Interior Melting of the C3B16 and C2B14− Clusters Between 1000 K and 2000 K
Abstract
1. Introduction
2. Computational Methods
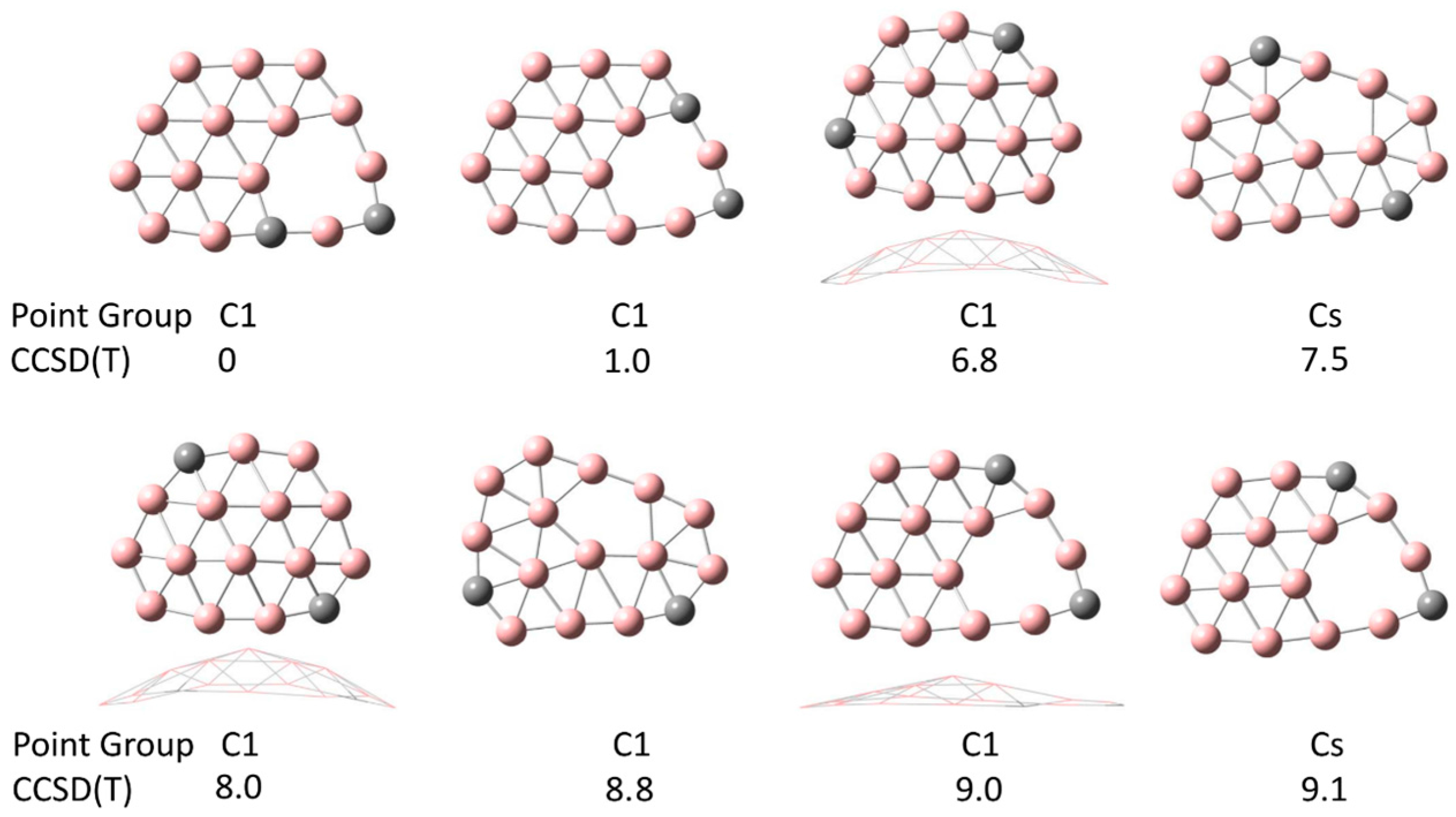
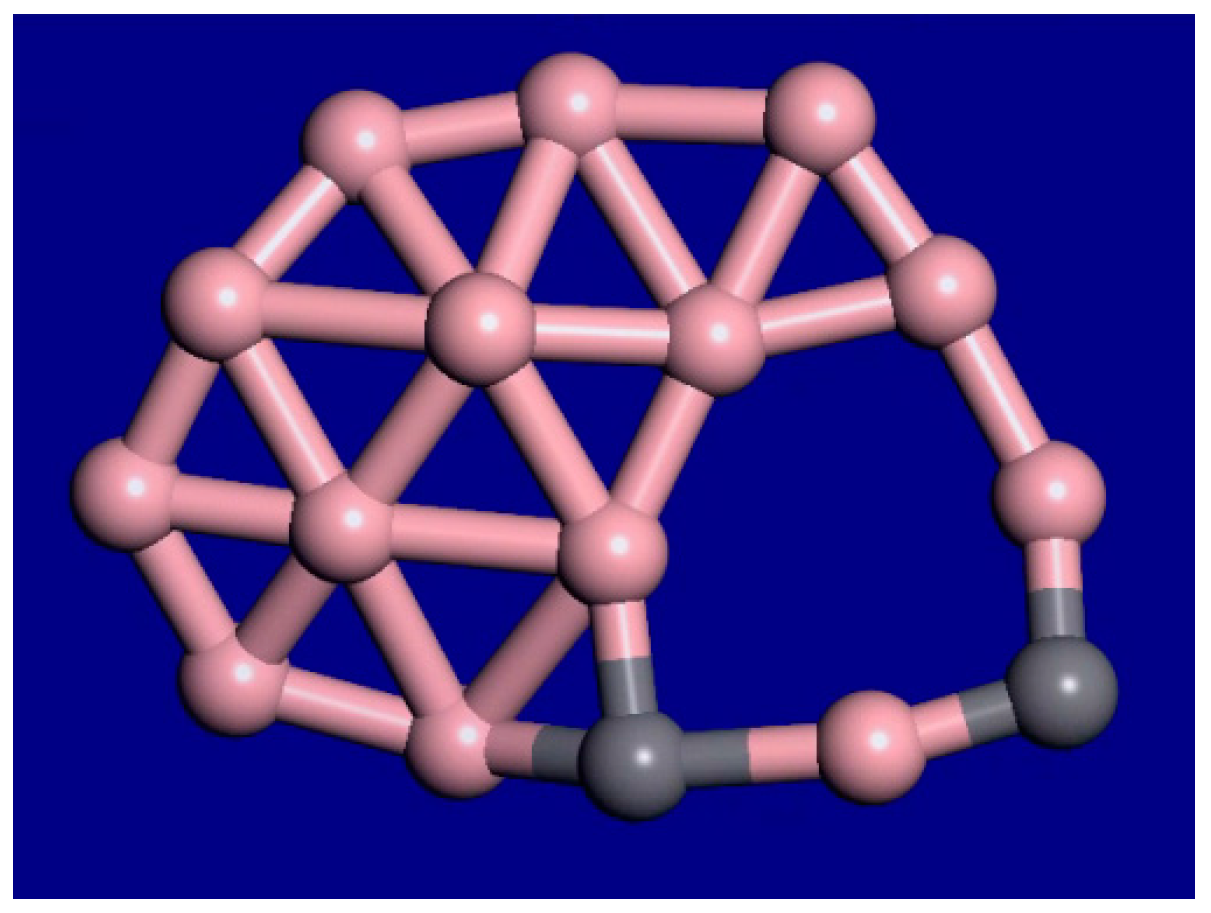
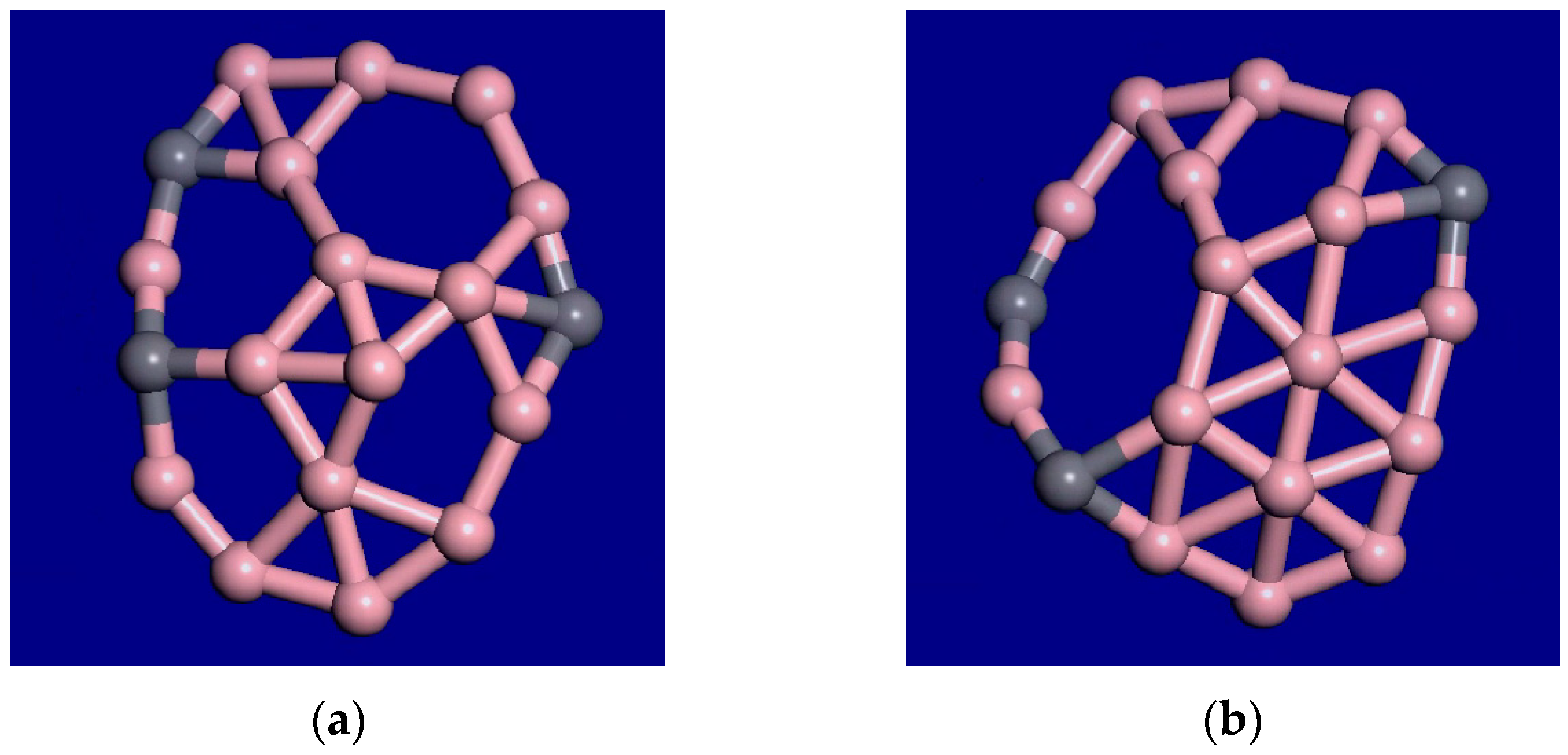
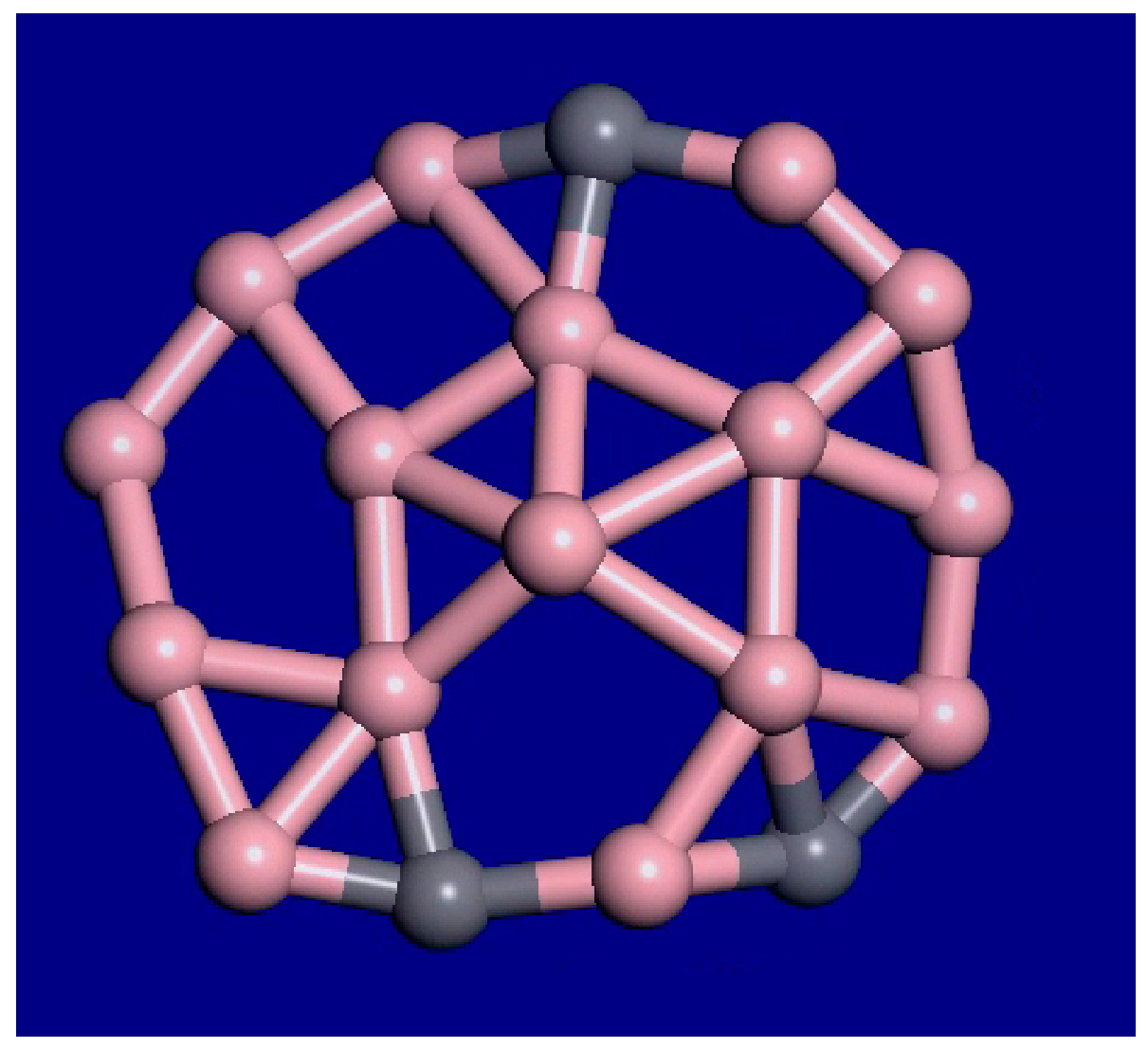
3. Results and Discussion
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Frenken, J.W.; van der Veen, J.F. Observation of Surface Melting. Phys. Rev. Lett. 1985, 54, 134–137. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Sergeeva, A.P.; Zhai, H.-J.; Averkiev, B.B.; Wang, L.-S.; Boldyrev, A.I. A Concentric Planar Doubly π-Aromatic B19− Cluster. Nat. Chem. 2010, 2, 202–206. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.P.; Zubarev, D.Y.; Zhai, H.-J.; Boldyrev, A.I.; Wang, L.-S. A Photoelectron Spectroscopic and Theoretical Study of B16− and B162−: An All-Boron Naphthalene. J. Am. Chem. Soc. 2008, 130, 7244–7246. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Zhao, X.-Y.; Chen, Q.; Zhai, H.-J.; Li, S.-D. B11(−): A Moving Subnanoscale Tank Tread. Nanoscale 2015, 7, 16054–16060. