1. Introduction
During the last decades of the new millennium, electronic device miniaturization reached the frontier in the nanoscale domain, an area most commonly known as nanoelectronics. At this scale, researchers demonstrated that size matters in increasing efficiency and high-throughput information processing, leading to large-scale consumption. Due to its high abundance and great performance in such devices, silicon is used as a semiconductor material; however, the high level of purity required for its application and overheating make its industrial application non-sustainable due to its charge losses, toxic environmental trail, and costs. For this reason, nanotechnology researchers are strengthening efforts to generate novel materials as substituents of silicon in this area [
1,
2] and apply them in new electronic devices, such as switches, diodes, rectifiers, solar cells, field effect transistors, and quantum wires, among other applications [
3,
4,
5], as well as in molecular electronics.
In searching for such devices, experimental and theoretical approaches have been reported in the literature over the years. The seminal work of Mann and Kuhn in 1971 [
6] demonstrated the electrical properties of cadmium carboxylate salt derivatives of fatty acids through the measurement of conductivity in a monolayer array of this system. In addition, Cooper et al. in 1971 found conductive behavior at low temperatures using a molecular system of tetrathiofuvalene (
) [
7,
8], and in the late 1970s, Macdiarmid et al. reported the conductive behavior of organic polymers [
9,
10], an effort recognized by the Nobel Prize in Chemistry in 2000.
On the other hand, instrumental technical advances in the 1980s led to robust analytical techniques, such as scanning tunneling microscopy (STM) and atomic force microscopy (AFM) [
11], allowing measurements of electrical properties of several nanoscopic systems, including molecules. However, some scenarios presented difficulties at the junctions of molecules with the tip probe due to the intrinsic conditions of the techniques for such malleable and soft systems. Fortunately, these issues were solved with the development of the mechanically controllable break junction (MCBJ) [
12], the scanning tunneling microscopic break junction (STMBJ), the electromigrated break junction (EBJ), and the thermoelectric atomic force microscope (ThAFM), thus enabling more precise data of electrical and thermal conductance in a single-molecule junction, leading to the invention of nanoscale organic thermoelectric devices [
2,
13]. Thus, in 2011, the conjugated polymer poly(3,4-ethylenedioxythiophene) (PEDOT) was characterized as one of the first systems with moderate thermal-to-electrical energy conversion efficiency [
14]. Since then, researchers have focused their efforts on increasing this conversion efficiency through the fabrication of new organic thermoelectric materials [
15]. 3,6-Disubstituted catechol has been used as the base of highly functionalized molecular rods possessing interesting electrical transport properties [
16]. In another study, poly-substituted catechol was used as an end arm of a long-carbon-chain hemiquinones (i.e., catechol covalently linked with ortho-benzoquinone), and STM analysis showed that catechol acts mainly as the donor, whereas the ortho-quinone serves as the charge acceptor [
17].
Likewise, circular wires were built by a catechol bridge between highly conjugated skeletons composed of aromatic rings and alkyne moieties covalently linked to two terminals. The redox pair catechol/quinone served as a molecular switch in an STM assembly, and thus, the researchers demonstrated that the electronic transport properties strongly depend on the oxidation state, which can be tuned by the degree of electronic delocalization and, therefore, the electronic conductance of the systems [
18]. In this work, Nicolas Weibel et al. set up the design and fabrication of potential advanced active polymeric materials, such as catechol-based organic electrodes containing reversible redox sites, which could be applied for economical and sustainable next-generation electrochemical energy storage (EES) devices [
19]. In addition, the 1,2-dihydroxybenzene scaffold is essential in a wide variety of organic dyes, typically large organic molecules, for this optical behavior. Therefore, despite the experimental evidence on how catechol plays a crucial role in the intramolecular electronic properties of such large molecules, there is a scarce fundamental understanding of its internal electronic structural features and how electronic transport proceeds within such systems [
20,
21,
22].
In terms of theoretical studies, the seminal work conducted by Aviram and Ratner in 1974 on electrical properties through organic molecules [
23] demonstrated their potential to act as possible rectifiers by studying the electrical and thermal transport processes in individual molecules, evidencing the essential role of aromatic systems in the semiconducting properties of these molecules [
24,
25]; however, such properties may vary depending on the configuration in which they are held between the metal contact or tips (i.e., symmetric or asymmetric). In addition, the electrical and magnetic properties can vary drastically [
26,
27], taking to account (or not) external stimuli such as impurities, vacancies, electric or magnetic fields, and temperature variations, among others.
