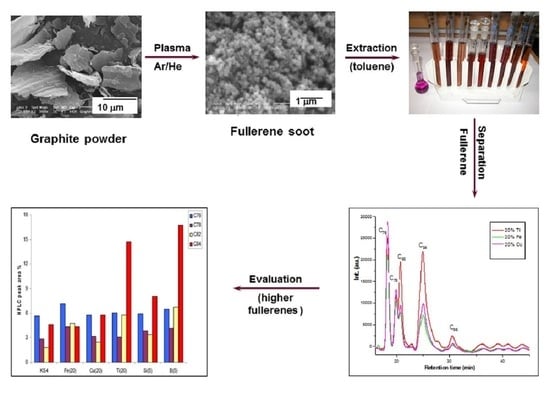
Effect of Metallic and Non-Metallic Additives on the Synthesis of Fullerenes in Thermal Plasma
Abstract
:1. Introduction
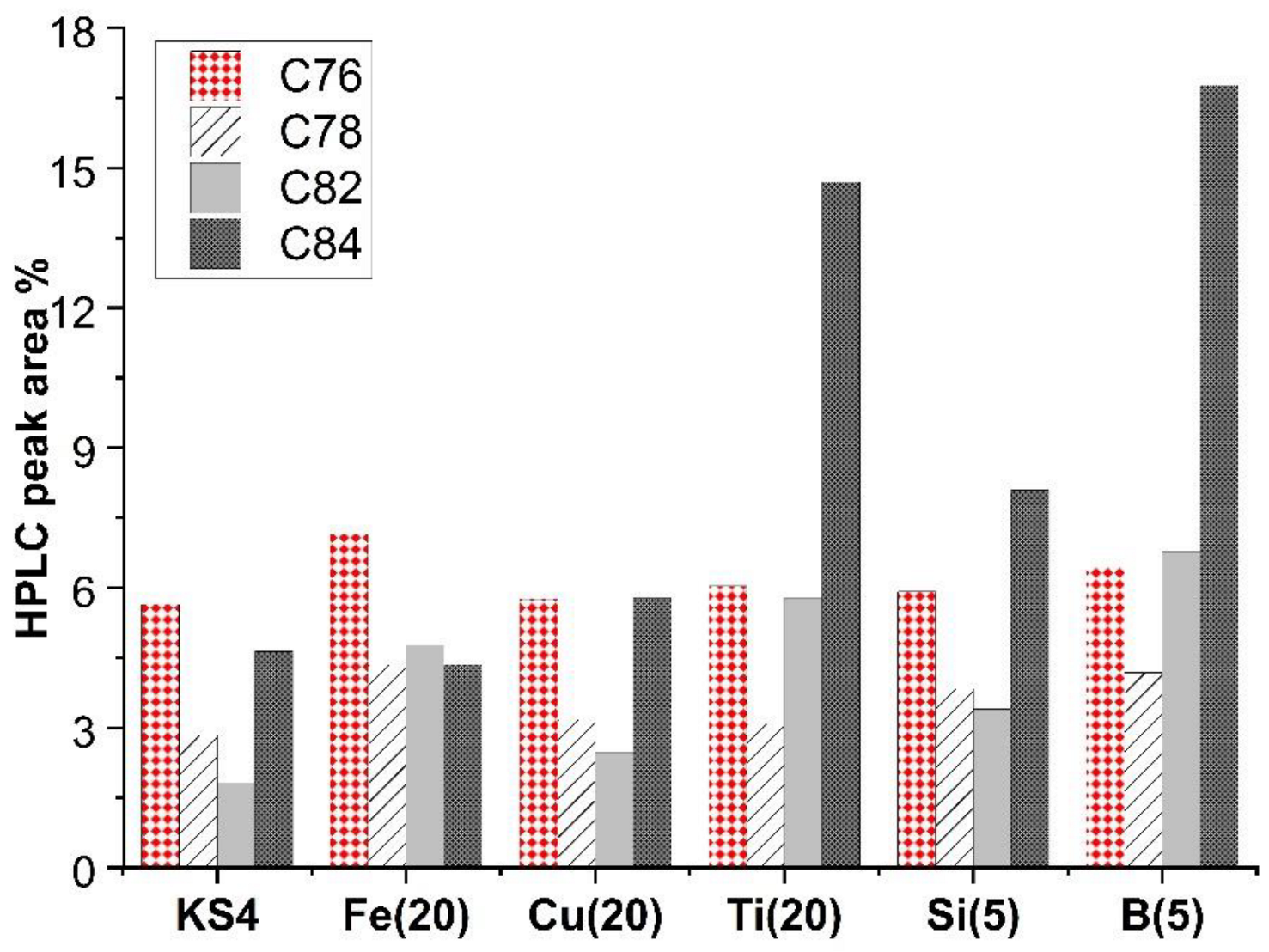
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kroto, H.W.; Heath, J.R.; O’Brien, S.C.; Curl, R.F.; Smalley, R.E. C60: Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Guldi, D.M.; Illescas, B.M.; Atienza, C.M.; Wielopolskia, M.; Martin, N. Fullerene for organic electronics. Chem. Soc. Rev. 2009, 38, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-Fullerene Bulk-Heterojunction Solar Cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Alsulam, I.K.; Alharbi, T.M.; Moussa, M.; Raston, C.L. High-Yield Continuous-Flow Synthesis of Spheroidal C60@ Graphene Composites as Supercapacitors. ACS Omega 2019, 4, 19279–19286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramezanitaghartapeh, M.; Achazi, A.J.; Soltani, A.; Miró, P.; Mahon, P.J.; Hollenkamp, A.F.; Musameh, M. Sustainable cyanide-C60 fullerene cathode to suppress the lithium polysulfides in a lithium-sulfur battery. Sustain. Mater. Technol. 2022, 32, e00403. [Google Scholar] [CrossRef]
- Shetti, N.P.; Mishra, A.; Basu, S.; Aminabhavi, T.M. Versatile fullerenes as sensor materials. Mater. Today Chem. 2021, 20, 100454. [Google Scholar] [CrossRef]
- Aoyagi, S.; Nishibori, E.; Sawa, H.; Sugimoto, K.; Takata, M.; Miyata, Y.; Kitaura, R.; Shinohara, H.; Okada, H.; Sakai, T.; et al. A layered ionic crystal of polar Li@C(60) superatoms. Nat. Chem. 2010, 2, 678–683. [Google Scholar] [CrossRef]