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-J.; Guo, J.-C.; Zhai, H.-J. Why Nanoscale Tank Treads Move? Structures, Chemical Bonding, and Molecular Dynamics of a Doped Boron Cluster B10C. Nanoscale 2017, 9, 9310–9316. [Google Scholar] [CrossRef] [PubMed]
- Jalife, S.; Liu, L.; Pan, S.; Cabellos, J.L.; Osorio, E.; Lu, C.; Heine, T.; Donald, K.J.; Merino, G. Dynamical Behavior of Boron Clusters. Nanoscale 2016, 8, 17639–17644. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.-C.; Feng, L.-Y.; Wang, Y.-J.; Jalife, S.; Vásquez-Espinal, A.; Cabellos, J.L.; Pan, S.; Merino, G.; Zhai, H.-J. Coaxial Triple-Layered versus Helical Be6B11− Clusters: Dual Structural Fluxionality and Multifold Aromaticity. Angew. Chem. 2017, 129, 10308–10311. [Google Scholar] [CrossRef]
- Wang, Y.-J.; Feng, L.-Y.; Guo, J.-C.; Zhai, H.-J. Dynamic Mg2B8 Cluster: A Nanoscale Compass. Chem. Asian J. 2017, 12, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Halla, J.O.C.; Islas, R.; Heine, T.; Merino, G. B19−: An Aromatic Wankel Motor. Angew. Chem. Int. Ed. 2010, 49, 5668–5671. