Temperature and Impurity Induced Stabilization of Cubic HfV2 Laves Phase
Abstract
1. Introduction
2. Methods
3. Results and Discussion
3.1. Ground State Stability
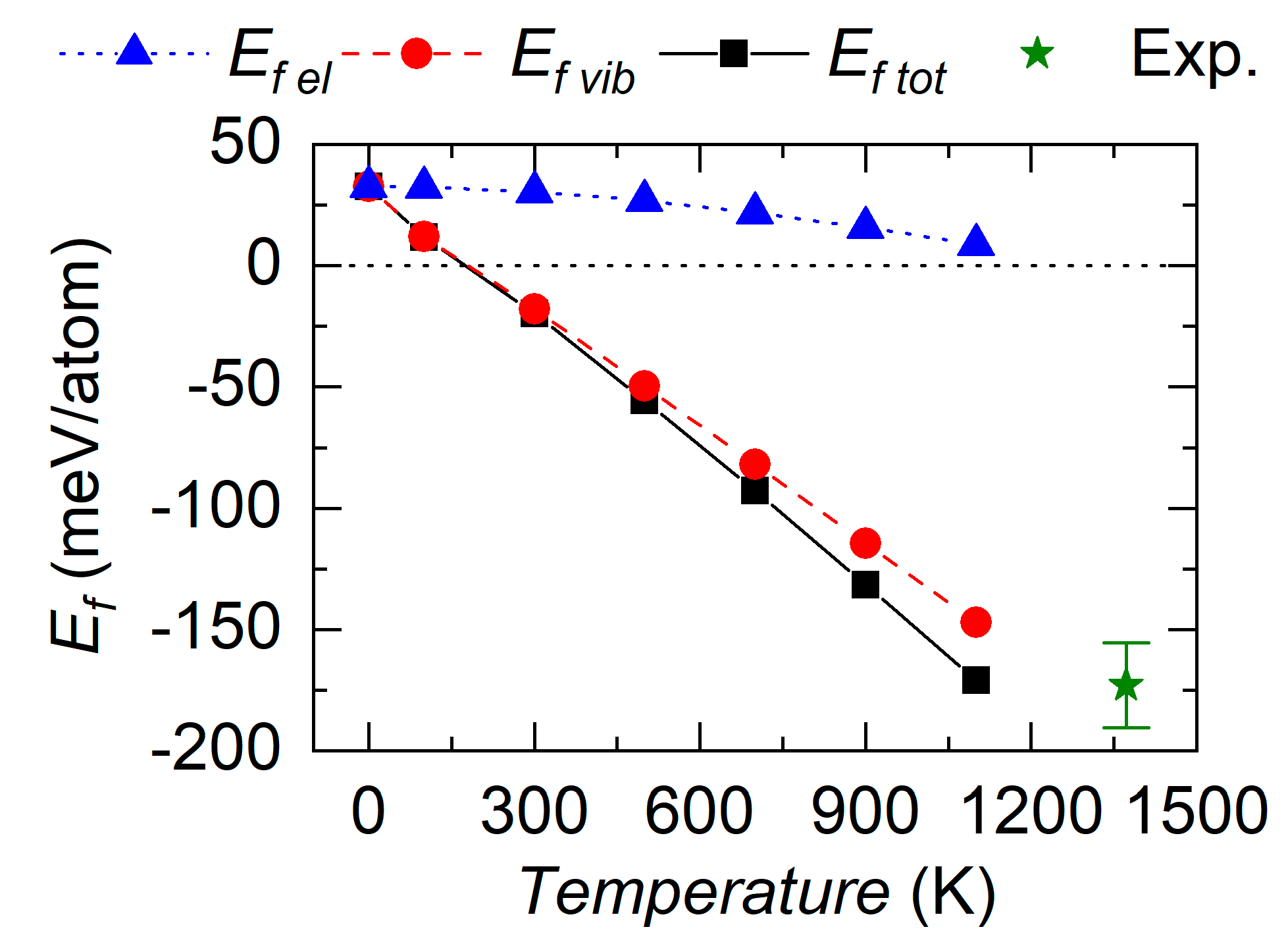
3.2. Temperature Effect on the Energetic Stability
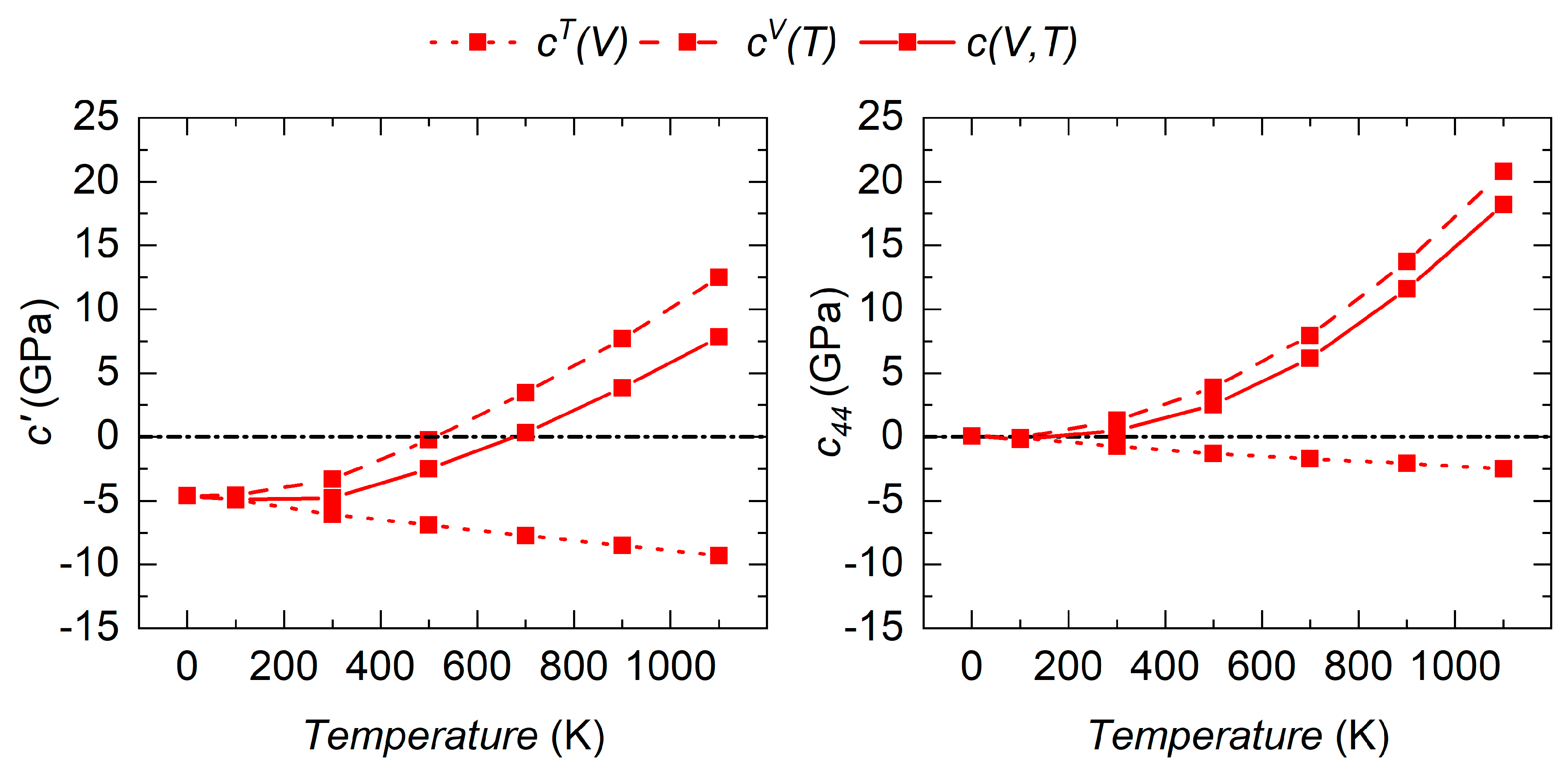
3.3. Temperature Effect on the Mechanical Stability
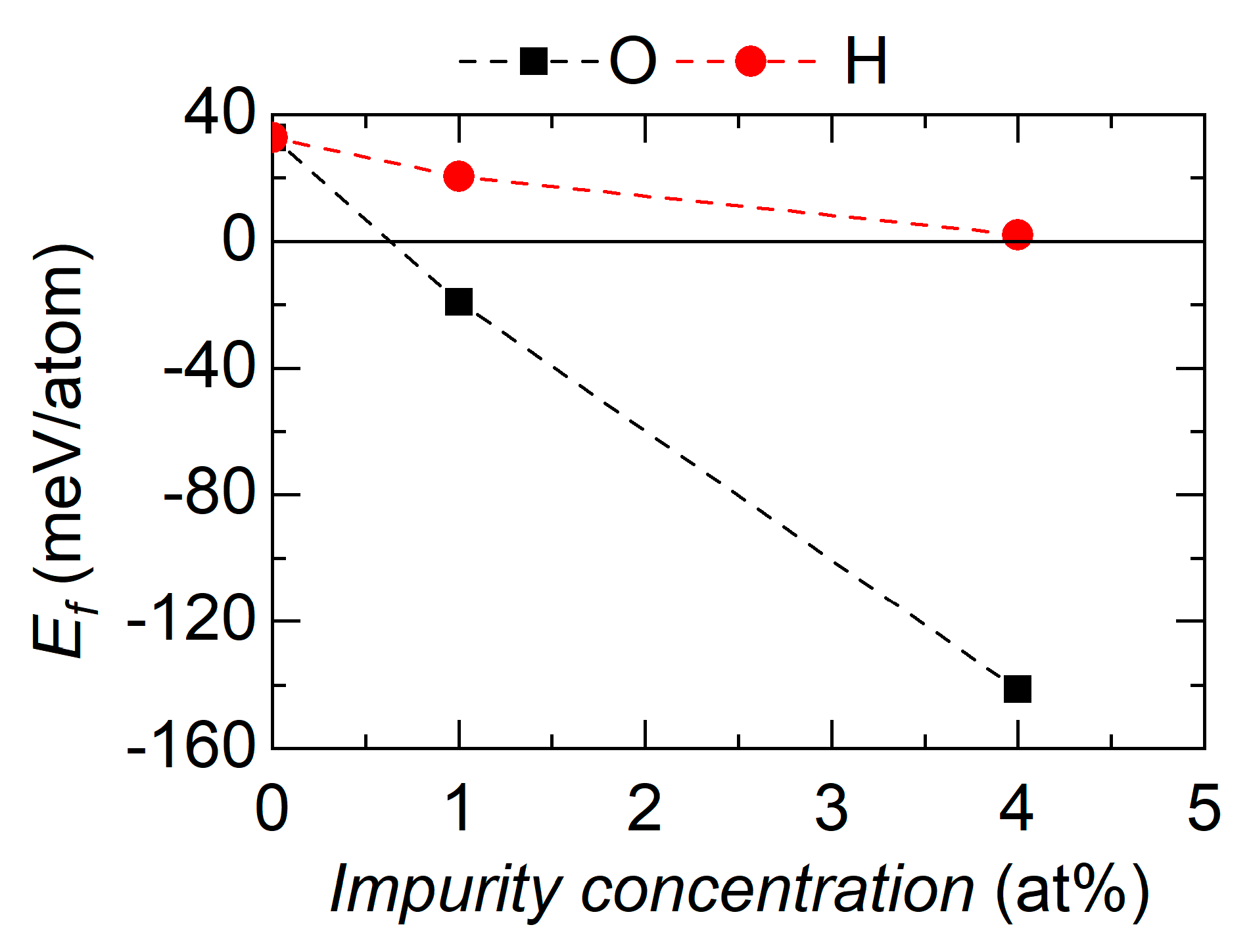
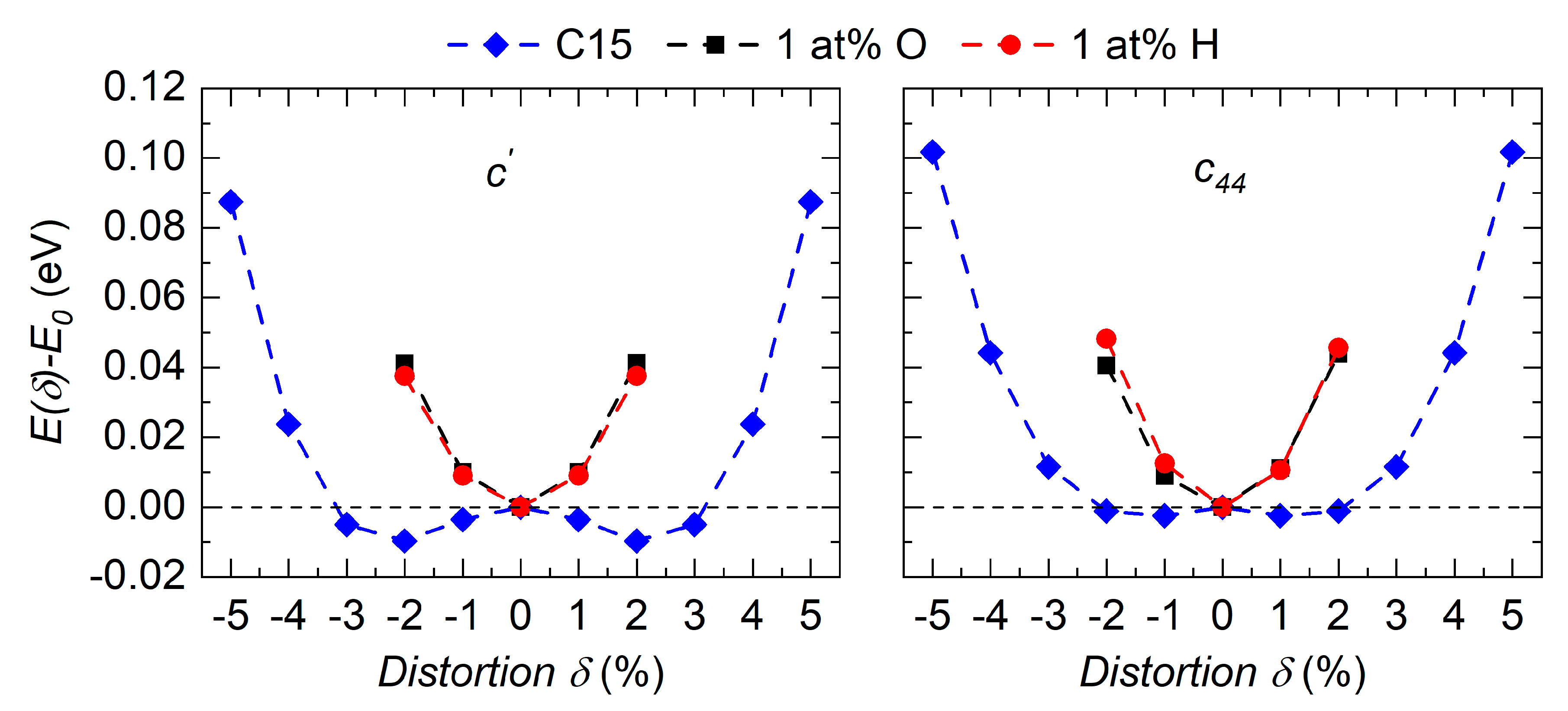
3.4. Effect of Impurities on the Ground State Stability
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lüthi, B.; Herrmann, M.; Assmus, W.; Schmidt, H.; Rietschel, H.; Wühl, H.; Gottwick, U.; Sparn, G.; Steglich, F. Normal-state and superconducting properties of HfV2. Z. Phys. B Condens. Matter 1985, 60, 387–392. [Google Scholar] [CrossRef]
- Drymiotis, F.R.; Lashley, J.C.; Kimura, T.; Lawes, G.; Smith, J.L.; Thoma, D.J.; Fisher, R.A.; Phillips, N.E.; Mudryk, Y.; Pecharsky, V.K.; et al. Specific heat of single-crystal HfV2: Strong-coupling conventional superconductivity and the effect of the martensitic transition. Phys. Rev. B 2005, 72, 024543. [Google Scholar] [CrossRef]
- Dreßler, S.; Taylor, J.W.; Ouladdiaf, B.; Neumann, K.U.; Ziebeck, K.R.A. Suppression of the martensitic phase transition and the effect on superconductivity in HfV2. Solid State Commun. 2000, 113, 649–651. [Google Scholar] [CrossRef]
- Parsons, M.J.; Brown, P.J.; Crangle, J.; Neumann, K.U.; Ouladdiaf, B.; Smith, T.J.; Zayer, N.K.; Ziebeck, K.R.A. A study of the structural phase transformation and superconductivity in HfV2. J. Phys. Condens. Matter 1998, 10, 8523. [Google Scholar] [CrossRef]
