Interfacial Crystallization within Liquid Marbles
Abstract
1. Introduction
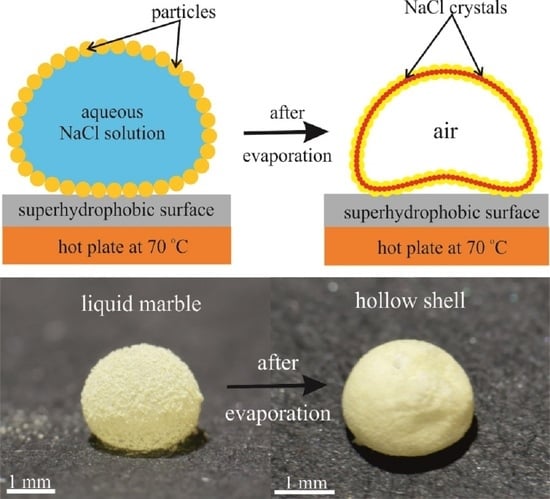
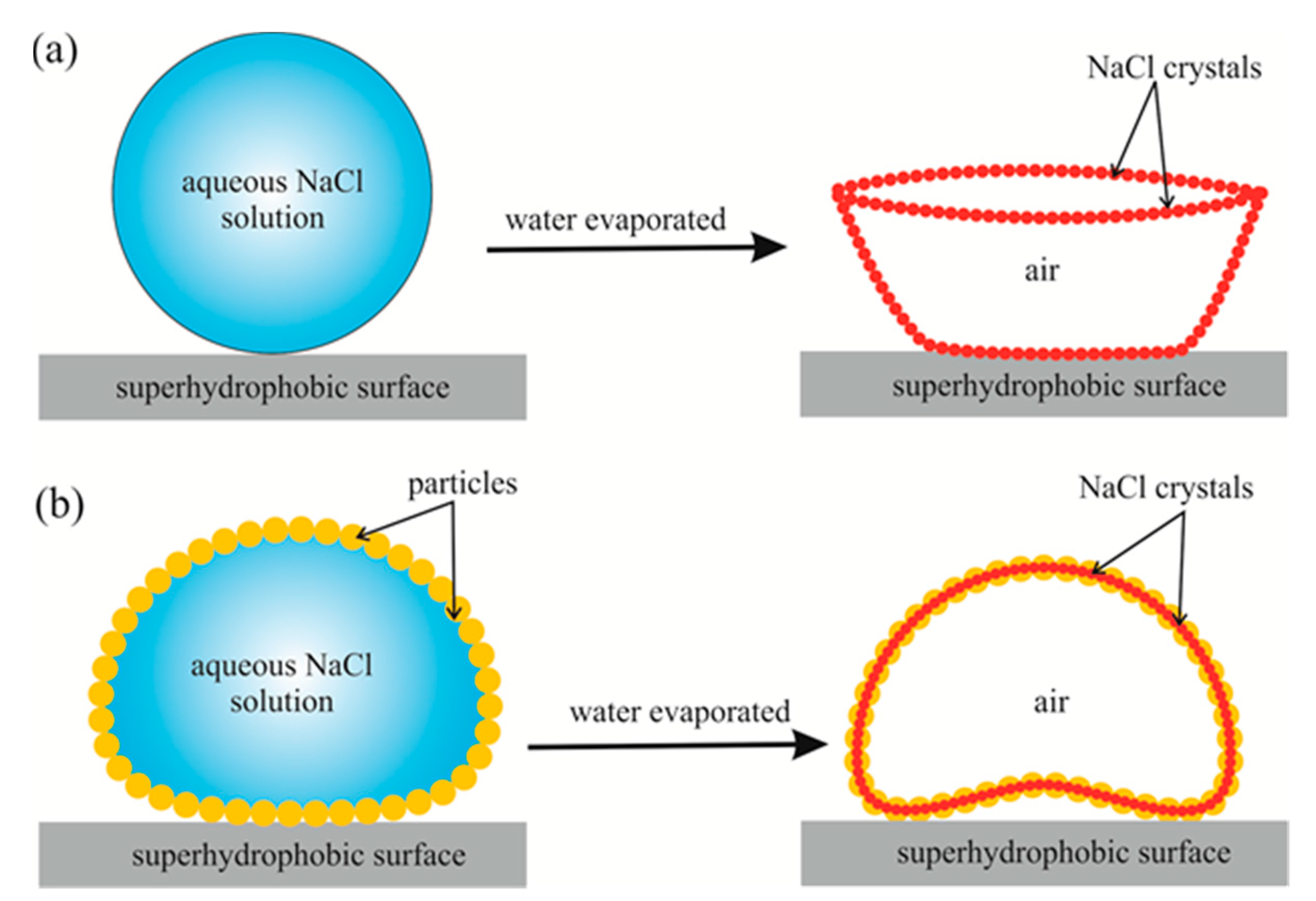
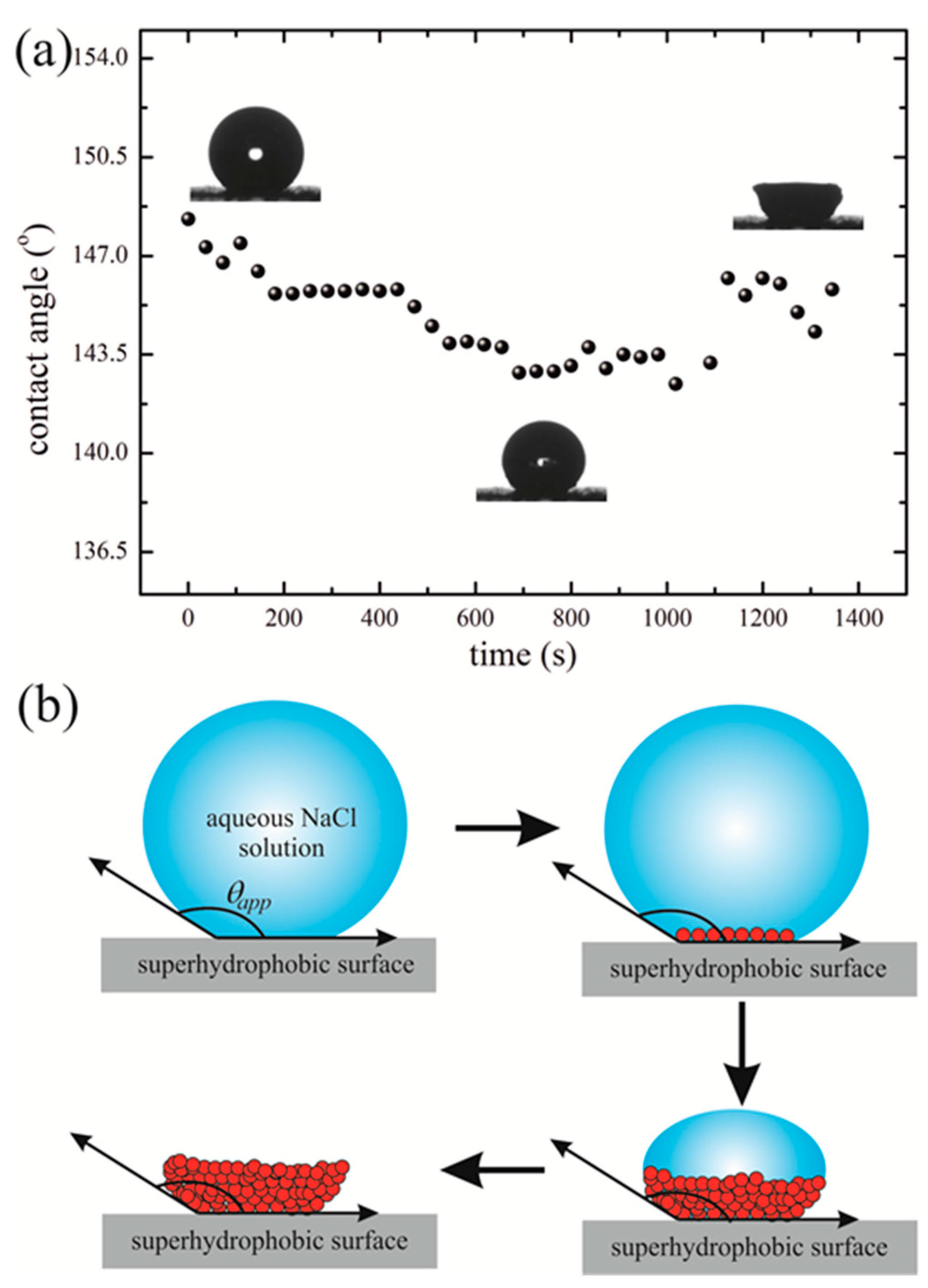
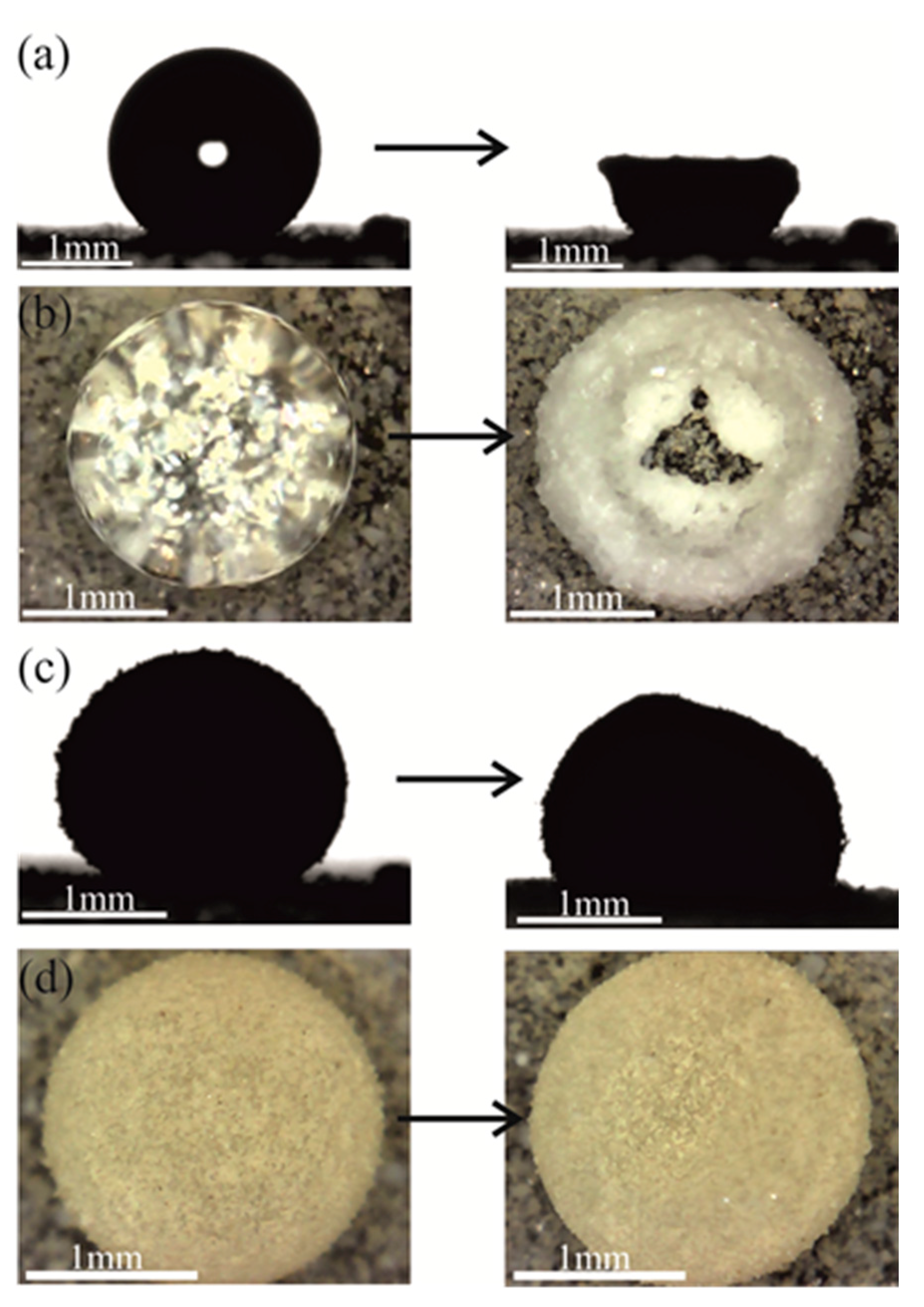
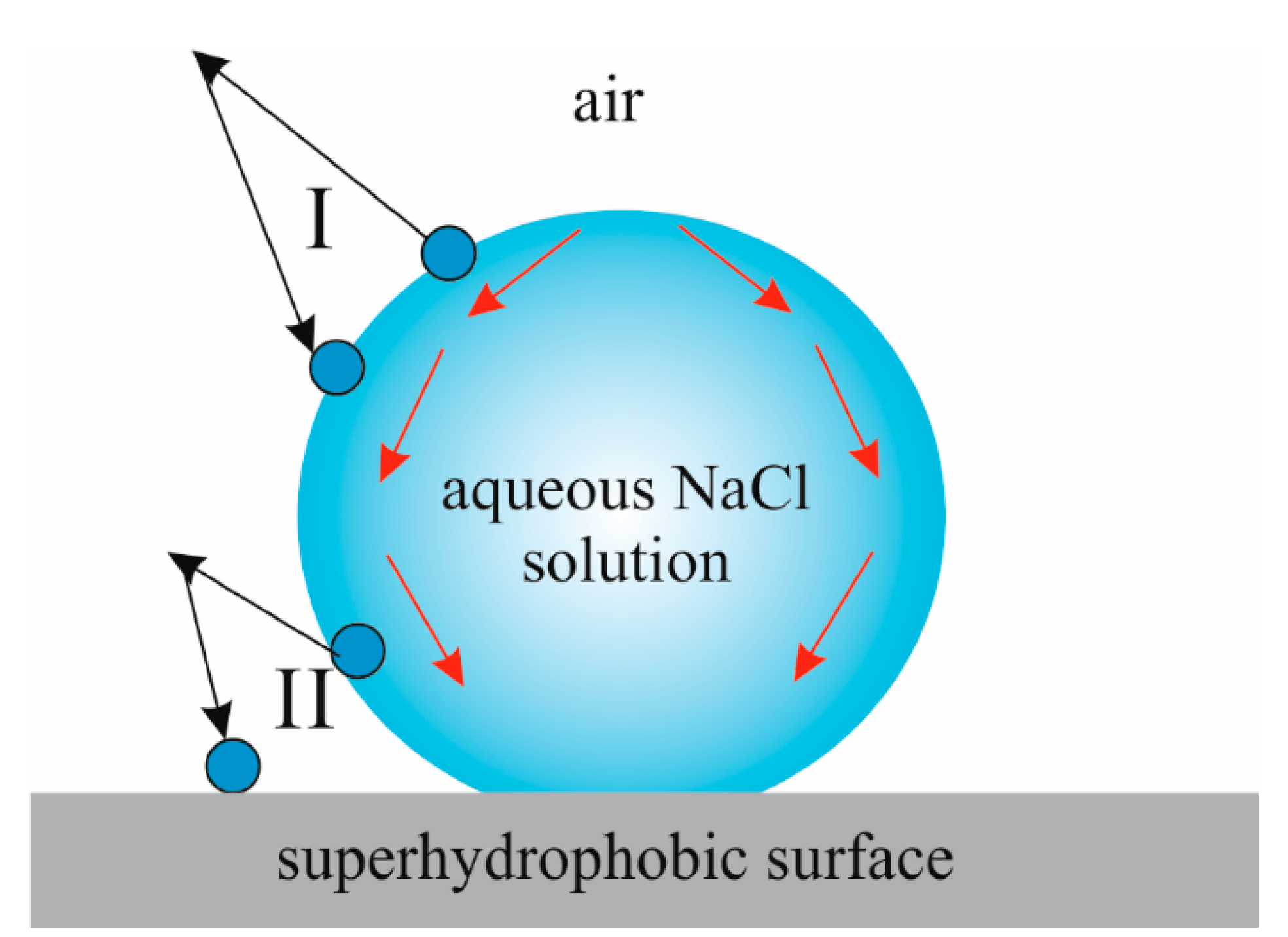
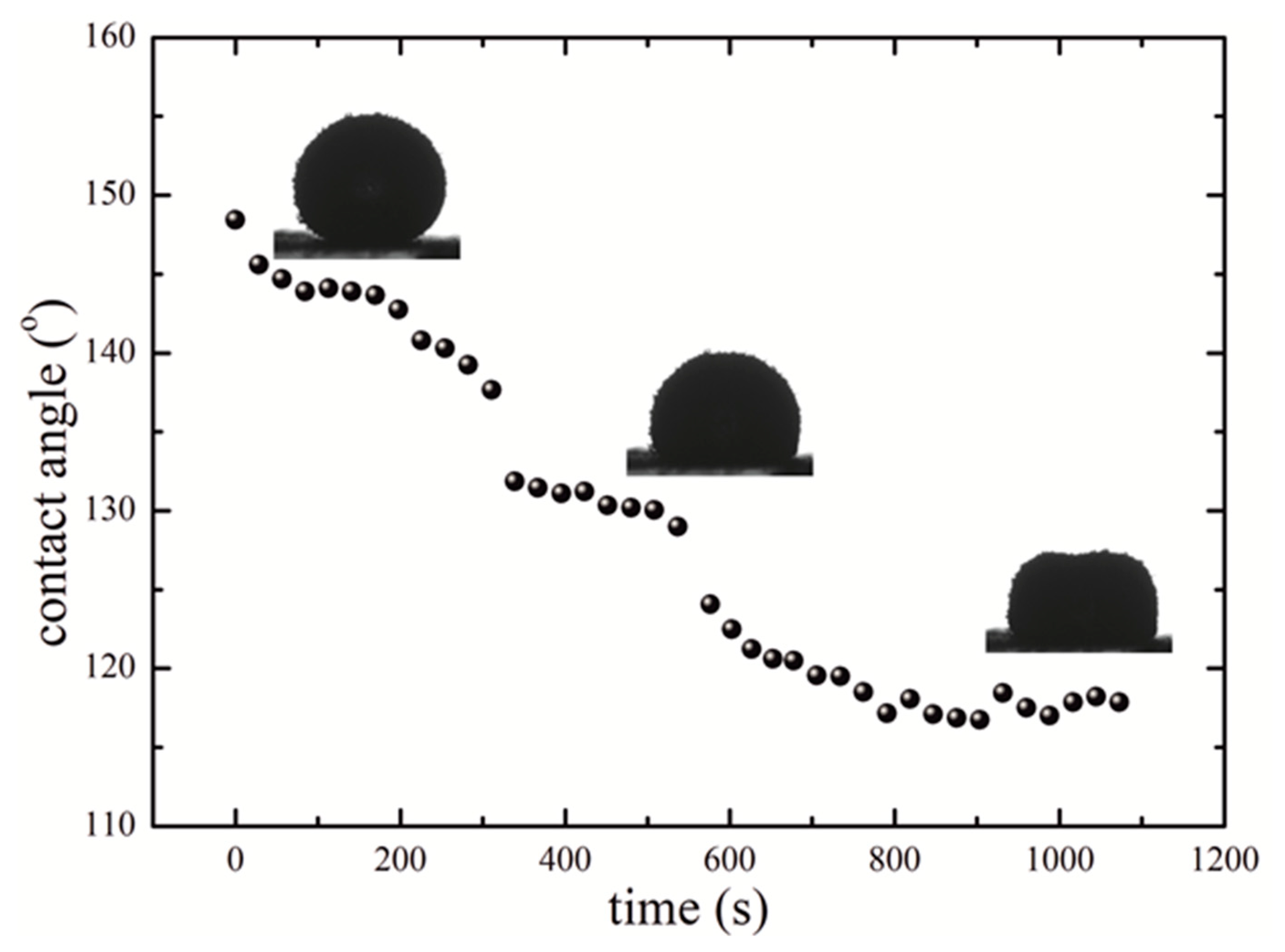
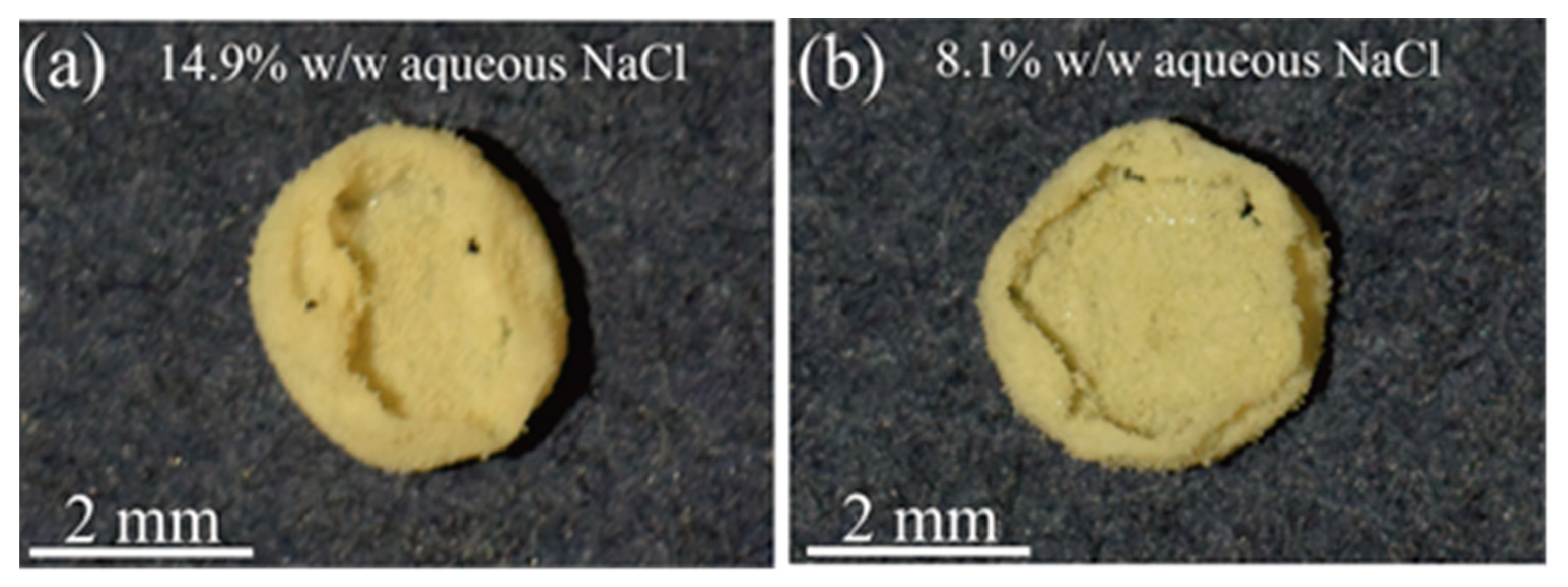
2. Results
3. Discussion
4. Conclusions
5. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ning, N.; Fu, S.; Zhang, W.; Chen, F.; Wang, K.; Deng, H.; Zhang, Q.; Fu, Q. Realizing the enhancement of interfacial interaction in semicrystalline polymer/filler composites via interfacial crystallization. Prog. Polym. Sci. 2012, 37, 1425–1455. [Google Scholar] [CrossRef]
- Ochoa, M.; Collazosa, N.; Lea, T.; Subramaniam, R.; Sanders, M.; Singh, R.P.; Depan, D. Nanocellulose-PE-b-PEG copolymer nanohybrid shish-kebab structure via interfacial crystallization. Carbohydr. Polym. 2017, 159, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gong, F.; He, G.; Li, Y.; Ding, L.; Nie, F.; Huan, F. Perfect Energetic Crystals with Improved Performances Obtained by Thermally Metastable Interfacial Self-Assembly of Corresponding Nanocrystals. Cryst. Growth Des. 2018, 18, 1657–1665. [Google Scholar] [CrossRef]
- Miyazawa, K.; Kuwasaki, Y.; Obayashi, A.; Kuwabara, K. C60 Nanowhiskers Formed by the Liquid–liquid Interfacial Precipitation Method. J. Mater. Res. 2002, 17, 83–88. [Google Scholar] [CrossRef]