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Guajardo, G.; Sergeeva, A.P.; Boldyrev, A.I.; Heine, T.; Ugalde, J.M.; Merino, G. Unravelling Phenomenon of Internal Rotation in B13+ through Chemical Bonding Analysis. Chem. Commun. 2011, 47, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Merino, G.; Heine, T. And Yet It Rotates: The Starter for a Molecular Wankel Motor. Angew. Chem. Int. Ed. 2012, 51, 10226–10227. [Google Scholar] [CrossRef] [PubMed]
- Moreno, D.; Pan, S.; Zeonjuk, L.L.; Islas, R.; Osorio, E.; Martínez-Guajardo, G.; Chattaraj, P.K.; Heine, T.; Merino, G.; Su, S.J.; et al. B182−: A Quasi-Planar Bowl Member of the Wankel Motor Family. Chem. Commun. 2014, 50, 8140–8143. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Navarro, F.; Martínez-Guajardo, G.; Osorio, E.; Moreno, D.; Tiznado, W.; Islas, R.; Donald, K.J.; Merino, G.; Merino, G. Stop Rotating! One Substitution Halts the B19− Motor. Chem. Commun. 2014, 50, 10680–10682. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-S. Photoelectron Spectroscopy of Size-Selected Boron Clusters: From Planar Structures to Borophenes and Borospherenes. Int. Rev. Phys. Chem. 2016, 35, 69–142. [Google Scholar] [CrossRef]