- Zhao, Y.; Chu, F.; Von Dreele, R.B.; Zhu, Q. Structural phase transitions of HfV2 at low temperatures. Acta Crystallogr. Sect. B Struct. Sci. 2000, 56, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Chu, F.; Thoma, D.J.; Mitchell, T.E.; Lin, C.L.; Šob, M. Phase stability of C15 MV2 (M=Zr, Hf or Ta): An electronic structure investigation. Philos. Mag. B 1998, 77, 121–136. [Google Scholar] [CrossRef]
- Forker, M.; Herz, W.; Simon, D.; Bedi, S.C. Perturbed-angular-correlation study of static and dynamic quadrupole interactions in the Laves-phase hydrides HfV2Hx. Phys. Rev. B 1995, 51, 15994–16007. [Google Scholar] [CrossRef]
- Däumer, W.; Khan, H.R.; Lüders, K. Electronic structure investigated by NMR and superconductivity of cubic Laves-phase hydrides: V2HfHx (0≤x≤4.5) and V2Hf0.5Zr0.5Hx(0≤x≤4.8). Phys. Rev. B 1988, 38, 4427–4436. [Google Scholar] [CrossRef]
- Heidinger, R.; Appel, H.; Then, G.; Thies, W.G. Observation of Radiation Damage in HfV2 and Its Hydrides by TDPAC. Phys. Status Solidi A 1990, 121, 445–454. [Google Scholar] [CrossRef]
- Radaković, J.; Belošević-Čavor, J.; Koteski, V. Hydrogen storage in Laves phases: First principles study of electronic structure and formation energies in HfV2 hydrides. Int. J. Hydrog. Energy 2013, 38, 9229–9235. [Google Scholar] [CrossRef]
- Finlayson, T.R.; Lanston, E.J.; Simpson, M.A.; Gibbs, E.E.; Smith, T.F. Elastic properties of (Hf,Zr)V2 superconducting compounds. J. Phys. F 1978, 8, 2269. [Google Scholar] [CrossRef]
- Balankin, A.S.; Skorov, D.M. Anomalies of elastic moduli in ZrV2 and HfV2 Laves phases at high temperatures. Sov. Phys. Solid State 1982, 24, 681–682. [Google Scholar]
- Zhang, C. Electronic structure and bonding properties for Laves-phase RV2 (R=Ti, Nb, Hf, and Ta) compounds. Phys. B 2008, 403, 2088–2092. [Google Scholar] [CrossRef]