- Tanaka, M.; Yamanaka, S.; Shirakawa, Y.; Shimosaka, A.; Hidaka, J. Preparation of porous particles by liquid–liquid interfacial crystallization. Adv. Powder Technol. 2011, 22, 125–130. [Google Scholar] [CrossRef]
- Kadota, K.; Shirakawa, Y.; Matsumoto, I.; Shimosaka, A.; Hidaka, J. Formation and morphology of asymmetric NaCl particles precipitated at the liquid-liquid interface. Adv. Powder Technol. 2007, 18, 775–785. [Google Scholar] [CrossRef]
- Tai, C.Y.; Wu, J.-F.; Rousseau, R.W. Interfacial supersaturation, secondary nucleation, and crystal growth. J. Cryst. Growth 1992, 116, 294–306. [Google Scholar] [CrossRef]
- Schmelzer, J.; Möller, J.; Gutzow, I.; Pascov, R.; Müller, R.; Pannhorst, W. Surface energy and structure effects on surface crystallization. J. Non-Cryst. Solids 1995, 183, 215–233. [Google Scholar] [CrossRef]
- Abyzov, A.S.; Schmelzer, J.W.P. Generalized Gibbs’ approach in heterogeneous nucleation. J. Chem. Phys. 2013, 138, 164504. [Google Scholar] [CrossRef]
- Abyzov, A.S.; Davydov, L.N.; Schmelzer, J.W.P. Heterogeneous Nucleation in Solutions on Rough Solid Surfaces: Generalized Gibbs Approach. Entropy 2019, 21, 782. [Google Scholar] [CrossRef]
- Aussillous, P.; Quéré, D. Liquid marbles. Nature 2001, 411, 924–927. [Google Scholar] [CrossRef] [PubMed]