- Wang, Y.-J.; You, X.-R.; Chen, Q.; Feng, L.-Y.; Wang, K.; Ou, T.; Zhao, X.-Y.; Zhai, H.-J.; Li, S.-D.; Wu, Y.B.; et al. Chemical Bonding and Dynamic Fluxionality of a B15+ Cluster: A Nanoscale Double-Axle Tank Tread. Phys. Chem. Chem. Phys. 2016, 18, 15774–15782. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Guajardo, G.; Luis Cabellos, J.; Díaz-Celaya, A.; Pan, S.; Islas, R.; Chattaraj, P.K.; Heine, T.; Merino, G. Dynamical Behavior of Borospherene: A Nanobubble. Sci. Rep. 2015, 5, 11287. [Google Scholar] [CrossRef] [PubMed]
- Averkiev, B.B.; Zubarev, D.Y.; Wang, L.-M.; Huang, W.; Wang, L.-S.; Boldyrev, A.I. Carbon Avoids Hypercoordination in CB6−, CB62−, and C2B5− Planar Carbon−Boron Clusters. J. Am. Chem. Soc. 2008, 130, 9248–9250. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-L.; Jian, T.; Chen, X.; Chen, T.-T.; Lopez, G.V.; Li, J.; Wang, L.-S. The Planar CoB18− Cluster as a Motif for Metallo-Borophenes. Angew. Chem. Int. Ed. 2016, 55, 7358–7363. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Moreno, D.; Osorio, E.; Castro, A.C.; Pan, S.; Chattaraj, P.K.; Heine, T.; Merino, G.; Nguyen, K.A.; Su, S.J.; et al. Structure and Bonding of IrB12−: Converting a Rigid Boron B12 Platelet to a Wankel Motor. RSC Adv. 2016, 6, 27177–27182. [Google Scholar] [CrossRef]