- Ormeci, A.; Chu, F.; Wills, J.M.; Mitchell, T.E.; Albers, R.C.; Thoma, D.J.; Chen, S.P. Total-energy study of electronic structure and mechanical behavior of C15 Laves phase compounds: NbCr2 and HfV2. Phys. Rev. B 1996, 54, 12753–12762. [Google Scholar] [CrossRef]
- Charifi, Z.; Ali Hussain, R.; Baaziz, H. Electronic band structures of AV2 (A = Ta, Ti, Hf and Nb) Laves phase compounds. J. Phys. Condens. Matter 2009, 21, 025502. [Google Scholar] [CrossRef]
- Radaković, J.; Belošević-Čavor, J.; Koteski, V. First principles study of HfV2 and ZrV2 phases: Structural analysis and site preference of Cd and Ta dopants. Intermetallics 2013, 32, 90–95. [Google Scholar] [CrossRef]
- Rudy, E.; Windisch, S. The phase diagrams hafnium-vanadium and hafnium-chromium. J. Less-Common Met. 1968, 15, 13–27. [Google Scholar] [CrossRef]
- Morant, C.; Galán, L.; Sanz, J.M. An XPS study of the initial stages of oxidation of hafnium. Surf. Interface Anal. 1990, 16, 304–308. [Google Scholar] [CrossRef]
- Chourasia, A.R.; Hickman, J.L.; Miller, R.L.; Nixon, G.A.; Seabolt, M.A. X-Ray Photoemission Study of the Oxidation of Hafnium. Int. J. Spectrosc. 2009, 2009, 6. [Google Scholar] [CrossRef]
- Alov, N.; Kutsko, D.; Spirovová, I.; Bastl, Z. XPS study of vanadium surface oxidation by oxygen ion bombardment. Surf. Sci. 2006, 600, 1628–1631. [Google Scholar] [CrossRef]
- Romanyuk, A.; Oelhafen, P. Oxidation of vanadium with reactive oxygen plasma: A photoelectron spectroscopy study of the initial stages of the oxide growth process. Thin Solid Film. 2007, 515, 6544–6547. [Google Scholar] [CrossRef]