- Tenjimbayashi, M.; Watanabe, Y.; Nakamura, Y.; Naito, M. Exceptional Robustness and Self-Reconfigurability of Liquid Marbles on Superhydrophobic Substrate. Adv. Mater. Interfaces 2020, 7, 2000160. [Google Scholar] [CrossRef]
- Li, X. Liquid marbles and liquid plasticines with nanoparticle monolayers. Adv. Colloid Interface Sci. 2019, 271, 101988. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E. Liquid Marbles, Elastic Nonstick Droplets: From Minireactors to Self-Propulsion. Langmuir 2017, 33, 663–669. [Google Scholar] [CrossRef] [PubMed]
- Arbatan, T.; Li, L.; Tian, J.; Shen, W. Liquid Marbles as Micro-bioreactors for Rapid Blood Typing. Adv. Healthc. Mater. 2012, 1, 80–83. [Google Scholar] [CrossRef]
- Tian, J.; Fu, N.; Chen, X.D.; Shen, W. Respirable liquid marble for the cultivation of microorganisms. Colloids Surf. B 2013, 106, 187–190. [Google Scholar] [CrossRef]
- Serrano, M.C.; Nardecchia, S.; Gutiérrez, M.C.; Ferrer, M.L.; del Monte, F. Mammalian Cell Cryopreservation by Using Liquid Marbles. ACS Appl. Mater. Interfaces 2015, 7, 3854–3860. [Google Scholar] [CrossRef]
- Vadivelu, R.K.; Ooi, C.H.; Yao, R.-Q.; Velasquez, J.T.; Pastrana, E.; Nido, J.D.; Lim, F.; Ekberg, J.A.K.; Nguyen, N.-T.; St John, J.A. Generation of three-dimensional multiple spheroid model of olfactory ensheathing cells using floating liquid marbles. Sci. Rep. 2015, 5, 1508. [Google Scholar] [CrossRef]