- Supady, A.; Blum, V.; Baldauf, C. First-Principles Molecular Structure Search with a Genetic Algorithm. J. Chem. Inf. Model. 2015, 55, 2338–2348. [Google Scholar] [CrossRef] [PubMed]
- Popov, I.A.; Jian, T.; Lopez, G.V.; Boldyrev, A.I.; Wang, L.-S. Cobalt-Centred Boron Molecular Drums with the Highest Coordination Number in the CoB16(−) Cluster. Nat. Commun. 2015, 6, 8654. [Google Scholar] [CrossRef] [PubMed]
- Sergeeva, A.P.; Averkiev, B.B.; Zhai, H.-J.; Boldyrev, A.I.; Wang, L.-S. All-Boron Analogues of Aromatic Hydrocarbons: B17− and B18−. J. Chem. Phys. 2011, 134, 224304. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.J.; Segall, M.D.; Pickard, C.J.; Hasnip, P.J.; Probert, M.I.J.; Refson, K.; Payne, M.C. First Principles Methods Using CASTEP. Z. Krist. Cryst. Mater. 2005, 220, 567–570. [Google Scholar] [CrossRef]
- Alexandrova, A.N.; Boldyrev, A.I.; Zhai, H.J.; Wang, L.S. All-Boron Aromatic Clusters as Potential New Inorganic Ligands and Building Blocks in Chemistry. Coord. Chem. Rev. 2006, 250, 2811–2866. [Google Scholar] [CrossRef]
