Exploring Low Energy Excitations in the d5 Iridate Double Perovskites La2BIrO6 (B = Zn, Mg)
Abstract
1. Introduction
2. Results and Discussion
2.1. Crystal Structure from X-Ray Powder Diffraction (XRPD)
2.2. Local Structure from Extended X-Ray Absorption Fine Structure (EXAFS) Spectroscopic Study
2.3. Determination of Ir-Oxidation State from X-Ray Absorption near Edge Structure (XANES) Spectroscopy
2.4. Electrical Resistivity
2.5. Temperature-Dependent DC Magnetic Susceptibility and Field-Dependent DC Magnetization
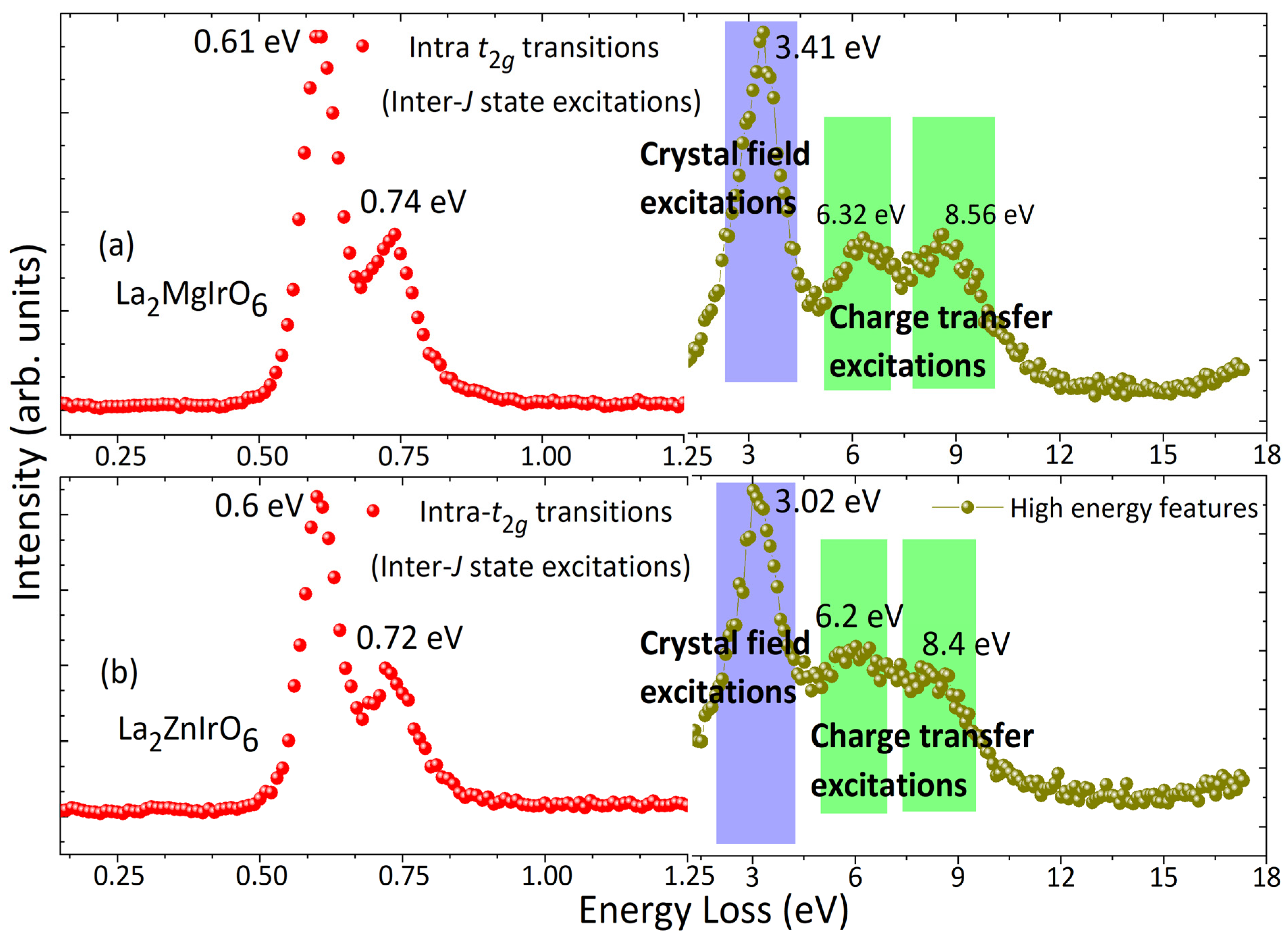
2.6. Ir L3-Edge Resonant Inelastic X-Ray Scattering (RIXS)
3. Conclusions
4. Materials and Methods
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kim, B.J.; Jin, H.; Moon, S.-J.; Kim, J.-Y.; Park, B.-G.; Leem, C.S.; Yu, J.; Noh, T.W.; Kim, C.; Oh, S.J.; et al. Novel Jeff = 1/2 Mott State Induced by Relativistic Spin-Orbit Coupling in Sr2IrO4. Phys. Rev. Lett. 2008, 101, 076402. [Google Scholar] [CrossRef]
- Pesin, D.; Balents, L. Mott physics and band topology in materials with strong spin–orbit interaction. Nat. Phys. 2010, 6, 376. [Google Scholar] [CrossRef]
- Jackeli, G.; Khaliullin, G. Mott Insulators in the Strong Spin-Orbit Coupling Limit: From Heisenberg to a Quantum Compass and Kitaev Models. Phys. Rev. Lett. 2009, 102, 017205. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Senthil, T. Twisted Hubbard Model for Sr2IrO4: Magnetism and Possible High Temperature Superconductivity. Phys. Rev. Lett. 2011, 106, 136402. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Turner, A.M.; Vishwanath, A.; Savrasov, S.Y. Topological semimetal and Fermi-arc surface states in the electronic structure of pyrochlore iridates. Phys. Rev. B 2011, 83, 205101. [Google Scholar] [CrossRef]
- Balents, L. Spin Liquids in frustrated magnets. Nature 2010, 464, 199. [Google Scholar] [CrossRef]
- Chaloupka, J.; Jackeli, G.; Khaliullin, G. Kitaev-Heisenberg Model on a Honeycomb Lattice: Possible Exotic Phases in Iridium Oxides A2IrO3. Phys. Rev. Lett. 2010, 105, 027204. [Google Scholar] [CrossRef]
- Pardo, V.; Pickett, W.E. Compensated magnetism by design in double perovskite oxides. Phys. Rev. B 2009, 80, 054415. [Google Scholar] [CrossRef]
- Saha-Dasgupta, T. Ferroic properties in bi-component perovskites: Artificial superlattices and naturally forming compounds. J. Phys. Condens. Matter. 2014, 26, 193201. [Google Scholar] [CrossRef]