- Chen, M.; Shah, M.P.; Shelper, T.B.; Nazareth, L.; Barker, M.; Velasquez, J.T.; Ekberg, J.A.K.; Vial, M.L.; St John, J.A. Naked Liquid Marbles: A Robust Three-Dimensional Low-Volume Cell-Culturing System. ACS Appl. Mater. Interfaces 2019, 11, 9814–9823. [Google Scholar] [CrossRef]
- Rychecký, O.; Majerská, M.; Král, V.; Štěpánek, F.; Čejková, J. Spheroid cultivation of HT-29 carcinoma cell line in liquid marbles. Chem. Pap. 2017, 71, 1055–1063. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Gorgannezhad, L.; Jin, J.; Ooi, C.H.; Stratton, H.; Dao, D.V.; Nguyen, N.-T. Liquid marbles as biochemical reactors for the polymerase chain reaction. Lab Chip 2019, 19, 3220–3227. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.K.; Ooi, C.H.; Singha, P.; Jin, J.; Sreejith, K.R.; Phan, H.P.; Nguyen, N.-T. Liquid Marbles as Miniature Reactors for Chemical and Biological Applications. Processes 2020, 8, 793. [Google Scholar] [CrossRef]
- Dandan, M.; Erbil, H.Y. Evaporation Rate of Graphite Liquid Marbles: Comparison with Water Droplets. Langmuir 2009, 25, 8362–8367. [Google Scholar] [CrossRef] [PubMed]
- Tosun, A.; Erbil, H.Y. Evaporation rate of PTFE liquid marbles. Appl. Surf. Sci. 2009, 256, 1278–1283. [Google Scholar] [CrossRef]