- Forker, M.; Herz, W.; Simon, D. Impurity trapping in the Laves phase HfV2 detected by perturbed angular correlations. J. Phys. Condens. Matter 1992, 4, 213. [Google Scholar] [CrossRef]
- Ivashchenko, V.I.; Turchi, P.E.A. Phonon softening and the phase transition in VN. Phys. Rev. B 2008, 78, 224113. [Google Scholar] [CrossRef]
- Stefanovich, E.V.; Shluger, A.L.; Catlow, C.R.A. Theoretical study of the stabilization of cubic-phase ZrO2 by impurities. Phys. Rev. B 1994, 49, 11560–11571. [Google Scholar] [CrossRef]
- Krčmar, M.; Fu, C.L. Effect of lattice anharmonicity in the structural phase transformation of Laves phase HfV2 alloy: A first-principles investigation. Acta Mater. 2013, 61, 7473–7480. [Google Scholar] [CrossRef]
- Chihi, T.; Fatmi, M.; Ghebouli, B. Ab Initio Calculations for Properties of Laves Phase V2M (M = Zr, Hf, Ta) Compounds. Am. J. Mod. Phys. 2013, 2, 88–92. [Google Scholar] [CrossRef]
- Levy, O.; Hart, G.L.W.; Curtarolo, S. Hafnium binary alloys from experiments and first principles. Acta Mater. 2010, 58, 2887–2897. [Google Scholar] [CrossRef]
- Vřešťál, J.; Pavlů, J.; Wdowik, U.D.; Šob, M. Modelling of phase equilibria in the Hf-V system below room temperature. J. Min. Metall. Sect. B Metall. 2017, 53, 239–247. [Google Scholar] [CrossRef][Green Version]
- Moncton, D.E. Lattice transformation in the superconductivity ZrV2 by neutron diffraction. Solid State Commun. 1973, 13, 1779–1782. [Google Scholar] [CrossRef]
- Smith, T.F.; Shelton, R.N.; Lawson, A.C. Superconductivity and structural instability of (Hf, Zr) V2 and (Hf, Ta) V2 alloys at high pressure. J. Phys. F 1973, 3, 2157. [Google Scholar] [CrossRef]
- Lumley, S.C.; Murphy, S.T.; Burr, P.A.; Grimes, R.W.; Chard-Tuckey, P.R.; Wenman, M.R. The stability of alloying additions in Zirconium. J. Nucl. Mater. 2013, 437, 122–129. [Google Scholar] [CrossRef]
