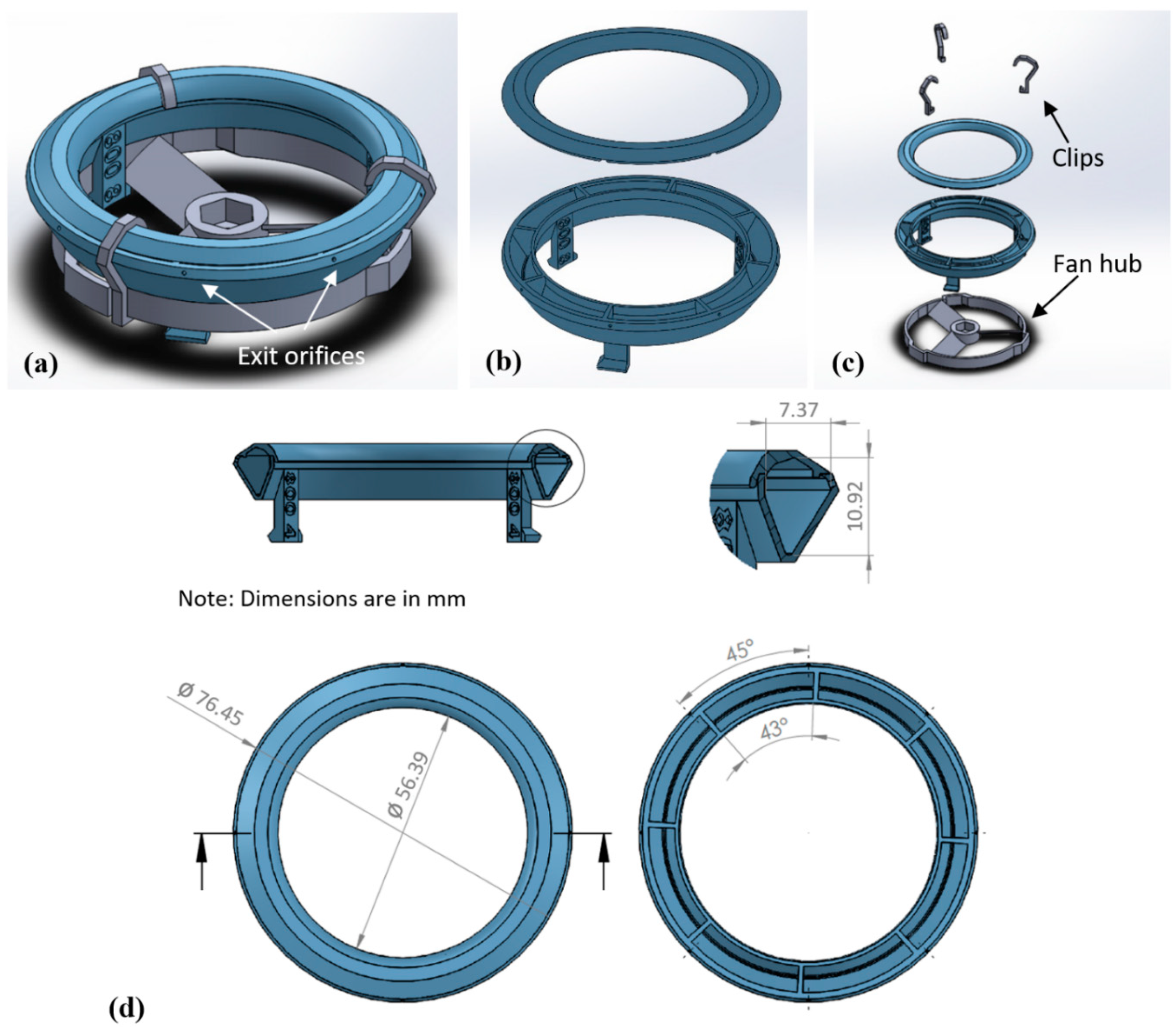
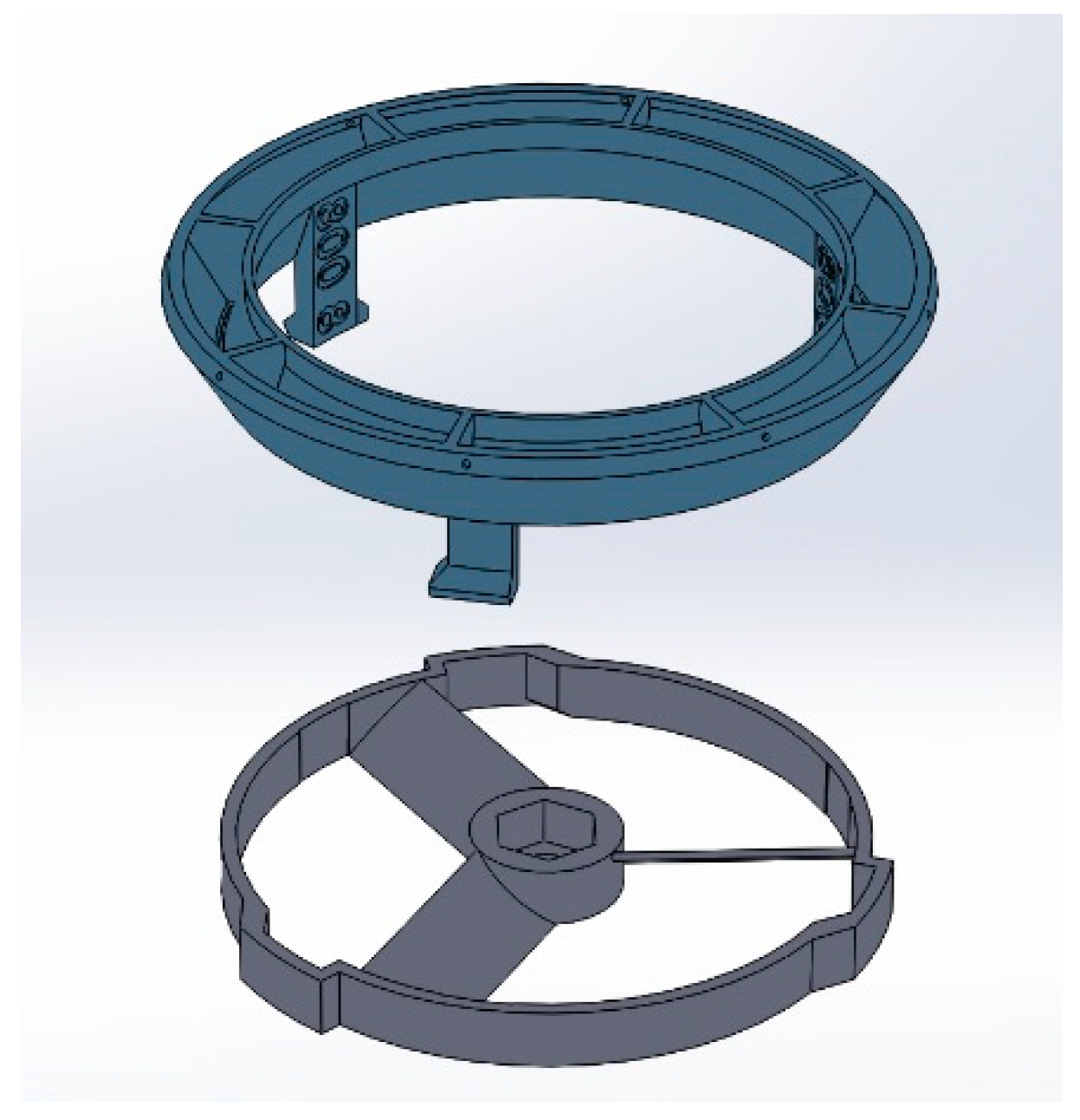
2.1.1. Spinneret and Fan Hub Assembly
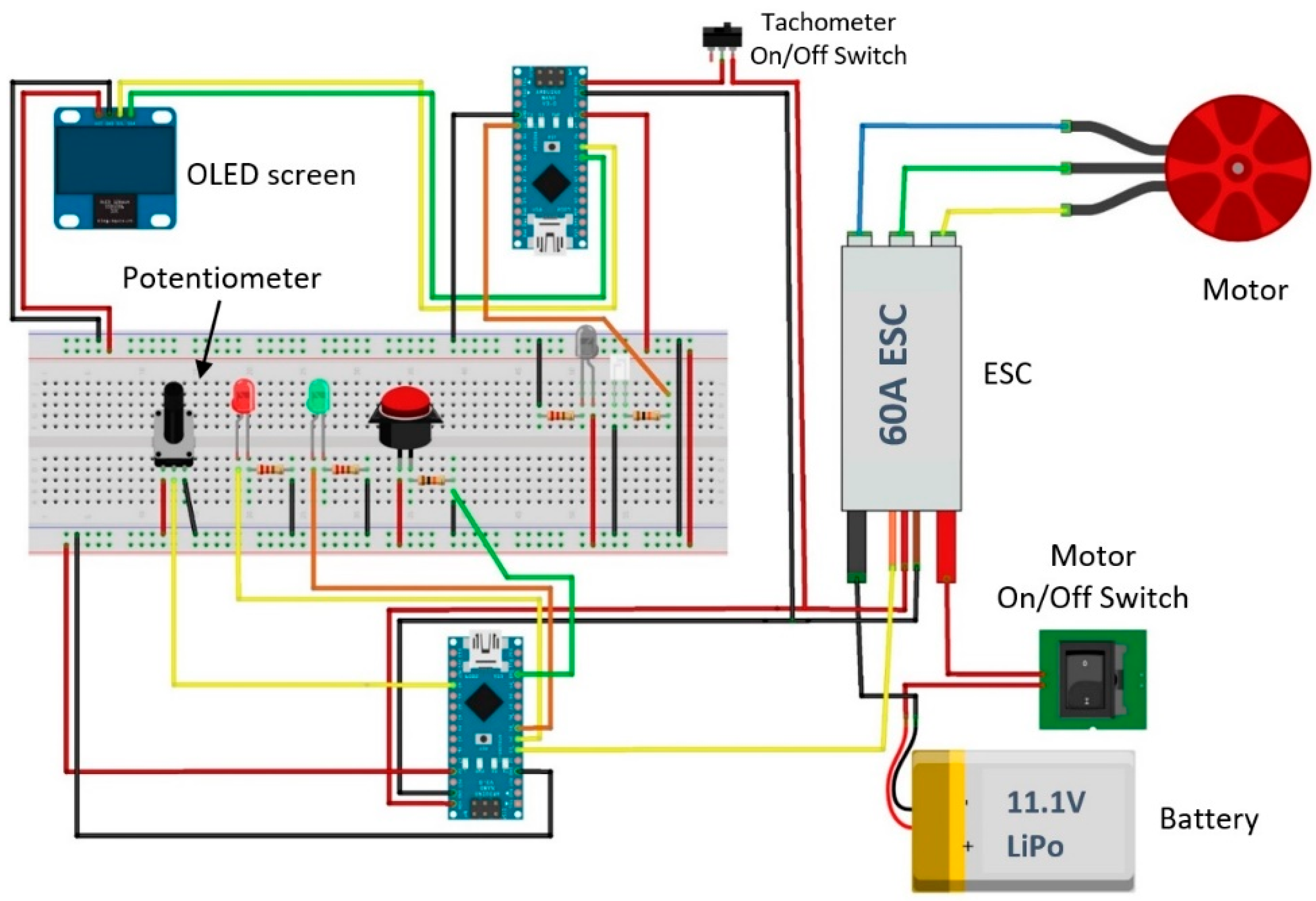
2.1.2. Drive System
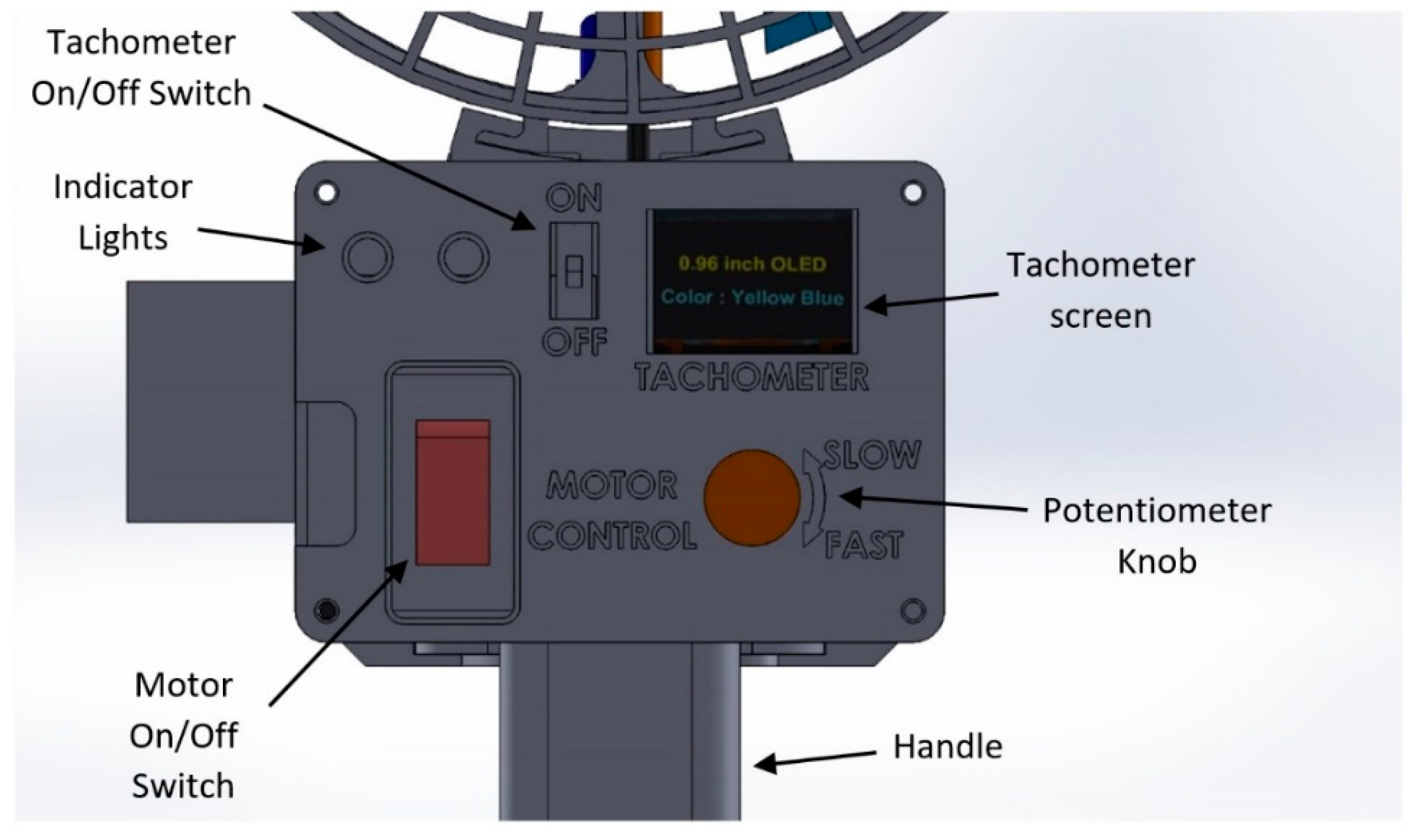
2.1.3. Housing and Handle
2.2. HD Performance Experiments
2.2.1. Materials
2.2.2. Experiments Design
2.2.3. Experimental Setup
3. Results
3.1. Design
3.2. HD Performance Experiments
3.2.1. Handheld Device Performance
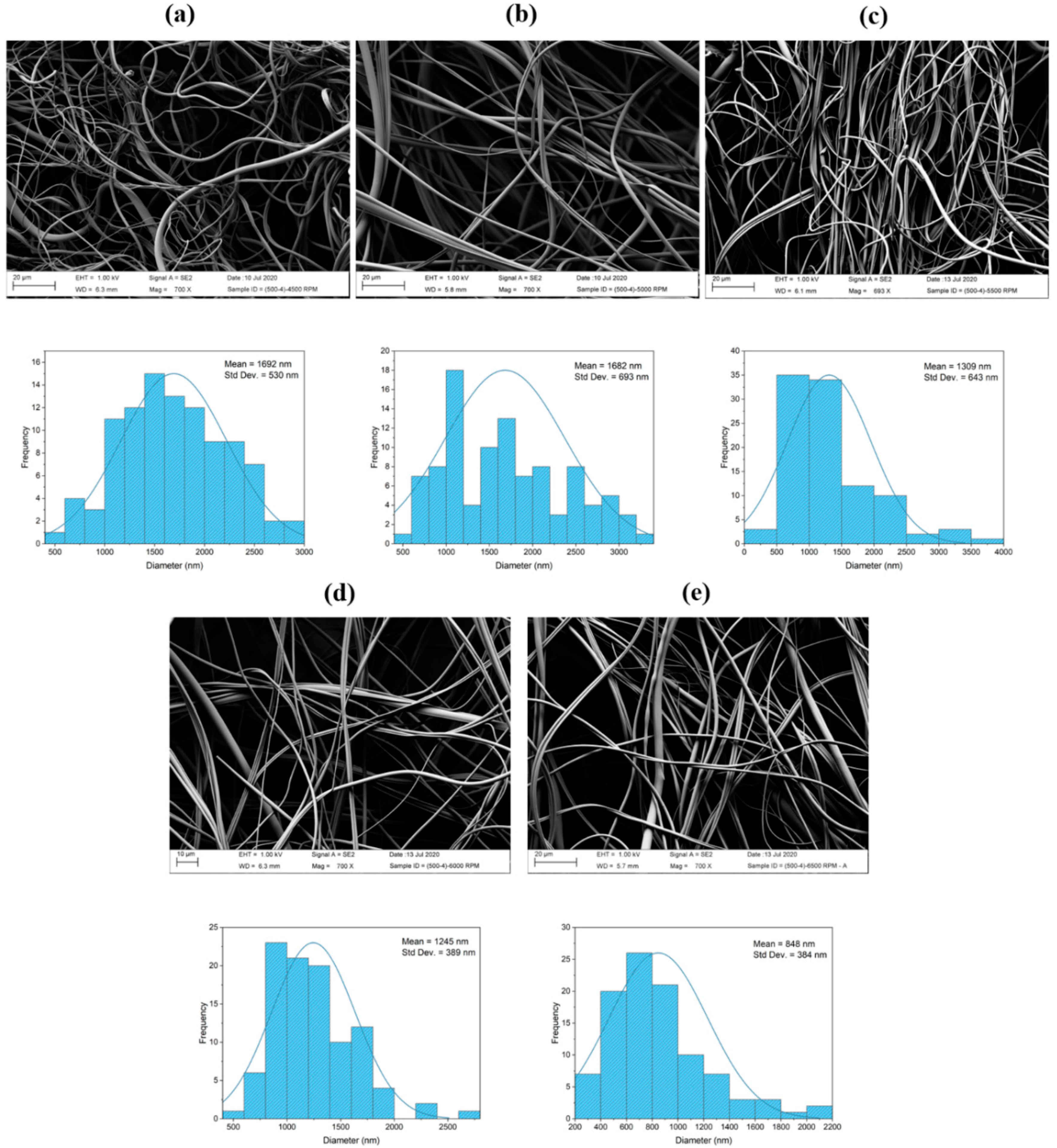
3.2.2. SEM Analysis
4. Conclusions
- Spinnerets with an orifice diameter of 500 microns yielded the greatest amount of fibers, measured gravimetrically. It was found that clogging can be reduced by using a small orifice diameter.
- The number of chambers also affected the amount of fibers produced. Spinnerets with two and four chambers produced more fibers than spinnerets with eight chambers.
- The fiber diameter size is dependent on the spinneret speed. It was found that, as the spinneret rotational speed increases, the fiber diameter decreases.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- McEachin, Z.; Lozano, K. Production and characterization of polycaprolactone nanofibers via forcespinningTM technology. J. Appl. Polym. Sci. 2012, 126, 473–479. [Google Scholar] [CrossRef]