Thus, we sought to study the electrical and thermal transport properties of catechol to understand how these transport processes depend on several variables and to determine its potential application in molecular conducting devices. To this end, we modeled catechol in the middle of two electrodes in two configurations (Model I and Model II) and calculated the electrical and thermal transport properties employing the Green’s function method through the use of the Dyson dynamic equation, based on a tight-binding Hamiltonian, within the renormalization framework or the decimation of the real system to an effective system. Then, we focused our interest on the variation in the position of the electrodes and the electrode–molecule coupling bond (strong and weak regimes) to calculate the properties of the transmission probability, the electric current, the electric conductance, the thermal conductance, the Seebeck coefficient, and the ZT factor. Very interestingly, we found significant differences between them, as described herein. The outline of this work is as follows. In
Section 2, we discuss the details of the analytical model used to determine the transport properties of the molecular system. In
Section 3, we describe the method, followed by
Section 4, where we present and describe the results of the transport properties. Finally, in
Section 5, we highlight the main conclusions of the work and provide final remarks.
4. Results
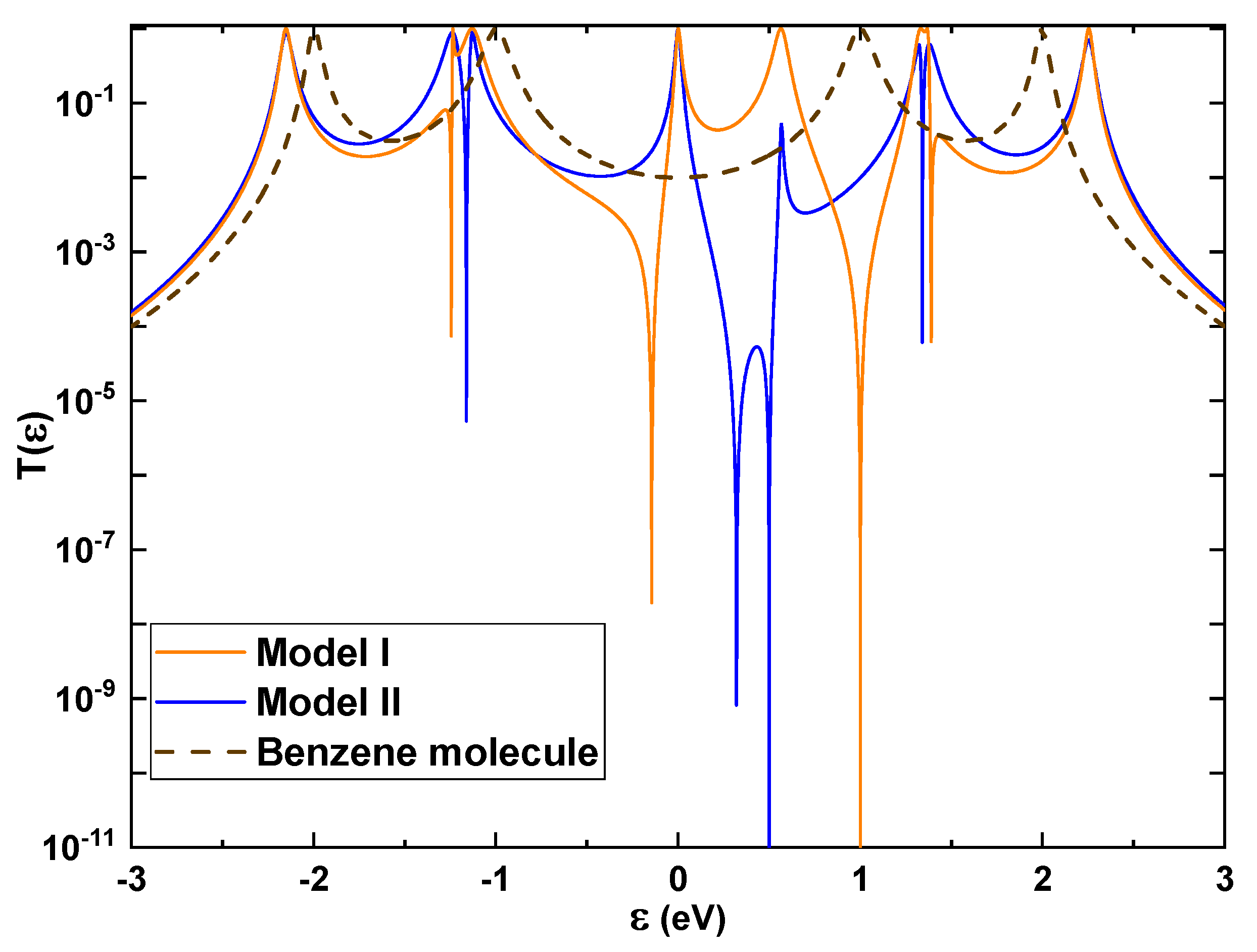
In order to understand the conducting behavior of Models I and II, we evaluated the transmission probability (
) as a function of the energy injected into the molecular system (
Figure 4), in contrast to a simpler aromatic system without any functionality (i.e., benzene), for values of
= 0.2 eV,
= 0.0 eV,
= 0.5 eV,
= 1.0 eV,
= 1.0 eV, and
= 1.0 eV. The resonant peaks occurring at weak coupling (i.e.,
) are closer to the eigenvalues of each molecular system, which are calculated by diagonalizing the Hamiltonian matrix