- Sreejith, K.R.; Ooi, C.H.; Dao, D.V.; Nguyen, N.-T. Evaporation dynamics of liquid marbles at elevated temperatures. RSC Adv. 2018, 8, 15436–15443. [Google Scholar] [CrossRef]
- Fullarton, C.; Draper, T.C.; Phillips, N.; Mayne, R.; de Lacy Costello, B.P.J.; Adamatzky, A. Evaporation, Lifetime, and Robustness Studies of Liquid Marbles for Collision-Based Computing. Langmuir 2018, 34, 2573–2580. [Google Scholar] [CrossRef]
- Fullarton, C.; Draper, T.C.; Phillips, N.; de Lacy Costello, B.P.J.; Adamatzky, A. Belousov-Zhabotinsky reaction in liquid marbles. J. Phys. Mater. 2019, 2, 015005. [Google Scholar] [CrossRef]
- Adamatzky, A.; Fullarton, C.; Phillips, N.; de Lacy Costello, B.; Draper, T.C. Thermal switch of oscillation frequency in Belousov-Zhabotinsky liquid marbles. R. Soc. Open Sci. 2019, 6, 190078. [Google Scholar] [CrossRef]
- Draper, T.C.; Fullarton, C.; Phillips, N.; de Lacy Costello, B.P.J.; Adamatzky, A. Liquid marble interaction gate for collision-based computing. Mater. Today 2017, 20, 561–568. [Google Scholar] [CrossRef]
- Draper, T.C.; Phillips, N.; Weerasekera, R.; Mayne, R.; Fullarton, C.; de Lacy Costello, B.P.J.; Adamatzky, A. Contactless sensing of liquid marbles for detection, characterization & computing. Lab Chip 2020, 20, 136–146. [Google Scholar] [PubMed]
- Paven, M.; Mayama, H.; Sekido, T.; Butt, H.J.; Nakamura, Y.; Fujii, S. Light-Driven Delivery and Release of Materials Using Liquid Marbles. Adv. Funct. Mater. 2016, 26, 3199–3206. [Google Scholar] [CrossRef]