- Yoshimoto, H.; Shin, Y.; Terai, H.; Vacanti, J.P. A biodegradable nanofiber scaffold by electrospinning and its potential for bone tissue engineering. Biomatererials 2003, 24, 2077–2082. [Google Scholar] [CrossRef]
- Ito, Y.; Hasuda, H.; Kamitakahara, M.; Ohtsuki, C.; Tanihara, M.; Kang, I.-K.; Kwon, O.H. A composite of hydroxyapatite with electrospun biodegradable nanofibers as a tissue engineering material. J. Biosci. Bioeng. 2005, 100, 43–49. [Google Scholar] [CrossRef]
- Graham, K.; Ouyang, M.; Raether, T.; Grafe, T.; McDonald, B.; Knauf, P. Fifteenth Annual Technical Conference & Expo of the American Filtration & Separations Society. Galveston TX 2002, 4, 9–12. [Google Scholar]
- Karube, Y.; Kawakami, H. Fabrication of well-aligned electrospun nanofibrous membrane based on fluorinated polyimide. Polym. Adv. Technol. 2010, 21, 861–866. [Google Scholar] [CrossRef]
- Li, D.; Xia, Y. Electrospinning of Nanofibers: Reinventing the Wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar] [CrossRef]
- Paneva, D.; Bougard, F.; Manolova, N.; Dubois, P.; Rashkov, I. Novel electrospun poly(ε-caprolactone)-based bicomponent nanofibers possessing surface enriched in tertiary amino groups. Eur. Polym. J. 2008, 44, 566–578. [Google Scholar] [CrossRef]