- Powell, A.V.; Gore, J.G.; Battle, P.D. The magnetic properties of iridium in mixed-metal oxides. J. Alloys Compd. 1993, 201, 73. [Google Scholar] [CrossRef]
- Manna, K.; Sarkar, R.; Fuchs, S.; Onykiienko, Y.A.; Bera, A.K.; Cansever, G.A.; Kamusella, S.; Maljuk, A.; Blum, C.G.F.; Corredor, L.T.; et al. Noncollinear antiferromagnetism of coupled spins and pseudospins in the double perovskite La2CuIrO6. Phys. Rev. B 2016, 94, 144437. [Google Scholar] [CrossRef]
- Narayanan, N.; Mikhailova, D.; Senyshyn, A.; Trots, D.M.; Laskowski, R.; Blaha, P.; Schwarz, K.; Fuess, H.; Ehrenberg, H. Temperature and composition dependence of crystal structures and magnetic and electronic properties of the double perovskites La2−xSrxCoIrO6 (0 ≤ x ≤ 2). Phys. Rev. B 2010, 82, 024403. [Google Scholar] [CrossRef]
- Kolchinskaya, A.; Komissinskiy, P.; Yazdi, M.B.; Vafaee, M.; Mikhailova, D.; Narayanan, N.; Ehrenberg, H.; Wilhelm, F.; Rogalev, A.; Alff, L. Magnetism and spin-orbit coupling in Ir-based double perovskites La2−xSrxCoIrO6. Phys. Rev. B 2012, 85, 224422. [Google Scholar] [CrossRef]
- Cao, G.; Subedi, A.; Calder, S.; Yan, J.-Q.; Yi, J.; Gai, Z.; Poudel, L.; Singh, D.J.; Lumsden, M.D.; Christianson, A.D.; et al. Magnetism and electronic structure of La2ZnIrO6 and La2MgIrO6: Candidate Jeff = 1/2 Mott insulators. Phys. Rev. B 2013, 87, 155136. [Google Scholar] [CrossRef]
- Zhu, W.K.; Lu, C.-K.; Tong, W.; Wang, J.M.; Zhou, H.D.; Zhang, S.X. Strong ferromagnetism induced by canted antiferromagnetic order in double perovskite iridates (La1−xSrx)2ZnIrO6. Phys. Rev. B 2015, 91, 144408. [Google Scholar] [CrossRef]
- Taylor, A.E.; Morrow, R.; Singh, D.J.; Calder, S.; Lumsden, M.D.; Woodward, P.M.; Christianson, A.D. Magnetic order and electronic structure of the 5d3 double perovskite Sr2ScOsO6. Phys. Rev. B 2015, 91, 100406. [Google Scholar] [CrossRef]
- Dzyaloshinskii, I. A Thermodynamic Theory of Weak Ferromagnetism of Antiferromagnetics. J. Phys. Chem. Solids 1958, 4, 241. [Google Scholar] [CrossRef]
- Moriya, T. Anisotropic Superexchange Interaction and Weak Ferromagnetism. Phys. Rev. 1960, 120, 91. [Google Scholar] [CrossRef]
- Aczel, A.A.; Cook, A.M.; Williams, T.J.; Calder, S.; Christianson, A.D.; Cao, G.-X.; Mandrus, D.; Kim, Y.-B.; Paramekanti, A. Highly anisotropic exchange interactions of jeff = 1/2 iridium moments on the fcc lattice in La2BIrO6 (B = Mg,Zn). Phys. Rev. B 2016, 93, 214426. [Google Scholar] [CrossRef]