- Todorović Marković, B.; Jokanović, V.; Jovanović, S.; Kleut, D.; Dramićanin, M.; Marković, Z. Surface chemical modification of fullerene by mechanochemical treatment. Appl. Surf. Sci. 2009, 255, 7537–7541. [Google Scholar] [CrossRef]
- Pilehvar, S.; De Wael, K. Recent Advances in Electrochemical Biosensors Based on Fullerene-C60 Nano-Structured Platforms. Biosensors 2015, 5, 712–735. [Google Scholar] [CrossRef] [Green Version]
- Wudl, F. Fullerene materials. J. Mater. Chem. 2002, 12, 1959–1963. [Google Scholar] [CrossRef]
- Maruyama, H.; Tomonoh, S.; Alford, J.M.; Karpuk, M.E. Fullerene Production in Tons and More: From Science to Industry. Fuller. Nanotub. Carbon Nanostruct. 2005, 1, 1–9. [Google Scholar] [CrossRef]
- Gruenberger, T.; Probst, N.; Fulcheri, L. Fullerene and carbon nanoparticle production via continuous AC plasma process. Chimica Oggi 2004, 22, 48–51. [Google Scholar] [CrossRef]
- Keypour, H.; Noroozi, M.; Rashidi, A. An improved method for the purification of fullerene from fullerene soot with activated carbon, celite, and silica gel stationary phases. J. Nanostruct. Chem. 2013, 3, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Kyesmen, P.I.; Onoja, A.; Amah, A.N. Fullerenes synthesis by combined resistive heating and arc discharge techniques. Chem. SpringerPlus 2016, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Kratschmer, W.; Lamb, L.D.; Fostiropoulos, K.; Huffman, D.R. Solid C60: A new form of carbon. Nature 1990, 347, 354–358. [Google Scholar] [CrossRef]