- Baji, A.; Mai, Y.-W.; Wong, S.-C.; Abtahi, M.; Chen, P. Electrospinning of polymer nanofibers: Effects on oriented morphology, structures and tensile properties. Compos. Sci. Technol. 2010, 70, 703–718. [Google Scholar] [CrossRef]
- Ramakrishna, S. An Introduction to Electrospinning and Nanofibers; World Scientific: Hackensack, NJ, USA, 2005. [Google Scholar]
- Lozano, K.; Sarkar, K. Methods and Apparatuses for Making Superfine Fibers. Patent WO2009117356A1, 24 September 2009. [Google Scholar]
- Sarkar, K.; Gómez, C.; Zambrano, S.; Ramirez, M.; De Hoyos, E.; Vasquez, H.; Lozano, K. Electrospinning to ForcespinningTM. Mater. Today 2010, 13, 12–14. [Google Scholar] [CrossRef]
- Padron, S.; Patlan, R.; Gutiérrez, J.; Santos, N.; Eubanks, T.; Lozano, K. Production and characterization of hybrid BEH-PPV/PEO conjugated polymer nanofibers by forcespinningTM. J. Appl. Polym. Sci. 2012, 125, 3610–3616. [Google Scholar] [CrossRef]
- Altecor, A.; Mao, Y.; Lozano, K. large-scale synthesis of tin-doped indium oxide nanofibers using water as solvent. Funct. Mater. Lett. 2012, 5, 1250020. [Google Scholar] [CrossRef]
- Rane, Y.; Altecor, A.; Bell, N.S.; Lozano, K. Preparation of Superhydrophobic Teflon® AF 1600 Sub-Micron Fibers and Yarns Using the Forcespinningtm Technique. J. Eng. Fibers Fabr. 2013, 8, 155892501300800420. [Google Scholar] [CrossRef]