- Fujii, S.; Yusa, S.I.; Nakamura, Y. Stimuli-Responsive Liquid Marbles: Controlling Structure, Shape, Stability, and Motion. Adv. Funct. Mater. 2016, 26, 7206–7223. [Google Scholar] [CrossRef]
- Bormashenko, E.; Bormashenko, Y.; Grynyov, R.; Aharoni, H.; Whyman, G.; Binks, B.P. Self-Propulsion of Liquid Marbles: Leidenfrost-like Levitation Driven by Marangoni Flow. J. Phys. Chem. C 2015, 119, 9910–9915. [Google Scholar] [CrossRef]
- Ooi, C.H.; Jin, J.; Nguyen, A.V.; Evans, G.M.; Nguyen, N.-T. Picking up and placing a liquid marble using dielectrophoresis. Microfluid. Nanofluid. 2018, 22, 142. [Google Scholar] [CrossRef]
- Jin, J.; Ooi, C.H.; Sreejith, K.R.; Dao, D.V.; Nguyen, N.-T. Dielectrophoretic Trapping of a Floating Liquid Marble. Phys. Rev. Appl. 2019, 11, 044059. [Google Scholar] [CrossRef]
- McBride, S.A.; Dash, S.; Varanasi, K.K. Evaporative Crystallization in Drops on Superhydrophobic and Liquid-Impregnated Surfaces. Langmuir 2018, 34, 12350–12358. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, Y.; Xi, J.; Zhu, Y.; Wang, N.; Xia, F.; Jiang, L. Petal Effect: A Superhydrophobic State with High Adhesive Force. Langmuir 2008, 24, 4114–4119. [Google Scholar] [CrossRef] [PubMed]