- Lange, A.; Huczko, A. Influence of nitrogen on carbon arc plasma and formation of fullerenes. Chem. Phys. Lett. 2001, 340, 1–6. [Google Scholar] [CrossRef]
- Churilov, G.N. Synthesis of fullerenes and other nanomaterials in arc discharge Fullerenes. Nanotub. Carbon Nanostruct. 2008, 16, 395–403. [Google Scholar] [CrossRef]
- Kareev, I.E.; Nekrasov, V.M.; Buhnov, V.P. Electric-arc synthesis of soot with a high content of higher fullerenes. Tech. Phys. 2015, 60, 102–106. [Google Scholar] [CrossRef]
- Chibante, L.P.F.; Thess, A.; Alford, J.M.; Diener, M.D.; Smalley, R.E. Solar generation of the fullerenes. J. Phys. Chem. 1993, 61, 8696–8700. [Google Scholar] [CrossRef]
- Mordkovich, V.Z.; Shiratori, Y.; Hiraoka, H.; Takeuchi, Y. Synthesis of multishell fullerenes by laser vaporization of composite carbon targets. Phys. Solid State 2002, 44, 603–606. [Google Scholar] [CrossRef]
- Todorovic-Markovic, B.; Markovic, Z.; Mohai, I.; Nikolic, Z.; Farkas, Z.; Szépvölgyi, J. Influence of Carbon Concentration and Rotational Temperature on Fullerene Yield in RF Reactor. Mater. Sci. Forum 2006, 518, 211–216. [Google Scholar] [CrossRef]
- Todorovic-Markovic, B.; Markovic, Z.; Mohai, I.; Károly, Z.; Gál, L.; Föglein, K.; Szabó, P.T.; Szépvölgyi, J. Efficient synthesis of fullerenes in RF thermal plasma reactor. Chem. Phys. Lett. 2003, 378, 434–439. [Google Scholar] [CrossRef]
- Yoshie, K.; Kasuya, S.; Eguchi, K.; Yoshida, T. Novel method for C60 synthesis: A thermal plasma at atmospheric pressure. Appl. Phys. Lett. 1992, 61, 2782–2783. [Google Scholar] [CrossRef]