- Vazquez, B.; Vasquez, H.; Lozano, K. Preparation and characterization of polyvinylidene fluoride nanofibrous membranes by forcespinningTM. Polym. Eng. Sci. 2012, 52, 2260–2265. [Google Scholar] [CrossRef]
- Raghavan, B.; Soto, H.; Lozano, K. Fabrication of Melt Spun Polypropylene Nanofibers by Forcespinning. J. Eng. Fibers Fabr. 2013, 8, 155892501300800100. [Google Scholar] [CrossRef]
- Padron, S.; Fuentes, A.; Caruntu, D.; Lozano, K. Experimental study of nanofiber production through forcespinning. J. Appl. Phys. 2013, 113, 24318. [Google Scholar] [CrossRef]
- Edmondson, D.; Cooper, A.; Jana, S.; Wood, D.; Zhang, M. Centrifugal electrospinning of highly aligned polymer nanofibers over a large area. J. Mater. Chem. 2012, 22, 18646. [Google Scholar] [CrossRef]
- Wang, L.; Ahmad, Z.; Huang, J.; Li, J.-S.; Chang, M.-W. Multi-compartment centrifugal electrospinning based composite fibers. Chem. Eng. J. 2017, 330, 541–549. [Google Scholar] [CrossRef]
- Wang, L.; Chang, M.-W.; Ahmad, Z.; Zheng, H.; Li, J.-S. Mass and controlled fabrication of aligned PVP fibers for matrix type antibiotic drug delivery systems. Chem. Eng. J. 2017, 307, 661–669. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, C.; Wang, H.