Correction of Geometrical Effects of a Knife-Edge Slit Camera for Prompt Gamma-Based Range Verification in Proton Therapy
Abstract
:1. Introduction
2. Materials and Methods
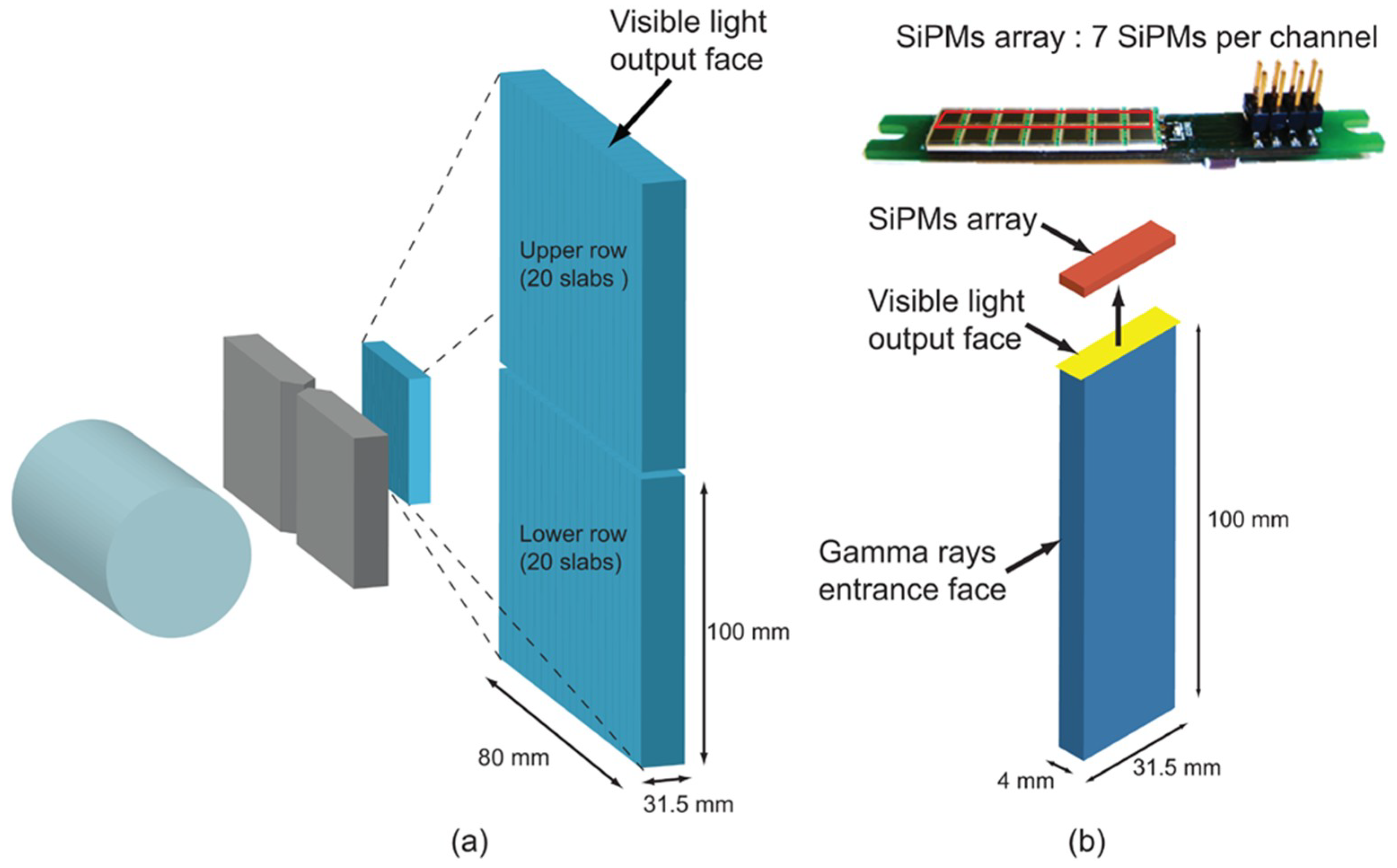
2.1. PG Camera
2.2. Analytical PG Simulation
2.3. Geometrical Correction
2.3.1. Parameters Influencing the Geometrical Correction
- the spatial distribution of the photon and neutron emission
- the geometry between the photon source and the camera
2.3.2. Monte Carlo Simulation to Determine the Geometrical Correction Function
2.3.3. Simulation Procedure
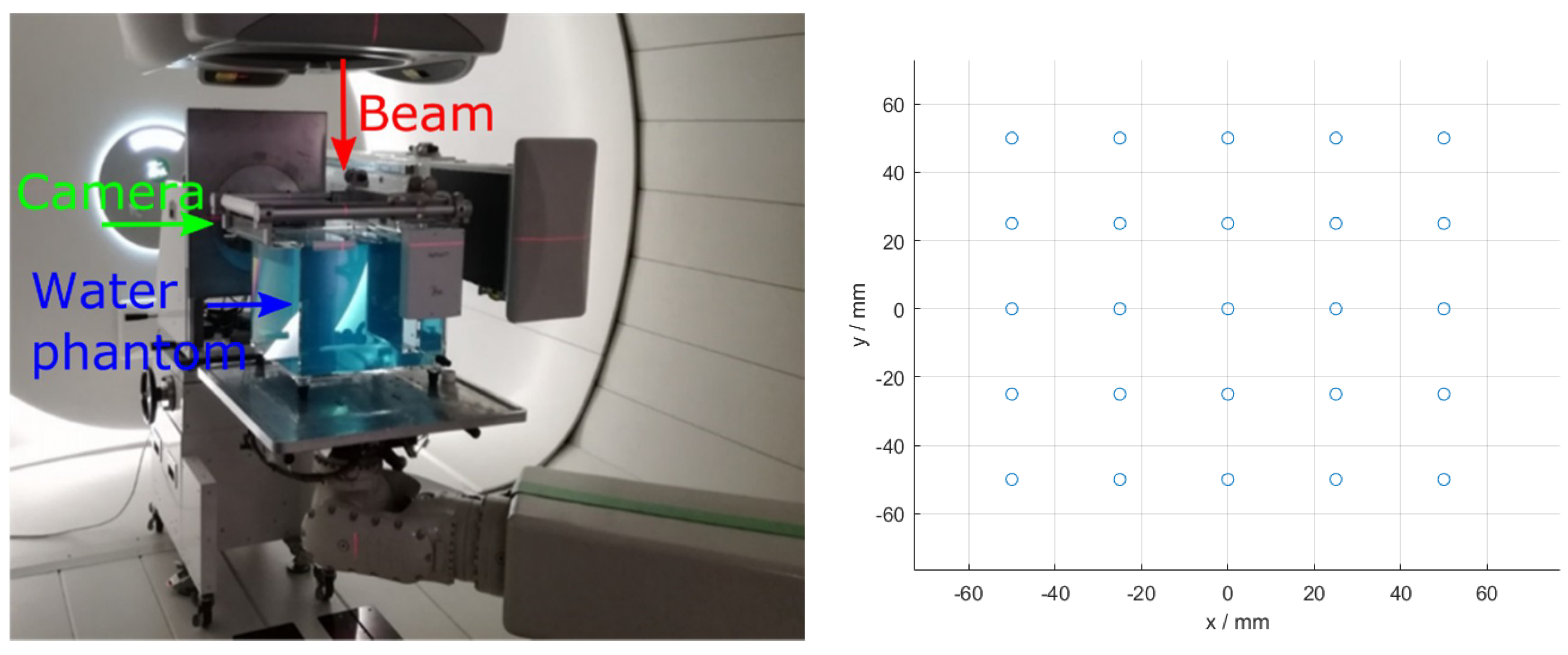
2.3.4. Validation of Model Using Benchmark Data
3. Results