- Howard, J.B.; Mckinnon, J.T.; Makarovsky, Y.; Lafleur, A.L.; Johnson, M.E. Fullerenes C60 and C70 in flames. Nature 1991, 352, 139–142. [Google Scholar] [CrossRef]
- Takehara, H.; Fujiwara, M.; Arikawa, M.; Diener, M.D.; Alford, J.M. Experimental study of industrial scale fullerene production by combustion synthesis. Carbon 2005, 43, 311–319. [Google Scholar] [CrossRef]
- Alford, J.M.; Diener, M.D.; Nabity, J.; Karpuk, M. Burners and combustion apparatus for carbon nanomaterial production. US Patent 72792317, 9 October 2007. [Google Scholar]
- Alford, J.M.; Bernal, C.; Cates, M.; Diener, M.D. Fullerene production in soothing flames from 1,2,3,4-tetrahydronaphthalene. Carbon 2008, 46, 1623–1625. [Google Scholar] [CrossRef]
- Amsharov, K.Y.; Jansen, M. A C78 Fullerene Precursor: Toward the Direct Synthesis of Higher Fullerenes. J. Org. Chem. 2008, 73, 2931–2934. [Google Scholar] [CrossRef]
- Otero, G.; Giulio, G.; Sánchez-Sánchez, B.C.; Caillard, R.; López, M.F.; Rogero, C.; Palomares, F.J.; Cabello, N.; Basanta, M.A.; Ortega, J.; et al. Fullerenes from aromatic precursors by surface-catalysed cyclodehydrogenation. Nature 2008, 454, 865–868. [Google Scholar] [CrossRef]
- Kabdulov, M.A.; Amsharov, K.Y.; Jansen, M. A step toward direct fullerene synthesis: C60 fullerene precursors with fluorine in key positions. Tetrahedron 2010, 66, 8587–8593. [Google Scholar] [CrossRef]
- Viñes, F.; Görling, A. Template-Assisted Formation of Fullerenes from Short-Chain Hydrocarbons by Supported Platinum Nanoparticles. Angew. Chem. Int. Ed. 2011, 50, 4611–4614. [Google Scholar] [CrossRef]