(Equation (
3)), where
N corresponds to the number of atomic sites
(6 carbon atoms and 2 atoms of the OH groups). Then, with the eigenvalues given at 2.26 eV, −2.15 eV, 1.37 eV, 1.33 eV, −1.24 eV, −1.13 eV, 0.57 eV, and 0.0 eV (see
Appendix A), we validated the renormalization for this molecular system [
27,
38] for the further determination of the electrical and thermal properties.
The resonant peak of
around the Fermi level (
) for both models represents the allowed electronic states at that level, thus clearing the path for the transport of charge carriers due to an increase in the density of states. Therefore, the forbidden band between the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO) tends to cancel out, pointing out that, under these conditions, catechol acts as a conducting material. This result contrasts with the simpler aromatic system of the benzene molecule, which behaves as a semiconducting material (
Figure 4, brown dashed line) with a gap around the Fermi level
= 0 eV [
24,
25]. Since the OH groups alter the hybridization of the aromatic ring’s orbitals of the molecular system, this clearly enhances the density of states around the Fermi level, turning it from a semiconducting to a conducting molecular skeleton.
When comparing the distinct behaviors of the two models, we can find evidence that the manner in which catechol’s OH groups are anchored to the leads alters the resistance of the conducting molecular system. Thus, the resonant peaks in Model I are broader than those in Model II, although their eingenvalues are very close. This difference is strongly related to the fact that in Model I, none of the OH groups are attached to the leads, indicating a greater probability of the charge carriers diffusing along the molecular system, whereas, with one OH group attached to a lead (Model II), we can observe a lower area under the curve, indicating a more significant resistance to conductivity.