-M.D.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. High throughput engineering and use of multi-fiber composite matrices for controlled active release. Mater. Today Commun. 2018, 17, 53–59. [Google Scholar] [CrossRef]
- Wang, L.; Wang, B.; Ahmad, Z.; Li, J.-S.; Chang, M.-W. Dual rotation centrifugal electrospinning: A novel approach to engineer multi-directional and layered fiber composite matrices. Drug Deliv. Transl. Res. 2018, 9, 204–214. [Google Scholar] [CrossRef]
- Liu, Y.; Tan, J.; Yu, S.; Yousefzadeh, M.; Lyu, T.; Jiao, Z.; Li, H.; Ramakrishna, S. High-efficiency preparation of polypropylene nanofiber by melt differential centrifugal electrospinning. J. Appl. Polym. Sci. 2019, 137, 137. [Google Scholar] [CrossRef]
- Merchiers, J.; Meurs, W.; Deferme, W.; Peeters, R.; Buntinx, M.; Reddy, N.K. Influence of Polymer Concentration and Nozzle Material on Centrifugal Fiber Spinning. Polymer 2020, 12, 575. [Google Scholar] [CrossRef] [PubMed]
- Coffee, R.A. A Dispensing Device and Method for Forming Material. CA Patent 2296334A1, 29 January 1998. [Google Scholar]
- Coffee, R.A.; Pirrie, A.B. Dispensing Device. U.S. Patent 6595208B1, 22 July 2003. [Google Scholar]