- Demazeau, G.; Siberchicot, B.; Matar, S.; Gayet, C.; Largeteau, A. A new ferromagnetic oxide La2MnIrO6: Synthesis, characterization, and calculation of its electronic structure. J. Appl. Phys. 1994, 75, 4617. [Google Scholar] [CrossRef]
- Uhl, M.; Matar, S.F.; Siberchicot, B. Calculated magnetic and electronic properties of the double perovskites La2TIrO6 (T = Mn, Fe, Co). J. Magn. Magn. Mater. 1998, 187, 201. [Google Scholar] [CrossRef]
- Currie, R.C.; Vente, J.F.; Frikkee, E.; Ijdo, D. The Structure and Magnetic Properties of La2MLrO6 with M = Mg, Co, Ni, and Zn. J. Solid State Chem. 1995, 116, 199. [Google Scholar] [CrossRef]
- Laguna-Marco, M.A.; Kayser, P.; Alonso, J.A.; Martinez-Lope, M.J.; van Veenendaal, M.; Choi, Y.; Haskel, D. Electronic structure, local magnetism, and spin-orbit effects of Ir(IV)-, Ir(V)-, and Ir(VI)-based compounds. Phys. Rev. B 2015, 91, 214433. [Google Scholar] [CrossRef]
- Popov, G.; Greenblatt, M.; Croft, M. Large effects of A-site average cation size on the properties of the double perovskites Ba2−xSrxMnReO6: A d5−d1 system. Phys. Rev. B 2003, 67, 024406. [Google Scholar] [CrossRef]
- Wiebe, C.R.; Greedan, J.E.; Luke, G.M.; Gardner, J.S. Spin-glass behavior in the S = ½ fcc ordered perovskite Sr2CaReO6. Phys. Rev. B 2002, 65, 144413. [Google Scholar] [CrossRef]
- Nag, A.; Bhowal, S.; Bert, F.; Hillier, A.D.; Itoh, M.; Carlomagno, I.; Meneghini, C.; Sarkar, T.; Mathieu, R.; Dasgupta, I.; et al. Ba3MIr2O9 hexagonal perovskites in the light of spin-orbit coupling and local structural distortions. Phys. Rev. B 2018, 97, 064408. [Google Scholar] [CrossRef]
- Meneghini, C.; Ray, S.; Liscio, F.; Bardelli, F.; Mobilio, S.; Sarma, D.D. Nature of “Disorder” in the Ordered Double Perovskite Sr2FeMoO6. Phys. Rev. Lett. 2009, 103, 046403. [Google Scholar] [CrossRef]
- Bufaiçal, L.; Ferreira, L.M.; Lora-Serrano, R.; Agüero, O.; Torriani, I.; Granado, E.; Pagliuso, P.G. Physical properties of disordered double perovskite Ca2−xLaxFeIrO6. J. Appl. Phys. 2008, 103, 07F716. [Google Scholar] [CrossRef]
- Jana, S.; Singh, V.; Kaushik, S.D.; Meneghini, C.; Pal, P.; Knut, R.; Karis, O.; Dasgupta, I.; Siruguri, V.; Ray, S. Atomic-scale chemical fluctuation in LaSrVMoO6, a proposed half-metallic antiferromagnet. Phys. Rev. B 2010, 82, 180407. [Google Scholar] [CrossRef]
- Jana, S.; Singh, V.; Nag, A.; Meneghini, C.; Dasgupta, I.; Aquilanti, G.; Ray, S. LaSrVMoO6: A case study for A-site covalency-driven local cationic order in double perovskites. Phys. Rev. B 2012, 86, 014203. [Google Scholar] [CrossRef]