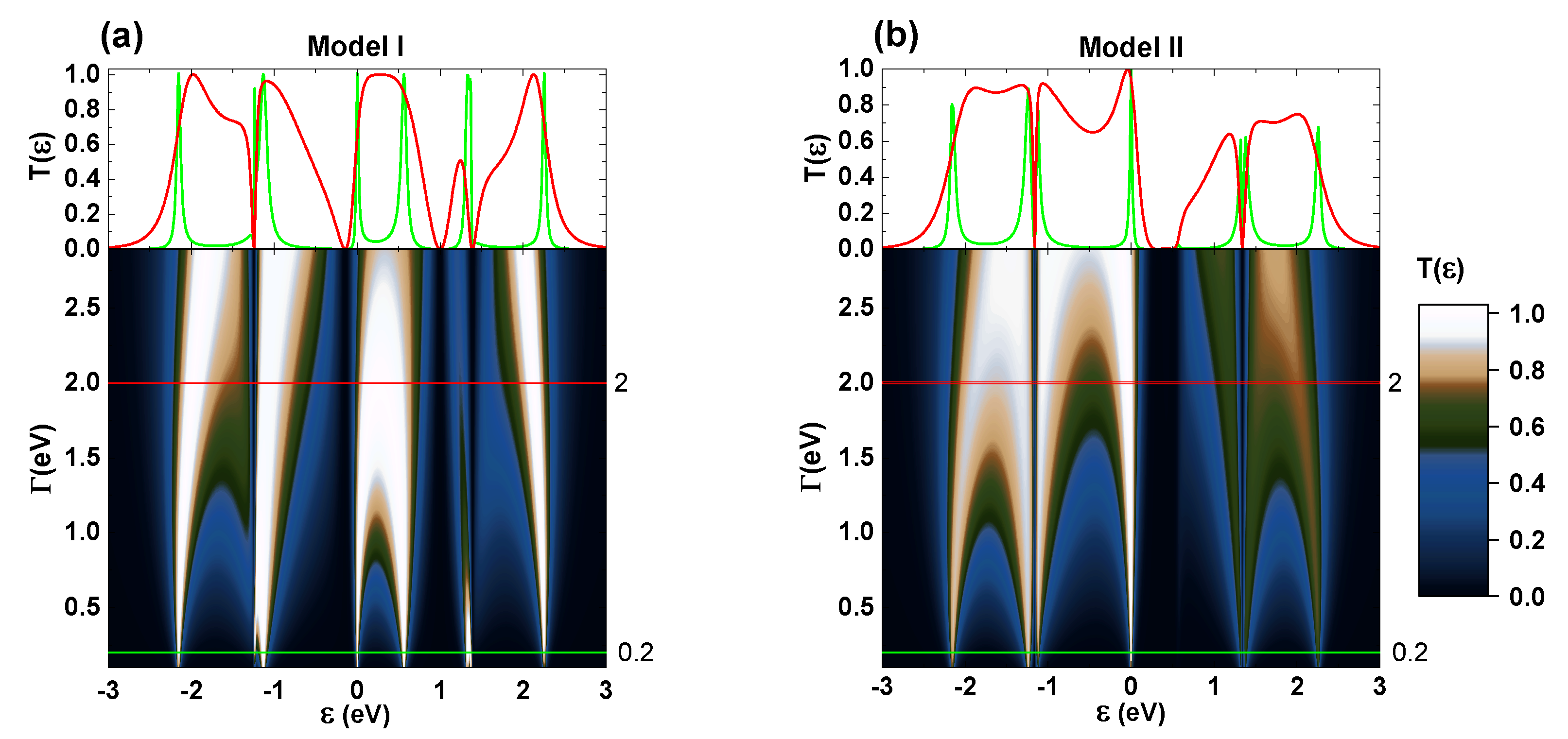
Because
strongly depends on the coupling potential of the molecule to the leads (
), we screened it using different values, with frontier values of 0.0 eV to 3.0 eV (
Figure 5), recalling that strong- and weak-coupling regimes are where
and
, respectively.
Figure 5 depicts the behavior of
for different coupling values, highlighting selected cases of weak coupling (
= 0.2 eV, green line) and strong coupling (
= 2.0 eV, red line) regimes. Hence, the areas under the red line curves are broader than those under the green lines, showing that in the strong-coupling regime, the resonant peaks are broadened due to hybridization between the discrete states of the molecular system and the delocalized states of the contacts, inducing an increment in conductivity. In addition, Model I presents higher transmission than Model II due to the manner in which catechol is anchored to the leads, as previously discussed.
Once we defined the conducting behavior of the molecular device under weak- and strong-coupling regimes, we studied the current passing through it depending on the applied voltage between the leads (
Figure 6). We observe lower currents with
= 0.2 eV for both models, in agreement with its semiconductor behavior, than with
= 2.0 eV when their conducting properties are enhanced. Notably, the current trend is similar in both models, with a larger amplitude for Model I (i.e., larger area under the curve), with an interesting stepped shape owing to the resonance peaks previously shown for the transmission probability (
Figure 4). The inset of
Figure 6 shows the electric current within the voltage limits from −0.5 V to 0.5 V, showcasing the very similar behaviors of the two models at weak coupling but differences at strong coupling, as well as a more linear performance canceling out the stepped shape, which remains at weak coupling. This linear behavior in both models is characteristic of conductive materials.