3.1. Parameters Influencing the Geometrical Correction
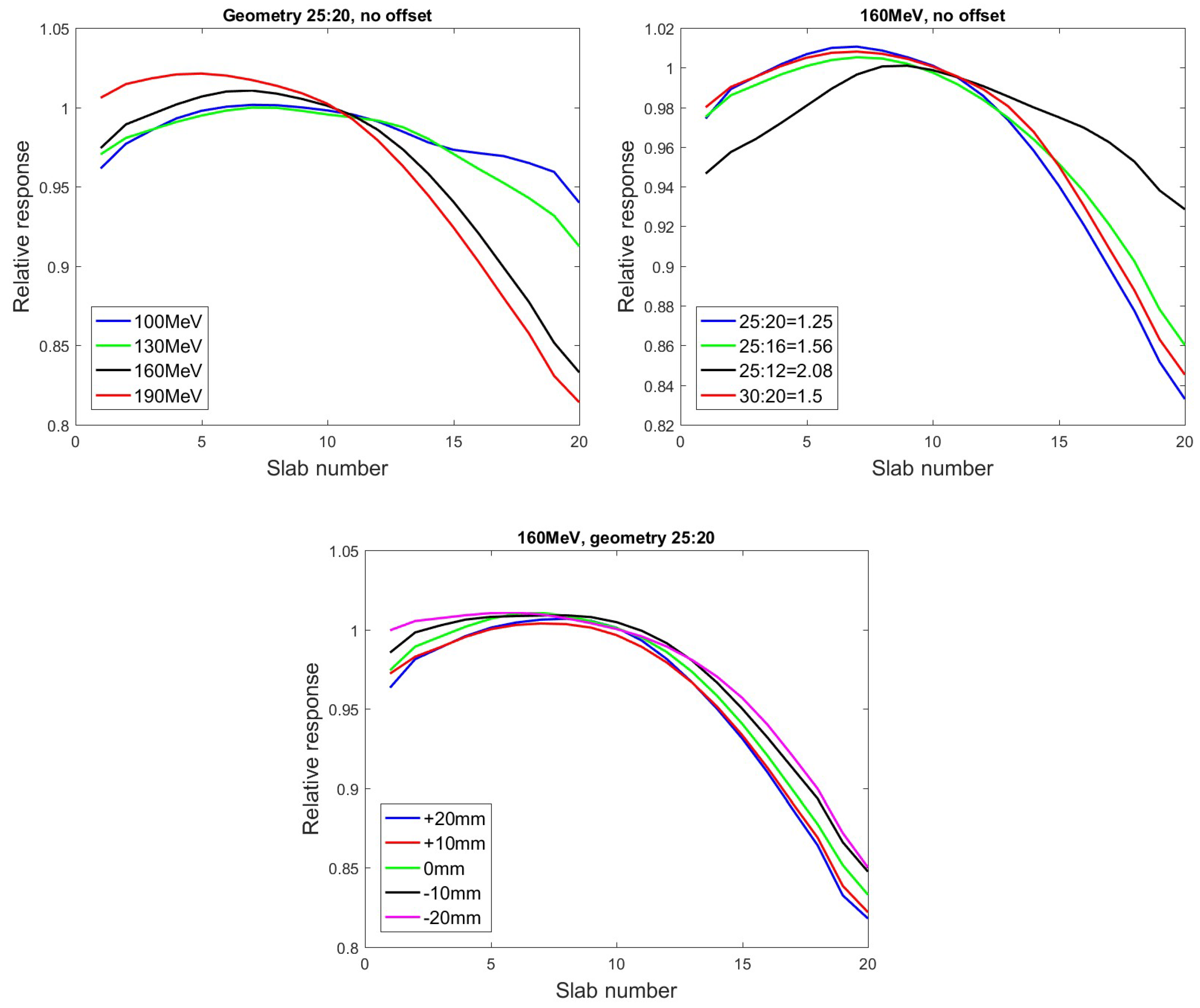
3.1.1. Beam Energy
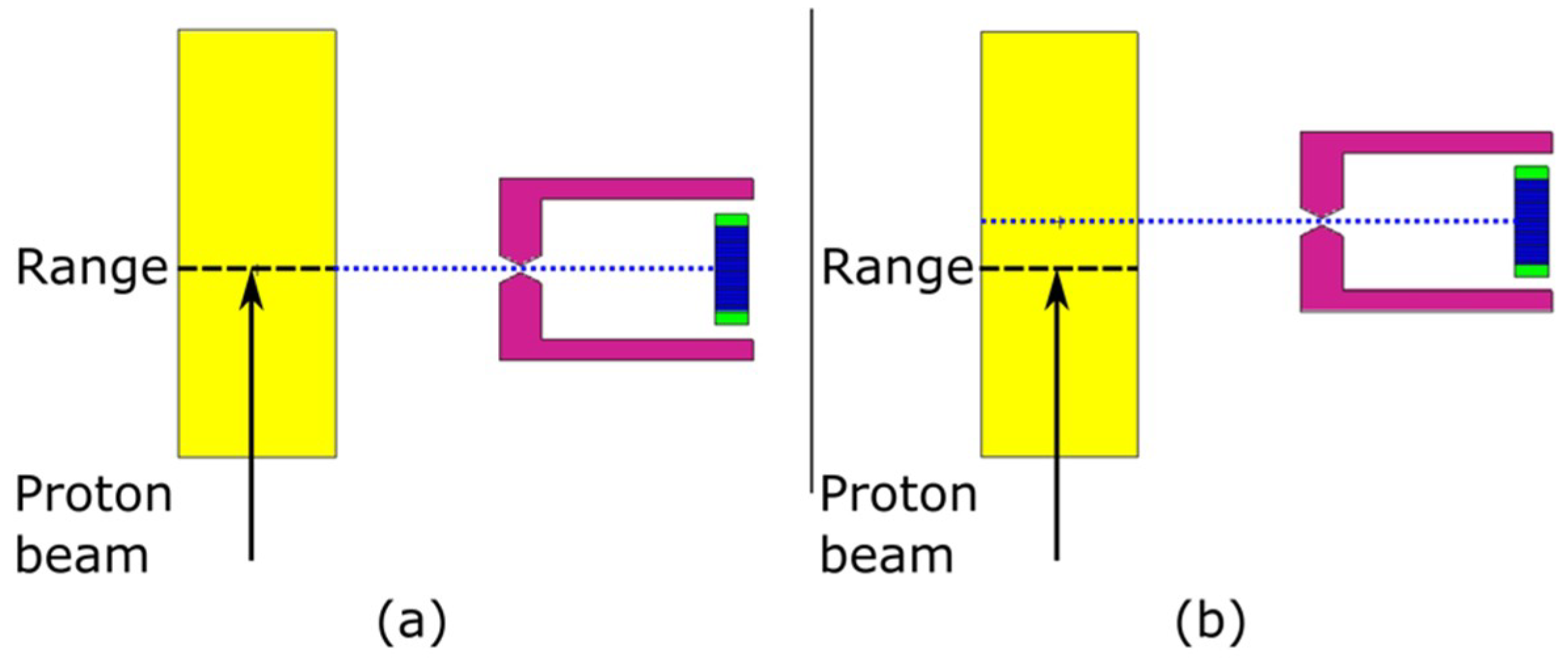
3.1.2. Camera Setup
3.1.3. Offset from Isocentre
3.2. Benchmark Experiment
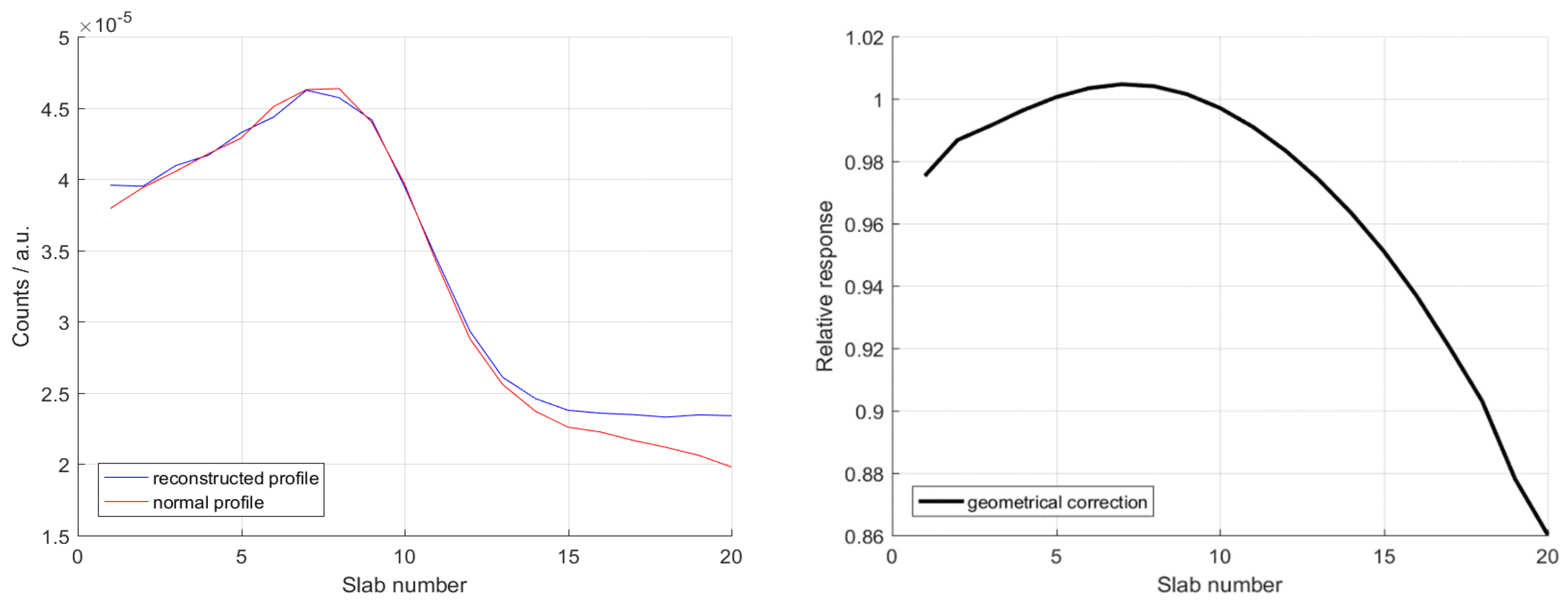
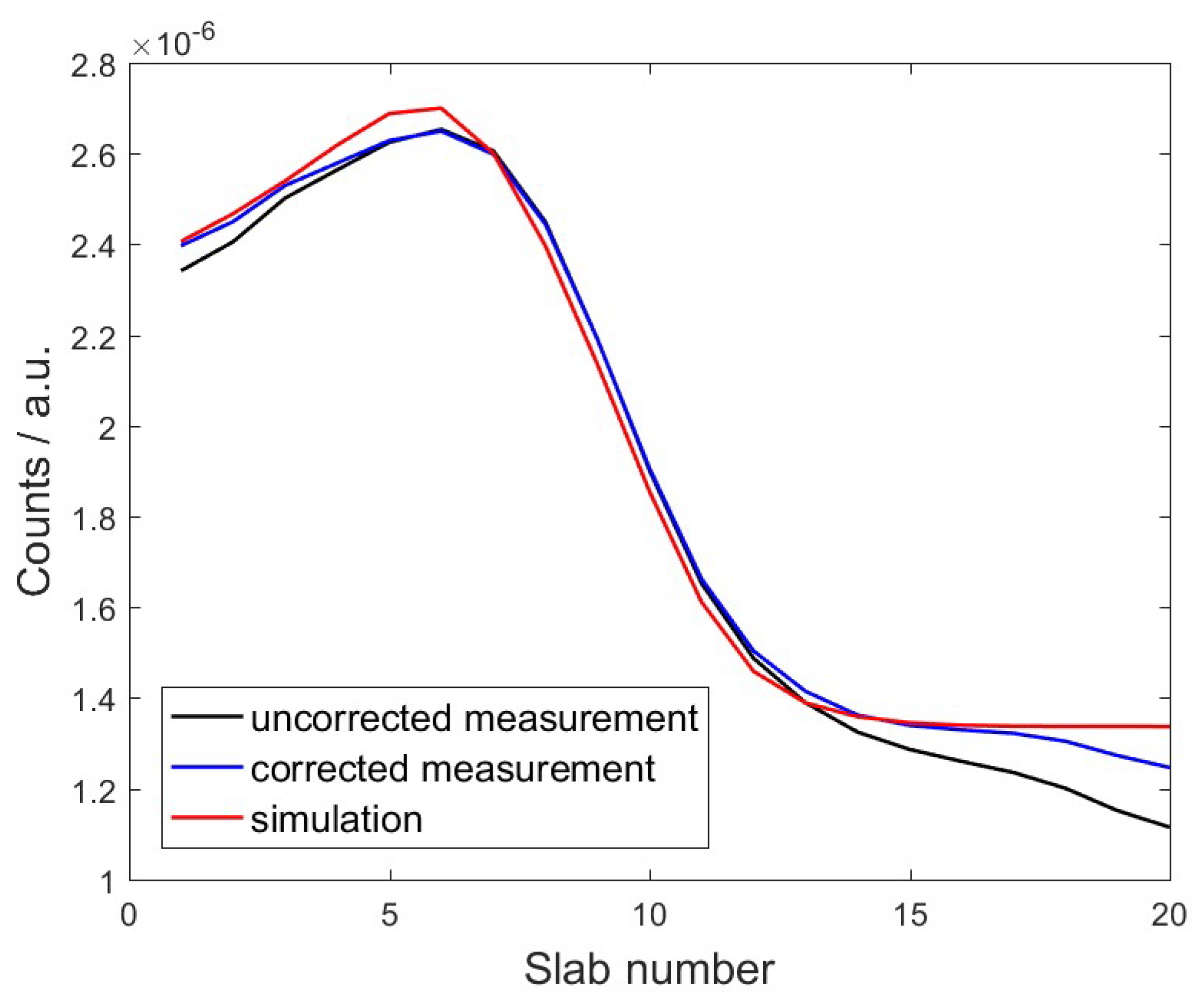
3.2.1. Profile Shape
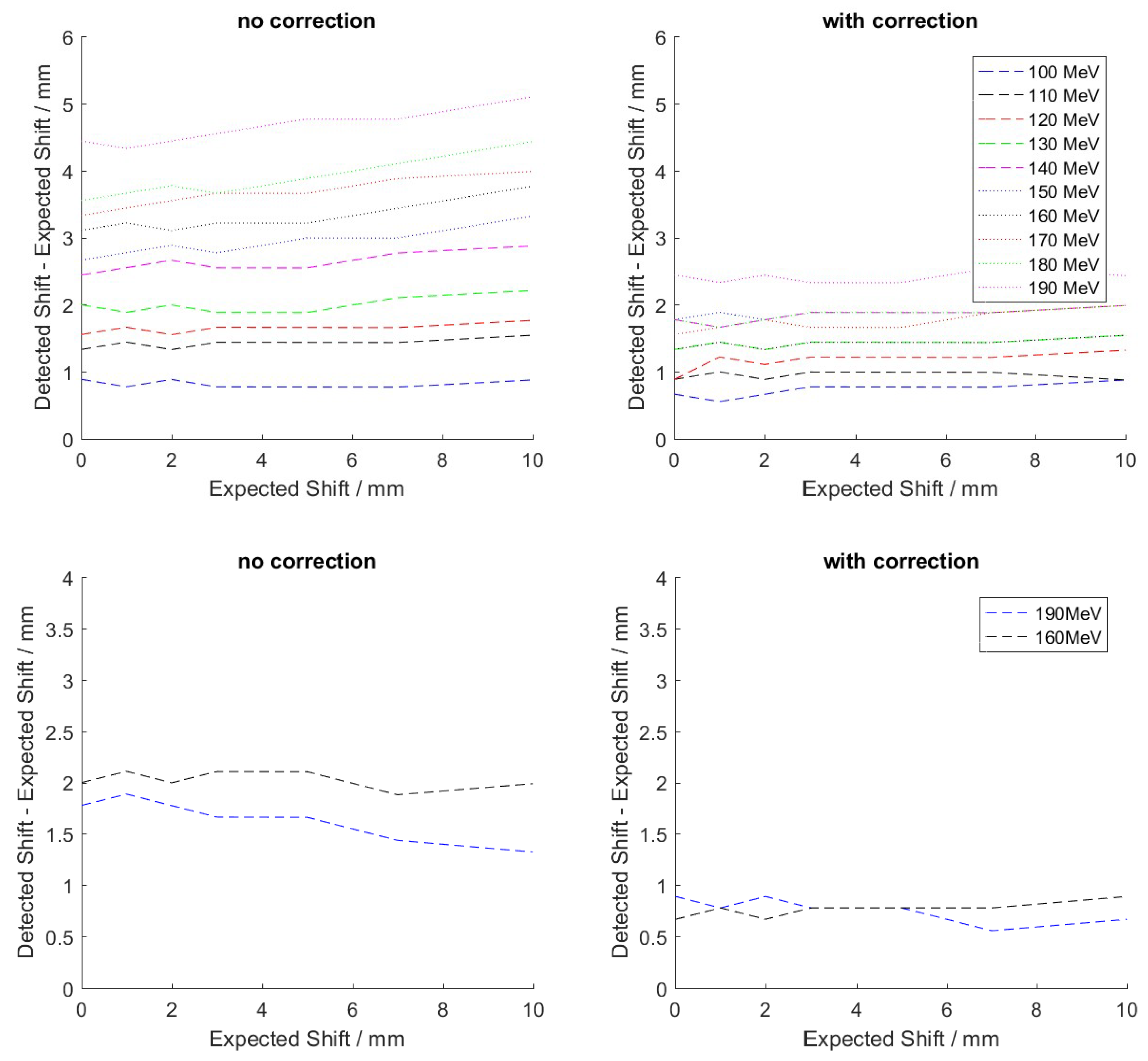
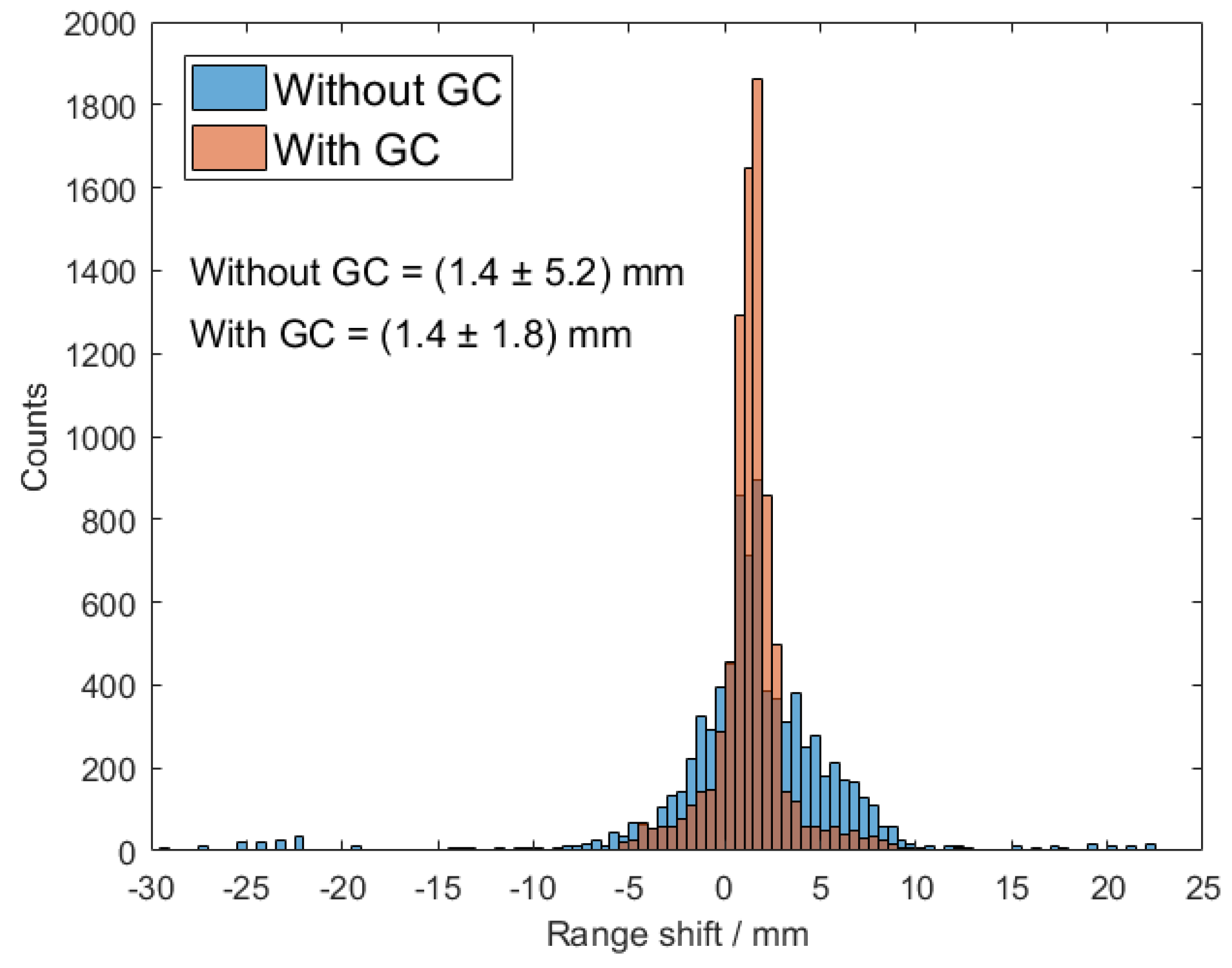
3.2.2. Range Retrieval
4. Discussion
4.1. Parameters Influencing the Geometrical Correction
4.2. Range Retrieval Analysis Using Benchmark Experiment Data
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Paganetti, H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys. Med. Biol. 2012, 57, R99. [Google Scholar] [CrossRef] [PubMed]