- Smith, D.J.; Reneker, D.H.; McManus, A.T.; Schreuder-Gibson, H.L.; Mello, C.; Sennett, M.S. Electrospun Fibers and an Apparatus Therefor. U.S. Patent 6753454B1, 22 June 2004. [Google Scholar]
- Greiner, A.; Wendorff, H.-J. Electrospinning: A Fascinating Method for the Preparation of Ultrathin Fibers. Angew. Chem. Int. Ed. 2007, 46, 5670–5703. [Google Scholar] [CrossRef]
- Sofokleous, P.; Stride, E.; Bonfield, W.; Edirisinghe, M. Design, construction and performance of a portable handheld electrohydrodynamic multi-needle spray gun for biomedical applications. Mater. Sci. Eng. C 2013, 33, 213–223. [Google Scholar] [CrossRef]
- Mouthuy, P.-A.; Groszkowski, L.; Ye, H. Performances of a portable electrospinning apparatus. Biotechnol. Lett. 2015, 37, 1107–1116. [Google Scholar] [CrossRef]
- Brako, F.; Luo, C.J.; Craig, D.Q.M.; Edirisinghe, M. An Inexpensive, Portable Device for Point-of-Need Generation of Silver-Nanoparticle Doped Cellulose Acetate Nanofibers for Advanced Wound Dressing. Macromol. Mater. Eng. 2018, 303, 1700586. [Google Scholar] [CrossRef]
- Pritchard, P.J.; Mitchell, J.W. Fox and McDonald’s Introduction to Fluid Mechanics, 9th ed.; Wiley: Hoboken, NJ, USA, 2015. [Google Scholar]