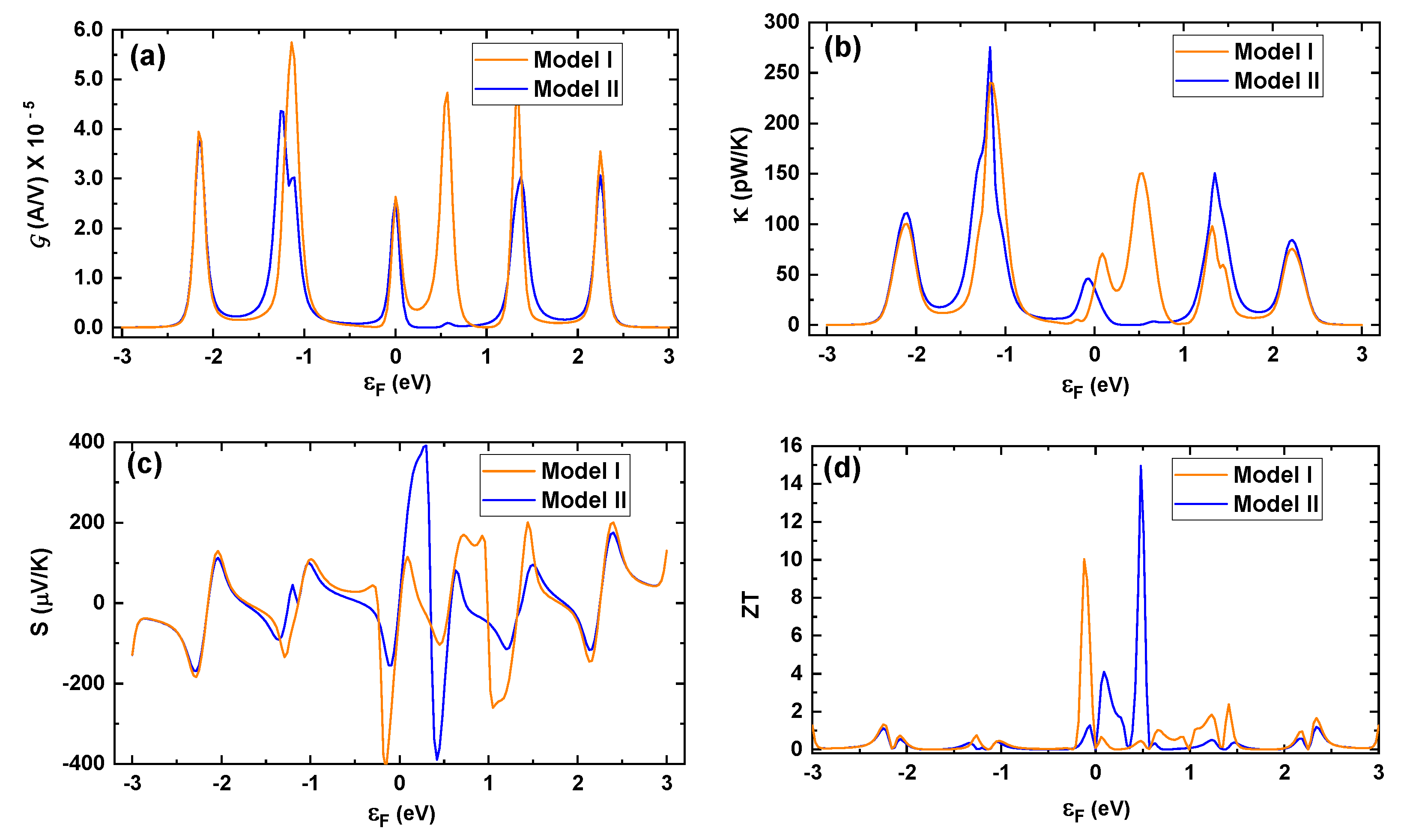
For the analysis of the thermal properties of conduction in both models, we determined the electrical conductance (
), thermal conductance (
), Seebeck coefficient (
S), and figure of merit (
) as a function of the Fermi energy (
Figure 7).
Figure 7a shows that
, calculated by Equation (
18), agrees with the transmission (see above,
Figure 5) according to Landauer’s formalism, where the conductance is proportional to
at low temperature and in the weak-coupling regime. Even though the conductance is pretty similar in both models, remarkably, at 0.5 V, Model II presents almost null conductance owing to the manner in which OH is anchored to the leads, suggesting the formation of an antiresonance state. Likewise, in
Figure 7b, we observe a similar trend in
to that in
in both models, noticing slight differences in thermal conductance, indicating a similar heat transfer capacity in both models throughout the calculated energy range.
The performance of the thermopower or
S as a function of the Fermi energy is very intriguing (
Figure 7c) since, around
= 0 eV, a clear difference is observed for both models. To explain this behavior, let us look back at
Figure 7a, where
, and thus
(
Figure 4), becomes more asymmetric for Model II than for Model I around
= 0 eV. This asymmetry causes the high value of
S, creating a strong dependence on it concerning the asymmetric nature of the transmission or
. Consequently, the higher the
S is, the larger the
will be due to their proportionality (see Equation (
21)). Therefore, to look for high thermoelectric efficiency (i.e., large
S value), we must seek high antisymmetrical transmission within the system. Thus, analyzing
Figure 7c, we observe that for energies far from
= 0 eV, the amplitude of
S decreases, and the transmission becomes more symmetrical or coherent [
39].