- Knopf, A.C.; Lomax, A. In vivo proton range verification: A review. Phys. Med. Biol. 2013, 58, R131. [Google Scholar] [CrossRef] [PubMed]
- Jongen, Y.; Stichelbaut, F. Verification of the proton beam position in the patient by the detection of prompt gamma-rays emission. In Proceedings of the 39th PTCOG conference, San Francisco, CA, USA, 26–29 October 2013. [Google Scholar]
- Min, C.H.; Kim, C.H.; Youn, M.; Kim, J.-W. Prompt gamma measurements for locating the dose falloff region in the proton therapy. Appl. Phys. Lett. 2006, 89, 3517. [Google Scholar] [CrossRef]
- Verburg, J.; Seco, J. Proton range verification through prompt gamma-ray spectroscopy. Phys. Med. Biol. 2014, 59, 7089. [Google Scholar] [CrossRef] [PubMed]
- Kelleter, L.; Wrońska, A.; Besuglow, J.; Konefał, A.; Laihem, K.; Leidner, J.; Magiera, A.; Parodi, K.; Rusiecka, K.; Stahl, A.; et al. Spectroscopic study of prompt-gamma emission for range verification in proton therapy. Phys. Med. 2017, 34, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Smeets, J.; Roellinghoff, F.; Prieels, D.; Stichelbaut, F.; Benilov, A.; Busca, P.; Fiorini, C.; Peloso, R.; Basilavecchia, M.; Frizzi, T.; et al. Prompt gamma imaging with a slit camera for real-time range control in proton therapy. Phys. Med. Biol. 2012, 57, 3371–3401. [Google Scholar] [CrossRef] [PubMed]