- Marković, Z.; Todorović- Marković, B.; Mohai, I.; Farkas, Z.; Kovats, E.; Szepvolgyi, J.; Otašević, D.; Scheier, P.; Feil, S.; Romčević, N. Comparative Process Analysis of Fullerene Production by the Arc and the Radio-Frequency Discharge Methods. J. Nanosci. Nanotechnol. 2007, 7, 1357–1369. [Google Scholar] [CrossRef]
- Szepvolgyi, J.; Marković, Z.; Todorović- Marković, B.; Nikolic, Z.; Mohai, I.; Farkas, Z.; Tóth, M.; Kováts, E.; Scheier, P.; Feil, S. Effects of precursors and plasma parameters on fullerene synthesis in RF thermal plasma reactor. Plasma Chem. Plasma Proc. 2006, 26, 597–608. [Google Scholar] [CrossRef]
- Wang, C.; Imahori, T.; Tanaka, Y.; Sakuta, T.; Takikawa, H.; Matsuo, H. Silicon inclusion effect on fullerene formation under induction thermal plasma condition. Thin Solid Films 2002, 407, 72–78. [Google Scholar] [CrossRef]
- Cota-Sanchez, G.; Soucy, G.; Huczko, A.; Beauvais, J.; Drouin, D. Effect of Iron Catalyst on the Synthesis of Fullerenes and Carbon Nanotubes in Induction Plasma. J. Phys. Chem. B 2004, 108, 19210–19217. [Google Scholar] [CrossRef]
- Guo, T.; Jin, C.M.; Smalley, R.E. Doping Bucky: Formation and Properties of Boron-Doped Buckminsterfullerene. J. Phys. Chem. 1991, 95, 4948–4950. [Google Scholar] [CrossRef]
- Lange, H.; Huczko, A.; Byszewski, P.; Mizera, E.; Shinohara, H. Influence of boron on carbon arc plasma and formation of fullerenes and nanotubes. Chem. Phys. Lett. 1998, 289, 174–180. [Google Scholar]