Finally, in
Figure 7d, we depict the
factor with the same energy range as in the previous graphics in
Figure 7. The fundamental differences between the two models occur for energies close to 0 eV. The maximum value reached for Model I is circa
= 10, while for Model II, the maximum value obtained is roughly
= 15, in accordance with the
S profile, where Model II has a higher maximum
S than Model I around these energies due to its asymmetry.
Thus, in
Figure 7a, we can observe marked differences between Models I and II at the value of
eV in the electrical conductance (
), thermal conductance (
), Seebeck coefficient (
S), and figure of merit (
). This result strongly agrees with the transmission probability (
Figure 4) since its value evaluated at
eV presents antiresonance for Model II, where the transmission probability decreases considerably and reaches almost zero, whereas Model I has non-zero transmission. In this sense, according to Equations (
18)–(
21), the electrical and thermal properties depend on the transmission integral (
) evaluated on the Fermi energy; therefore, at
eV, the largest differences between the models are shown for each of these properties (
Figure 7).
To evaluate the dependence of electrical conductance (
) on the Fermi energy (
) and the temperature (
), we studied its variation in defined intervals of interest within a weak-coupling regime (
Figure 8a,b). We observe that at low temperatures, the electrical conductance is higher owing to the lower resistance for the passage of electrons through the molecule due to less vibrational motion of the atoms within its chemical skeleton. Thus, at higher temperatures, the resistance is higher and the electrical conductance decreases. This pattern is evident for both models, depicting similar behavior. On the other hand, in
Figure 8c,d, we present the maximum value of
as a function of temperature at
= 0.2 eV and as a function of
at
= 300 K, respectively. For this analysis, we have studied the variation in the Fermi energy within a window of (
eV
eV), because the most important behavior is observed when the Fermi energy is fixed around the inner edges of the allowed bands.
These maximum values of