- Perali, I.; Celani, A.; Bombelli, L.; Fiorini, C.; Camera, F.; Clementel, E.; Henrotin, S.; Janssens, G.; Prieels, D.; Roellinghoff, F.; et al. Prompt gamma imaging of proton pencil beams at clinical dose rate. Phys. Med. Biol. 2014, 59, 5849–5871. [Google Scholar] [CrossRef] [PubMed]
- Priegnitz, M.; Helmbrecht, S.; Janssens, G.; Perali, I.; Smeets, J.; Vander Stappen, F.; Sterpin, E.; Fiedler, F. Measurement of prompt gamma profiles in inhomogeneous targets with a knife-edge slit camera during proton irradiation. Phys. Med. Biol. 2015, 60, 4849–4871. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, L.; Priegnitz, M.; Janssens, G.; Petzoldt, J.; Wohlfahrt, P.; Trezza, A.; Smeets, J.; Pausch, G.; Richter, C. Sensitivity of a prompt-gamma slit-camera to detect range shifts for proton treatment verification. Radiother. Oncol. 2017, 125, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Priegnitz, M.; Helmbrecht, S.; Janssens, G.; Perali, I.; Smeets, J.; Vander Stappen, F.; Sterpin, E.; Fiedler, F. Detection of mixed-range proton pencil beams with a prompt gamma slit camera. Phys. Med. Biol. 2016, 61, 855–871. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Pausch, G.; Barczyk, S.; Priegnitz, M.; Keitz, I.; Thiele, J.; Smeets, J.; Stappen, F.V.; Bombelli, L.; Fiorini, C.; et al. First clinical application of a prompt gamma based in vivo proton range verification system. Radiother. Oncol. 2016, 118, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Bentefour, E.H.; Janssens, G.; Smeets, J.; Vander Stappen, F.; Hotoiu, L.; Yin, L.; Dolney, D.; Avery, S.; O’Grady, F.; et al. Prompt Gamma Imaging for In Vivo Range Verification of Pencil Beam Scanning Proton Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017, 99, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Petzoldt, J.; Janssens, G.; O’Grady, F.; Yin, L.; Bentefour, E.H.; Smeets, J.; Prieels, D.; Lustig, R.A.; Lin, A.; et al. Prompt Gamma Imaging Signature of Anatomic Change during Base of Skull Proton Therapy. Radiother. Oncol. Submitted.