- Cota-Sanchez, G.; Soucy, G.; Huczko, A.; Lange, H. Induction plasma synthesis of fullerenes and nanotubes using carbon black–nickel particles. Carbon 2005, 43, 3153–3166. [Google Scholar] [CrossRef]
- Kim, K.S.; Moradian, A.; Mostaghimi, J.; Soucy, G. Modeling of Induction Plasma Process for Fullerene Synthesis: Effect of Plasma Gas Composition and Operating Pressure. Plasma Chem. Plasma Proc. 2010, 30, 91–110. [Google Scholar] [CrossRef]
- Wang, C.; Inazaki, A.; Shirai, T.; Tanaka, Y.; Sakuta, T.; Takikawa, H.; Matsuo, H. Effect of ambient gas and pressure on fullerene synthesis in induction thermal plasma. Thin Solid Films 2003, 425, 41–48. [Google Scholar] [CrossRef]
- Törpe, A.; Belton, D.J. Improved Spectrophotometric Analysis of Fullerenes C60 and C70 in High-solubility Organic Solvents. Anal. Sci. 2015, 31, 125–130. [Google Scholar]
- Kozlov, V.S.; Suyasova, M.; Lebedev, V.T. Synthesis, extraction, and chromatographic purification of higher empty fullerenes and endohedral gadolinium metallofullerenes. Russ. J. Appl. Chem. 2014, 87, 121–127. [Google Scholar] [CrossRef]
- Tsetkova, L.V.; Keskinov, V.A.; Charykov, N.A.; Alekseev, N.I.; Gruzinskaya, E.G.; Semenov, K.N.; Postnov, V.N.; Krokhina, O.A. Extraction of fullerene mixture from fullerene soot with organic solvents. Russ. J. Gen. Chem. 2011, 81, 920–926. [Google Scholar] [CrossRef]
- Raebiger, J.W.; Alford, J.M.; Bolskar, R.D.; Diener, M.D. Chemical redox recovery of giant, small-gap and other fullerenes. Carbon 2011, 49, 37–46. [Google Scholar] [CrossRef]
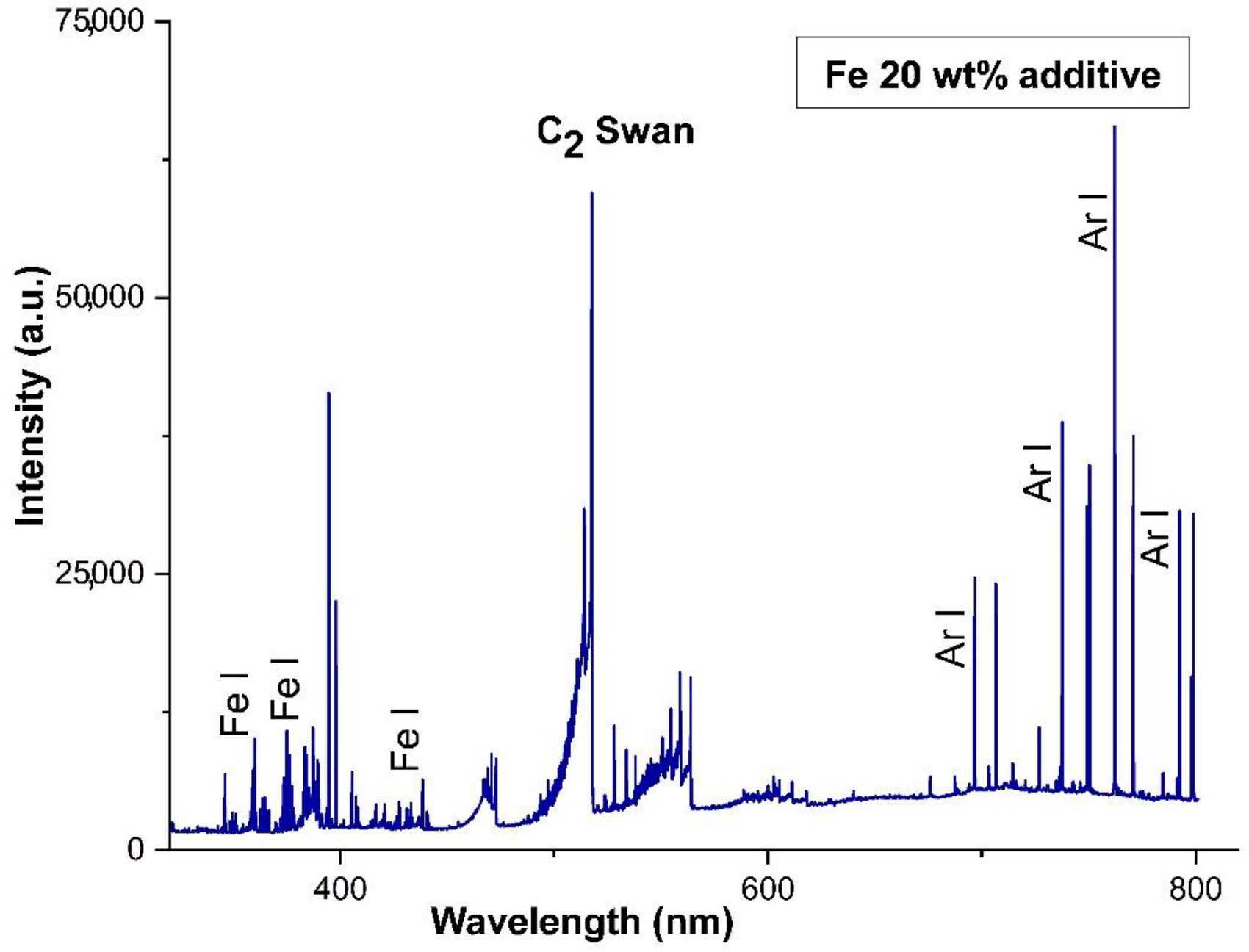