are within the same range for both models, and they steeply decrease as the temperature increases (
Figure 8c). This behavior is expected due to power dissipation effects. Notably, the order of magnitude for the calculated electrical conductance agrees with that reported by Kolivoska et al. [
16], who studied electrical transport through molecular rods functionalized with catechol and proposed that the low conductance is attributed to molecules trapped in an energy gap.
In contrast, the maximum values of
increase as the coupling increases (
) due to the hybridization of the discrete states of the molecule with the delocalized states of the contacts. The difference between the two models is an average value of 1
A/V over all
values, being higher for Model I (
Figure 8d).
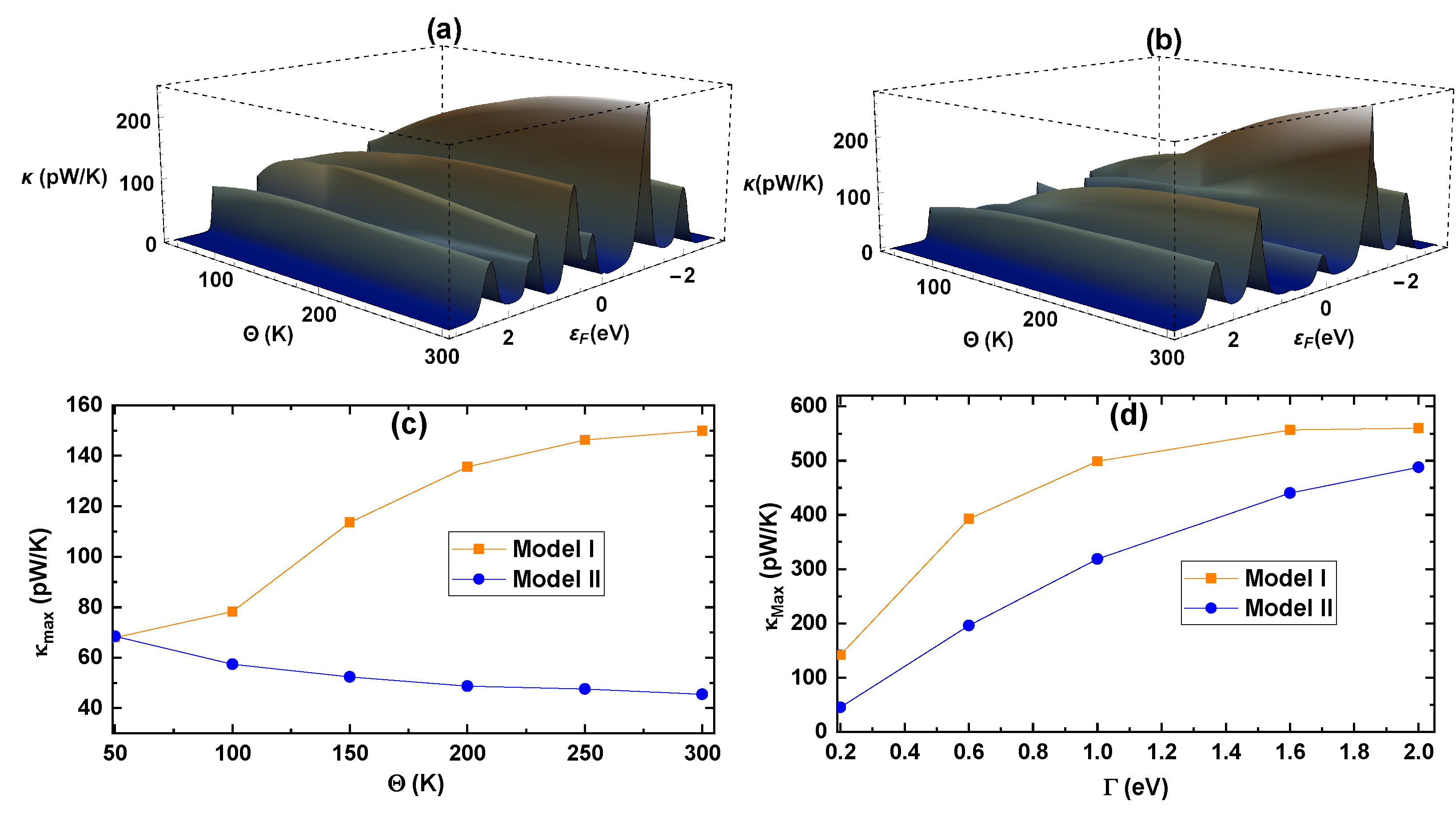
We evaluated the thermal conductance (
) at several temperatures (
) and various Fermi energy values (
) to understand its dependence on these two factors (
Figure 9a,b). In accordance with expectations, we observe that
increases with the increase in temperature, reaching its maximum at 300 K with 246.2 pW/K and 275.9 pW/K for Models I and II, respectively, at
= −1.17 eV. Extracting the maximum values of
with respect to the temperature (
Figure 9c) and to the coupling potential (
) at a constant temperature (
Figure 9d) within the range of Fermi energies (
eV
eV), we clearly defined its dependence on these two factors. Looking at
Figure 9c, Model I presents an evident proportionality between
and the temperature, whereas, for Model II,
is indirectly proportional to the temperature. This behavior is because the thermal conductance around the Fermi energy
= 0 eV for Model II is very small, and as the energy window around this value increases,
decreases slightly and is almost constant when the temperature rises; then, within this energy range, the temperature has a minor influence on Model II’s thermal conductance. When analyzing the dependence of the maximum conductance with respect to
, we observe similar behavior for both models, increasing with higher potentials, resembling what we observed in
Figure 8d, where, in this case,
= 560 pW/K is reached by Model I (see
Figure 9d).