- Mo, X.; Xu, C.Y.; Kotaki, M.; Ramakrishna, S. Electrospun P (LLA-CL) nanofiber: A biomimetic extracellular matrix for smooth muscle cell and endothelial cell proliferation. Biomatererials 2004, 25, 1883–1890. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potter, G.; Barbosa, R.; Villarreal, A.; Salinas, A.; Guzman, H.; De Leon, H.; Ortega, J.A.; Lozano, K. Design and Validation of a Portable Handheld Device to Produce Fine Fibers Using Centrifugal Forces. Instruments 2020, 4, 27. https://doi.org/10.3390/instruments4030027
Potter G, Barbosa R, Villarreal A, Salinas A, Guzman H, De Leon H, Ortega JA, Lozano K. Design and Validation of a Portable Handheld Device to Produce Fine Fibers Using Centrifugal Forces. Instruments. 2020; 4(3):27. https://doi.org/10.3390/instruments4030027
Chicago/Turabian StylePotter, Gregory, Raul Barbosa, Alexa Villarreal, Alexandra Salinas, Hector Guzman, Heriberto De Leon, Javier A. Ortega, and Karen Lozano. 2020. "Design and Validation of a Portable Handheld Device to Produce Fine Fibers Using Centrifugal Forces" Instruments 4, no. 3: 27. https://doi.org/10.3390/instruments4030027
APA StylePotter, G., Barbosa, R., Villarreal, A., Salinas, A., Guzman, H., De Leon, H., Ortega, J. A., & Lozano, K. (2020). Design and Validation of a Portable Handheld Device to Produce Fine Fibers Using Centrifugal Forces. Instruments, 4(3), 27. https://doi.org/10.3390/instruments4030027