- Widom, M. Prediction of Structure and Phase Transformations. In High-Entropy Alloys: Fundamentals and Applications; Gao, M.C., Yeh, J.-W., Liaw, P.K., Zhang, Y., Eds.; Springer: Cham, Germany, 2016; pp. 267–298. [Google Scholar] [CrossRef]
- Grimvall, G. Thermophysical Properties of Materials; Elsevier: Amsterdam, The Netherlands, 1999. [Google Scholar] [CrossRef]
- Moruzzi, V.L.; Janak, J.F.; Schwarz, K. Calculated thermal properties of metals. Phys. Rev. B 1988, 37, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Söderlind, P.; Nordström, L.; Yongming, L.; Johansson, B. Relativistic effects on the thermal expansion of the actinide elements. Phys. Rev. B 1990, 42, 4544–4552. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B 1993, 48, 13115–13118. [Google Scholar] [CrossRef] [PubMed]
- Kresse, G.; Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal-amorphous-semiconductor transition in germanium. Phys. Rev. B 1994, 49, 14251–14269. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Blöchl, P.E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Birch, F. Finite Elastic Strain of Cubic Crystals. Phys. Rev. 1947, 71, 809–824. [Google Scholar] [CrossRef]
- Murnaghan, F.D. The Compressibility of Media under Extreme Pressures. Proc. Natl. Acad. Sci. USA 1944, 30, 244–247. [Google Scholar] [CrossRef] [PubMed]
- Fast, L.; Wills, J.M.; Johansson, B.; Eriksson, O. Elastic constants of hexagonal transition metals: Theory. Phys. Rev. B 1995, 51, 17431–17438. [Google Scholar] [CrossRef] [PubMed]