Continuing with our analysis of the thermoelectrical properties for Models I and II, we determined the Seebeck coefficient (
S) as a function of Fermi energy (
) and temperature (
) (
Figure 10a,b). As expected, as the temperature rises, S increases in both models, which is seen more explicitly in
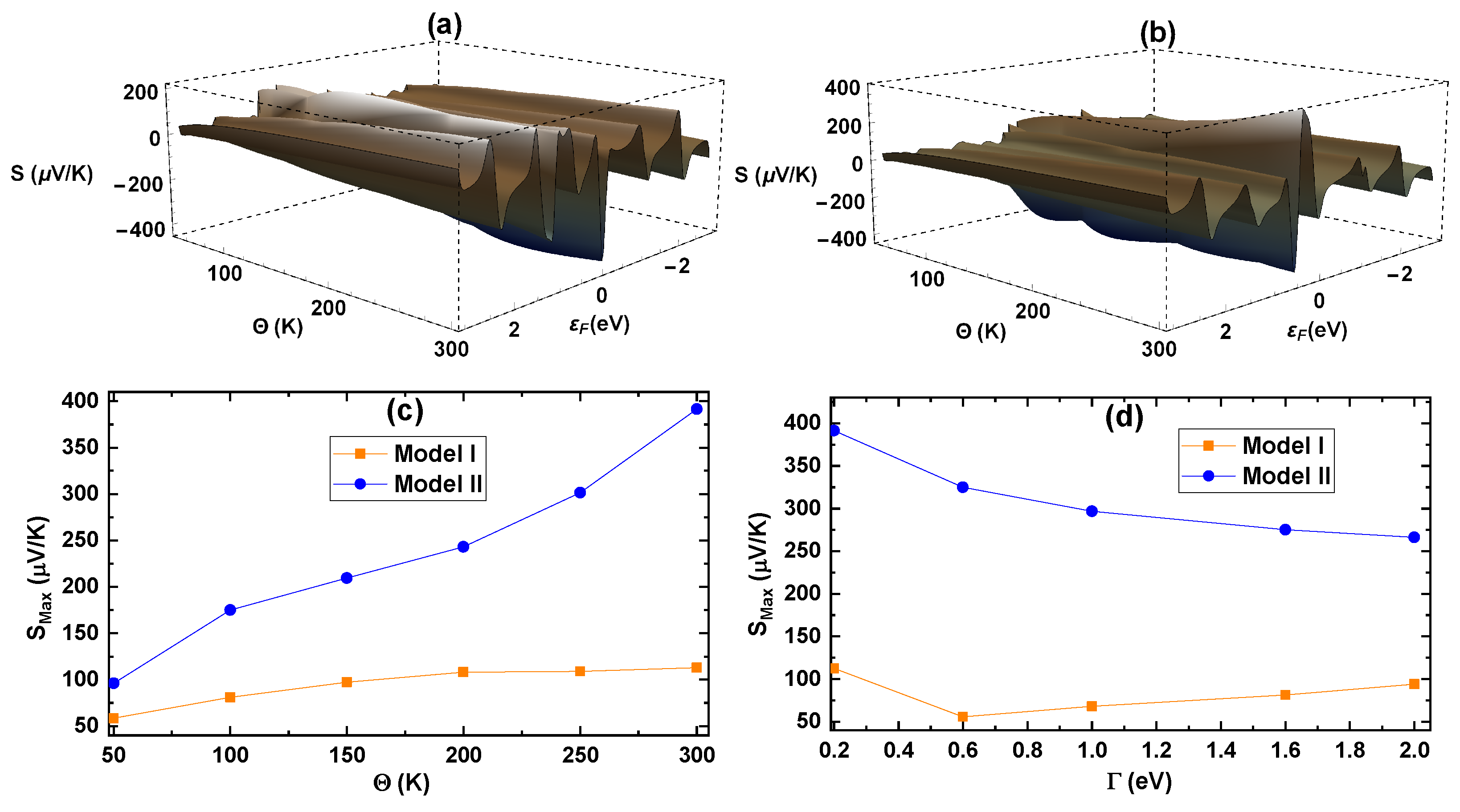
Figure 10c, where we depict the maximum of the Seebeck coefficient as a function of the temperature with
= 0.2 eV in an energy range of
eV
0.5 eV. The increment in the Seebeck coefficient is more significant in Model II, reaching its maximum at 391.5 µV/K versus 112.8 µV/K for Model I. This result is because as the temperature increases around the Fermi level
= 0 eV, the transmission probability becomes more asymmetric for Model II, as we previously described. Additionally, when we evaluate the variation in S
with varying
coupling (
Figure 10d), we find that it steeply decreases for Model II, while it slightly decreases for Model I. This result is directly related to the higher symmetry of the transmission probability around the Fermi energy in stronger coupling regimes.
Finally, we determined the figure of merit (ZT) as a function of temperature (
) and Fermi energy (
Figure 11a,b). Additionally, for a more explicit analysis, we extracted their maximum values as a function of
(
Figure 11c) and
(
Figure 11d). At first sight, we observe a similar behavior to that obtained for S, as ZT is proportional to it. What is interesting to note is that the ZT
values for both models have the highest performance in the low-coupling regime (
= 0.2 eV), 10 and 14.7 for Model I and Model II, respectively. Conversely, in higher-coupling regimes, ZT
decreases to a value of about 1.5 for both models. This result is strongly related to the transmission probability values in strong-coupling regimes, where it becomes more symmetrical, and electron transport is more coherent. Therefore, we conclude that the maximum efficiency for the models evaluated, in terms of energy conversion, occurs for the system at the highest evaluated temperature (i.e.,
= 300 K) and in a weak-coupling regime (
= 0.2 eV).