- Hueso-González, F.; Rabe, M.; Ruggieri, T.A.; Bortfeld, T.; Verburg, J.M. A full-scale clinical prototype for proton range verification using prompt gamma-ray spectroscopy. Phys. Med. Biol. 2018, 63, 5019. [Google Scholar] [CrossRef] [PubMed]
- Ferrero, V.; Fiorina, E.; Morrocchi, M.; Pennazio, F.; Baroni, G.; Battistoni, G.; Belcari, N.; Camarlinghi, N.; Ciocca, M.; del Guerra, A.; et al. Online proton therapy monitoring: Clinical test of a Silicon-photodetector-based in-beam PET. Nat. Sci. Rep. 2018, 8, 4100. [Google Scholar] [CrossRef] [PubMed]
- Sterpin, E.; Janssens, G.; Smeets, J.; Vander Stappen, F.; Prieels, D.; Priegnitz, M.; Perali, I.; Vynckier, S. Analytical computation of prompt gamma ray emission and detection for proton range verification. Phys. Med. Biol. 2015, 60, 4915–4946. [Google Scholar] [CrossRef] [PubMed]
- OpenREGGUI. Available online: www.openreggui.org (accessed on 26 September 2018).
- Pelowitz, D.B. (Ed.) MCNPX Users Manual Version 2.7.0; LA-CP-11-00438; Los Alamos National Laboratory: Los Alamos, NM, USA, 2011. [Google Scholar]
| Meas. ID | Geometry | Energy (MeV) | Energy Window Delivered (MeV) |
|---|---|---|---|
| 1 | 25:20 | 100 | 100:125 |
| 2 | 25:20 | 110 | 100:135 |
| 3 | 25:20 | 120 | 100:145 |
| 4 | 25:20 | 130 | 105:155 |
| 5 | 25:20 | 140 | 115:165 |
| 6 | 25:20 | 150 | 125:175 |
| 7 | 25:20 | 160 | 135:185 |
| 8 | 25:20 | 170 | 145:190 |
| 9 | 25:20 | 180 | 155:190 |
| 10 | 25:20 | 190 | 165:190 |
| 11 | 25:12 | 190 | 100:190 |
| 12 | 25:12 | 160 | 100:190 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petzoldt, J.; Janssens, G.; Nenoff, L.; Richter, C.; Smeets, J. Correction of Geometrical Effects of a Knife-Edge Slit Camera for Prompt Gamma-Based Range Verification in Proton Therapy. Instruments 2018, 2, 25. https://doi.org/10.3390/instruments2040025
Petzoldt J, Janssens G, Nenoff L, Richter C, Smeets J. Correction of Geometrical Effects of a Knife-Edge Slit Camera for Prompt Gamma-Based Range Verification in Proton Therapy. Instruments. 2018; 2(4):25. https://doi.org/10.3390/instruments2040025
Chicago/Turabian StylePetzoldt, Johannes, Guillaume Janssens, Lena Nenoff, Christian Richter, and Julien Smeets. 2018. "Correction of Geometrical Effects of a Knife-Edge Slit Camera for Prompt Gamma-Based Range Verification in Proton Therapy" Instruments 2, no. 4: 25. https://doi.org/10.3390/instruments2040025
APA StylePetzoldt, J., Janssens, G., Nenoff, L., Richter, C., & Smeets, J. (2018). Correction of Geometrical Effects of a Knife-Edge Slit Camera for Prompt Gamma-Based Range Verification in Proton Therapy. Instruments, 2(4), 25. https://doi.org/10.3390/instruments2040025