- Music, D.; Takahashi, T.; Vitos, L.; Asker, C.; Abrikosov, I.A.; Schneider, J.M. Elastic properties of Fe–Mn random alloys studied by ab initio calculations. Appl. Phys. Lett. 2007, 91, 191904. [Google Scholar] [CrossRef]
- Chung, D.H.; Buessem, W.R. The Voigt-Reuss-Hill Approximation and Elastic Moduli of Polycrystalline MgO, CaF2, β-ZnS, ZnSe, and CdTe. J. Appl. Phys. 1967, 38, 2535–2540. [Google Scholar] [CrossRef]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Dugdale, J.S.; MacDonald, D.K.C. The Thermal Expansion of Solids. Phys. Rev. 1953, 89, 832–834. [Google Scholar] [CrossRef]
- Huang, L.; Vitos, L.; Kwon, S.K.; Johansson, B.; Ahuja, R. Thermoelastic properties of random alloys from first-principles theory. Phys. Rev. B 2006, 73, 104203. [Google Scholar] [CrossRef]
- Huang, L.; Ramzan, M.; Vitos, L.; Johansson, B.; Ahuja, R. Anomalous temperature dependence of elastic constant c44 in V., Nb, Ta, Pd, and Pt. J. Phys. Chem. Solids 2010, 71, 1065–1068. [Google Scholar] [CrossRef][Green Version]
- Pushkarev, E.A.; Petrenko, N.S.; Finkel, V.A. Thermal expansion of the superconducting compound HfV2 at low temperatures. Phys. Status Solidi A 1978, 47, K145–K148. [Google Scholar] [CrossRef]
- Keuter, P.; Music, D.; Schnabel, V.; Stuer, M.; Schneider, J.M. From qualitative to quantitative description of the anomalous thermoelastic behavior of V, Nb, Ta, Pd and Pt. J. Phys. Condens. Matter 2019, 31, 225402. [Google Scholar] [CrossRef] [PubMed]
- Keuter, P.; Music, D.; Stuer, M.; Schneider, J.M. Electronic structure tuning of the anomalous thermoelastic behavior in Nb-X (X = Zr, V, Mo) solid solutions. J. Appl. Phys. 2019, 125, 215103. [Google Scholar] [CrossRef]