- Parriger, C.G.; Plemmons, D.H.; Hornkohl, J.O.; Lewis, J.W.L. Spectroscopic temperature measurements in a decaying laser-induced plasma using the C2 Swan system. J. Quant. Spectrosc. Radiat. Transfer. 1994, 52, 707–711. [Google Scholar] [CrossRef]
- Parriger, C.G.; Hornkohl, J.O.; Keszler, A.M.; Nemes, L. Measurement and analysis of atomic and diatomic carbon spectra from laser ablation of graphite. Appl. Opt. 2003, 42, 6192–6198. [Google Scholar]
- Selvan, R.; Pradeep, T. Towards the synthesis and characterization of metallocarbohedrenes. Chem. Phys. Lett. 1999, 309, 149–156. [Google Scholar] [CrossRef]
- Taylor, J.C. Computer Programs for Standardless Quantitative Analysis of Minerals Using the Full Powder Diffraction Profile. Powder Diffr. 1991, 6, 2–9. [Google Scholar] [CrossRef] [Green Version]

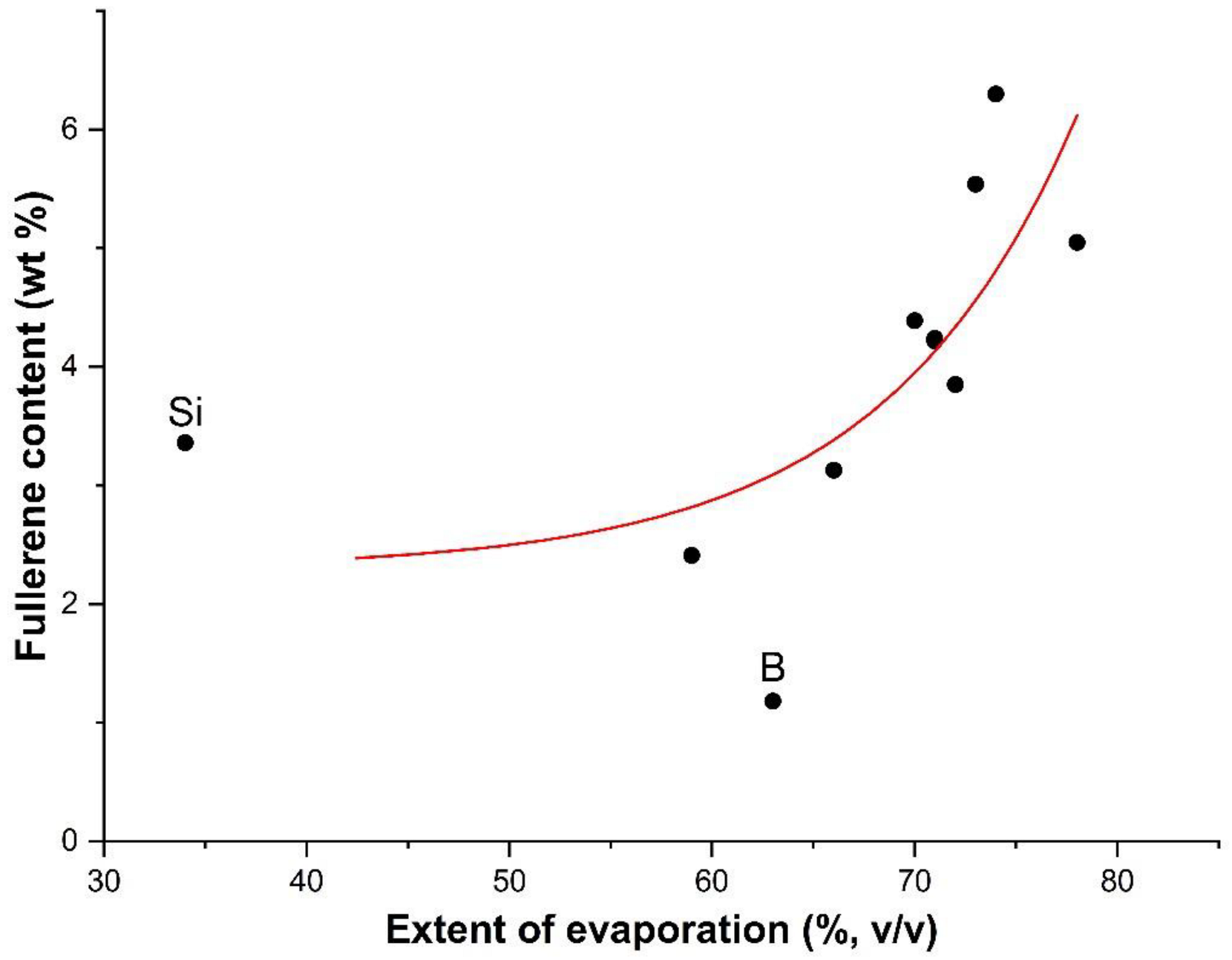
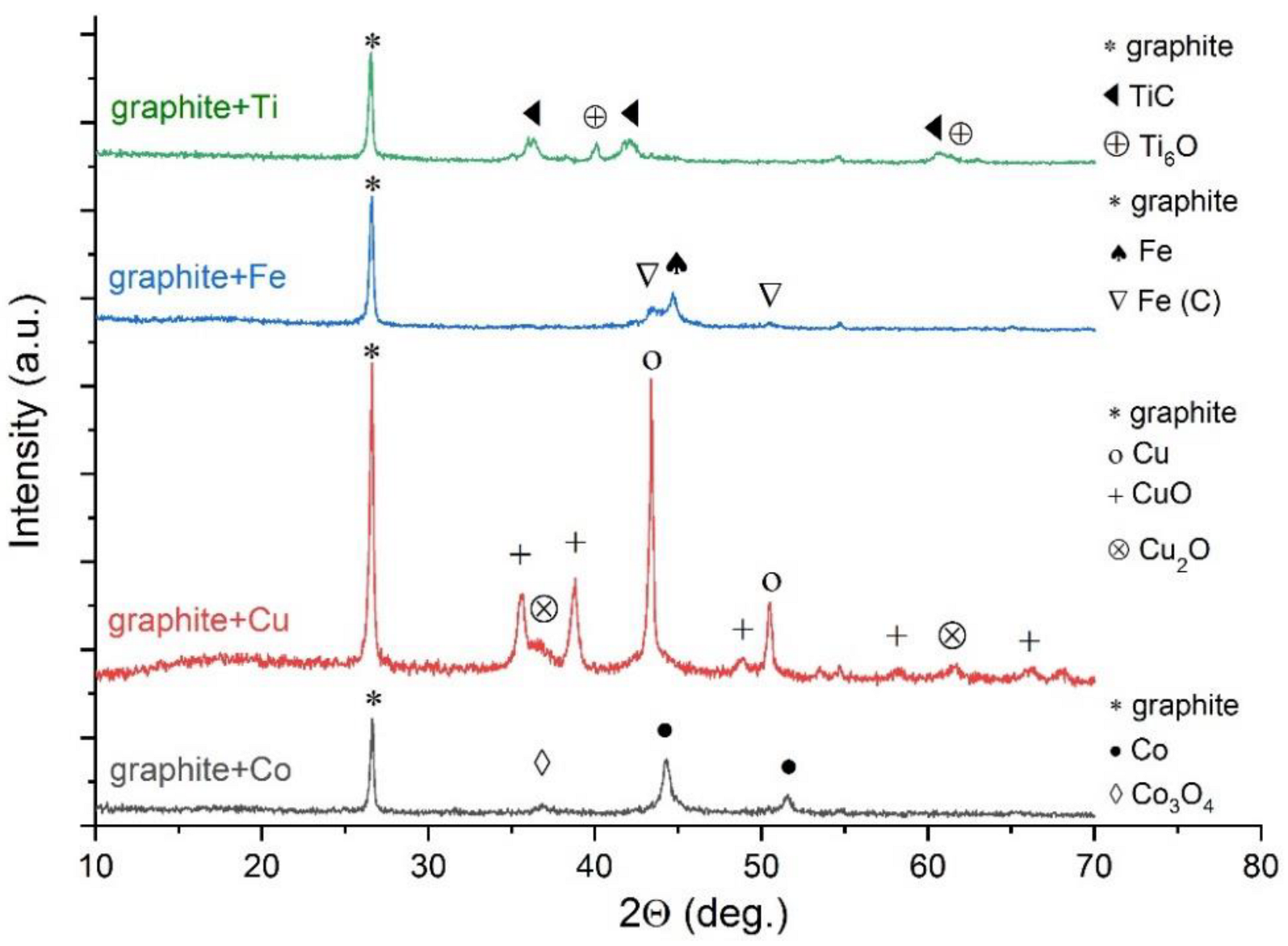

| Run | Additive (wt%) | Feed Rate (g·h−1) | Espec (kWh·g−1) | Evaporation Ratio (%) | C70/C60 Ratio | Tvib-rot (K) | Fullerene Content (wt%) |
|---|---|---|---|---|---|---|---|
| 1 | - | 19 | 1.47 | 78 | 0.269 | 4500 | 5.1 |
| 2 | Fe (5) | 13 | 2.15 | 71 | 0.261 | - | 4.2 |
| 3 | Fe (5) | 46 | 0.61 | 72 | 0.259 | - | 3.9 |
| 4 | Fe (20) | 17 | 1.65 | 74 | 0.277 | 5100 | 6.2 |
| 5 | Cu (20) | 23 | 1.21 | 73 | 0.281 | 4000 | 5.5 |
| 6 | Ni (20) | 21 | 1.33 | 71 | 0.282 | 5800 | 4.3 |
| 7 | Co (20) | 22 | 1.27 | 70 | 0.288 | 5600 | 4.4 |
| 8 | Ti (20) | 22 | 1.27 | 59 | 0.465 | 4600 | 2.5 |
| 9 | Si (5) | 33 | 0.85 | 34 | 0.307 | 3900 | 3.3 |