- Bormashenko, E.; Stein, T.; Pogreb, R.; Aurbach, D. “Petal Effect” on Surfaces Based on Lycopodium: High-Stick Surfaces Demonstrating High Apparent Contact Angles. J. Phys. Chem. C 2009, 113, 5568–5572. [Google Scholar] [CrossRef]
- Bhushan, B.; Nosonovsky, M. The rose petal effect and the modes of superhydrophobicity. Philos. Trans. R. Soc. A 2010, 368, 4713–4728. [Google Scholar] [CrossRef]
- Bormashenko, E. Progress in understanding wetting transitions on rough surfaces. Adv. Colloid Interface Sci. 2015, 222, 92–103. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Capillary flow as the cause of ring stains from dried liquid drops. Nature 1997, 389, 827–829. [Google Scholar] [CrossRef]
- Deegan, R.D.; Bakajin, O.; Dupont, T.F.; Huber, G.; Nagel, S.R.; Witten, T.A. Contact line deposits in an evaporating drop. Phys. Rev. E 2000, 62, 756–765. [Google Scholar] [CrossRef] [PubMed]
- Misyura, S.Y. The crystallization behavior of the aqueous solution of CaCl2 salt in a drop and a layer. Sci. Rep. 2020, 10, 256. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Kaiqi Zhang, K.; Kong, S.; Wang, X.; Yong, L.; Mi, M. Evaporation and crystallization process for sessile saline droplets during depressurization. Eur. Phys. J. E 2020, 43, 36. [Google Scholar] [CrossRef] [PubMed]
- Balderas-López, J.A.; Mandelis, A.; Garcia, J.A. Thermal-wave resonator cavity design and measurements of the thermal diffusivity of liquids. Rev. Sci. Instrum. 2000, 71, 2933–2937. [Google Scholar] [CrossRef]