- Belozerov, A.S.; Poteryaev, A.I.; Anisimov, V.I. Evidence for strong Coulomb correlations in the metallic phase of vanadium dioxide. JETP Lett. 2011, 93, 70–74. [Google Scholar] [CrossRef]
- Isaacs, E.B.; Marianetti, C.A. Electronic correlations in monolayer VS2. Phys. Rev. B 2016, 94, 035120. [Google Scholar] [CrossRef]
- Tomczak, J.M.; Biermann, S. Multi-orbital effects in optical properties of vanadium sesquioxide. J. Phys. Condens. Matter 2009, 21, 064209. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.L.; Sun, Z.M.; Hashimoto, H.; Barsoum, M.W. Electron correlation effects in the MAX phase Cr2AlC from first-principles. J. Appl. Phys. 2011, 109, 063707. [Google Scholar] [CrossRef]
- Ramzan, M.; Lebègue, S.; Ahuja, R. Correlation effects in the electronic and structural properties of Cr2AlC. Phys. Status Solidi RRL 2011, 5, 122–124. [Google Scholar] [CrossRef]
- Grimvall, G.; Magyari-Köpe, B.; Ozoliņš, V.; Persson, K.A. Lattice instabilities in metallic elements. Rev. Mod. Phys. 2012, 84, 945–986. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. The standard enthalpies of formation of some intermetallic compounds of transition metals by high temperature direct synthesis calorimetry. J. Alloy. Compd. 2006, 415, 143–149. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, Z.; Wang, S.; Li, H.; Dong, J.; Xing, N.; Guo, Y.; Li, W. First-principles study of electronic structure for the Laves-phase compounds HfFe2 and HfV2. J. Alloy. Compd. 2008, 448, 53–58. [Google Scholar] [CrossRef]
- Jaffe, J.E.; Bachorz, R.A.; Gutowski, M. Low-temperature polymorphs of ZrO2 and HfO2: A density-functional theory study. Phys. Rev. B 2005, 72, 144107. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, X.; Duan, D.; Tian, F.; Liu, H.; Li, D.; Zhao, Z.; Sha, X.; Yu, H.; Zhang, H.; et al. First-principles study on the structural and electronic properties of metallic HfH2 under pressure. Sci. Rep. 2015, 5, 11381. [Google Scholar] [CrossRef] [PubMed]