| 10 | B (5) | 12 | 2.33 | 63 | 0.332 | 4500 | 1.2 |
| 11 | Cu (5) | 44 | 0.64 | 66 | 0.271 | 3900 | 3.2 |
| Pressure (kPa) | Fullerene Content % | |
|---|---|---|
| No Additive | Cu Additive | |
| 50 | 0.1 | 0.1 |
| 72 | 1.7 | 1.5 |
| 92 | 5.05 | 5.54 |
| Additive (wt%) | Fullerene Content % | ||
|---|---|---|---|
| 0 Days | 7 Days | 30 Days | |
| - | 5.05 | 3.38 | 2.47 |
| Fe (5) | 4.22 | 3.37 | 2.53 |
| Fe (20) | 6.30 | 4.28 | 3.213 |
| Cu (20) | 5.54 | 3.87 | 2.65 |
| Si (5) | 3.36 | 2.35 | 1.78 |
| B (5) | 1.18 | 1.10 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keszler, A.M.; Kováts, É.; Bódis, E.; Károly, Z.; Szépvölgyi, J. Effect of Metallic and Non-Metallic Additives on the Synthesis of Fullerenes in Thermal Plasma. Condens. Matter 2022, 7, 44. https://doi.org/10.3390/condmat7030044
Keszler AM, Kováts É, Bódis E, Károly Z, Szépvölgyi J. Effect of Metallic and Non-Metallic Additives on the Synthesis of Fullerenes in Thermal Plasma. Condensed Matter. 2022; 7(3):44. https://doi.org/10.3390/condmat7030044
Chicago/Turabian StyleKeszler, Anna Mária, Éva Kováts, Eszter Bódis, Zoltán Károly, and János Szépvölgyi. 2022. "Effect of Metallic and Non-Metallic Additives on the Synthesis of Fullerenes in Thermal Plasma" Condensed Matter 7, no. 3: 44. https://doi.org/10.3390/condmat7030044
APA StyleKeszler, A. M., Kováts, É., Bódis, E., Károly, Z., & Szépvölgyi, J. (2022). Effect of Metallic and Non-Metallic Additives on the Synthesis of Fullerenes in Thermal Plasma. Condensed Matter, 7(3), 44. https://doi.org/10.3390/condmat7030044