- Shahidzadeh-Bonn, N.; Rafa, S.; Bonn, D.; Wegdam, G. Salt Crystallization during Evaporation: Impact of Interfacial Properties. Langmuir 2008, 24, 8599–8605. [Google Scholar] [CrossRef]
- Burton, J.C.; Sharpe, A.L.; van der Veen, R.C.A.; Franco, A.; Nagel, S.R. Geometry of the Vapor Layer Under a Leidenfrost Drop. Phys. Rev. Lett. 2012, 109, 074301. [Google Scholar] [CrossRef]
- Liu, J.L.; Zuo, P.C. Wetting and elasto-plasticity based sculpture of liquid marbles. Eur. Phys. J. E 2016, 39, 1–6. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bormashenko, E.; Roy, P.K.; Shoval, S.; Legchenkova, I. Interfacial Crystallization within Liquid Marbles. Condens. Matter 2020, 5, 62. https://doi.org/10.3390/condmat5040062
Bormashenko E, Roy PK, Shoval S, Legchenkova I. Interfacial Crystallization within Liquid Marbles. Condensed Matter. 2020; 5(4):62. https://doi.org/10.3390/condmat5040062
Chicago/Turabian StyleBormashenko, Edward, Pritam Kumar Roy, Shraga Shoval, and Irina Legchenkova. 2020. "Interfacial Crystallization within Liquid Marbles" Condensed Matter 5, no. 4: 62. https://doi.org/10.3390/condmat5040062
APA StyleBormashenko, E., Roy, P. K., Shoval, S., & Legchenkova, I. (2020). Interfacial Crystallization within Liquid Marbles. Condensed Matter, 5(4), 62. https://doi.org/10.3390/condmat5040062