- Feng, L.-Y.; Zhai, H.-J. Wheel-Like, Elongated, Circular, and Linear Geometries in Boron-Based CnB7−n (n = 0–7) Clusters: Structural Transitions and Aromaticity. Phys. Chem. Chem. Phys. 2017, 19, 24284–24293. [Google Scholar] [CrossRef] [PubMed]


© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, L.-M.; Ganz, E. Interior Melting of the C3B16 and C2B14− Clusters Between 1000 K and 2000 K. Condens. Matter 2017, 2, 35. https://doi.org/10.3390/condmat2040035
Yang L-M, Ganz E. Interior Melting of the C3B16 and C2B14− Clusters Between 1000 K and 2000 K. Condensed Matter. 2017; 2(4):35. https://doi.org/10.3390/condmat2040035
Chicago/Turabian StyleYang, Li-Ming, and Eric Ganz. 2017. "Interior Melting of the C3B16 and C2B14− Clusters Between 1000 K and 2000 K" Condensed Matter 2, no. 4: 35. https://doi.org/10.3390/condmat2040035
APA StyleYang, L.-M., & Ganz, E. (2017). Interior Melting of the C3B16 and C2B14− Clusters Between 1000 K and 2000 K. Condensed Matter, 2(4), 35. https://doi.org/10.3390/condmat2040035