- Aboud, S.; Wilcox, J. A Density Functional Theory Study of the Charge State of Hydrogen in Metal Hydrides. J. Phys. Chem. C 2010, 114, 10978–10985. [Google Scholar] [CrossRef]

| C15 | Code | Ef (meV/atom) | a (Å) | B (GPa) | |||
|---|---|---|---|---|---|---|---|
| This Work | VASP PBE | 32.7 | 7.316 | 143 | 137 | 146 | 0 |
| VASP LDA | 20.4 | 7.140 | |||||
| DFT [28] | VASP PBE | 34.8 | 7.278 | ||||
| VASP LDA | 26.8 | 7.109 | |||||
| Wien2k PBE | 27.1 | 7.330 | |||||
| DFT [15] | Wien2k PBE | −18.0 | 7.319 | ||||
| DFT [14] | LMTO LDA-RPA | −15.7 | 171 | ||||
| Exp. [1] | - | 7.38 | 111 * | 128 * | 102 * | 17 * |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Keuter, P.; Music, D.; Stuer, M.; Schneider, J.M. Temperature and Impurity Induced Stabilization of Cubic HfV2 Laves Phase. Condens. Matter 2019, 4, 63. https://doi.org/10.3390/condmat4030063
Keuter P, Music D, Stuer M, Schneider JM. Temperature and Impurity Induced Stabilization of Cubic HfV2 Laves Phase. Condensed Matter. 2019; 4(3):63. https://doi.org/10.3390/condmat4030063
Chicago/Turabian StyleKeuter, Philipp, Denis Music, Michael Stuer, and Jochen M. Schneider. 2019. "Temperature and Impurity Induced Stabilization of Cubic HfV2 Laves Phase" Condensed Matter 4, no. 3: 63. https://doi.org/10.3390/condmat4030063
APA StyleKeuter, P., Music, D., Stuer, M., & Schneider, J. M. (2019). Temperature and Impurity Induced Stabilization of Cubic HfV2 Laves Phase. Condensed Matter, 4(3), 63. https://doi.org/10.3390/condmat4030063