- Jana, S.; Meneghini, C.; Sanyal, P.; Sarkar, S.; Dasgupta, T.S.; Karis, O.; Ray, S. Signature of an antiferromagnetic metallic ground state in heavily electron-doped Sr2FeMoO6. Phys. Rev. B 2012, 86, 054433. [Google Scholar] [CrossRef]
- Sarma, D.D.; Mahadevan, P.; Saha-Dasgupta, T.; Ray, S.; Kumar, A. Electronic Structure of Sr2FeMoO6. Phys. Rev. Lett. 2000, 85, 2549. [Google Scholar] [CrossRef] [PubMed]
- Das, H.; Sanyal, P.; Saha-Dasgupta, T.; Sarma, D.D. Origin of magnetism and trend in Tc in Cr-based double perovskites: Interplay of two driving mechanisms. Phys. Rev. B 2011, 83, 104418. [Google Scholar] [CrossRef]
- Hemery, E.K.; Williams, G.V.M.; Trodahl, H.J. La-induced changes in the magnetic and electronic properties of Sr2−xLaxFeMoO6. Phys. Rev. B 2006, 74, 054423. [Google Scholar] [CrossRef]
- Cao, G.; Durairaj, V.; Chikara, S.; De Long, L.E.; Parkin, S.; Schlottmann, P. Non-Fermi-liquid behavior in nearly ferromag netic SrIrO3 single crystals. Phys. Rev. B 2007, 76, 100402. [Google Scholar] [CrossRef]
- Kayser, P.; Martínez-Lope, M.J.; Alonso, J.A.; Retuerto, M.; Croft, M.; Ignatov, A.; Fernández-Díaz, M.T. Crystal and Magnetic Structure of Sr2MIrO6 (M = Ca, Mg) Double Perovskites—A Neutron Diffraction Study. Eur. J. Inorg. Chem. 2013, 2014, 178. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Chakraborty, A.; Bhowal, S.; Kumar, V.; Sala, M.M.; Efimenko, A.; Bert, F.; Biswas, P.K.; Meneghini, C.; Büttgen, N.; et al. Breakdown of atomic spin-orbit coupling picture in an apparently isolated pseudo-one-dimensional iridate: Sr3NaIrO6. Phys. Rev. B 2022, 105, 104431. [Google Scholar] [CrossRef]
- Nag, A.; Middey, S.; Bhowal, S.; Panda, S.K.; Mathieu, R.; Orain, J.C.; Bert, F.; Mendels, P.; Freeman, G.; Mansson, M.; et al. Origin of the Spin-Orbital Liquid State in a Nearly J = 0 Iridate Ba3ZnIr2O9. Phys. Rev. Lett. 2016, 116, 097205. [Google Scholar] [CrossRef]
- Khan, M.S.; Bandyopadhyay, A.; Nag, A.; Kumar, V.; Mahajan, A.V.; Ray, S. Magnetic ground state of the distorted 6H perovskite Ba3CdIr2O9. Phys. Rev. B 2019, 100, 064423. [Google Scholar] [CrossRef]
- Banerjee, A.; Sannigrahi, J.; Giri, S.; Majumdar, S. Observation of non-Fermi liquid behavior in hole-doped Eu2Ir2O7. Phys. Rev. B 2017, 96, 224426. [Google Scholar] [CrossRef]
- Porter, Z.; Zoghlin, E.; Britner, S.; Husremovic, S.; Ruff, J.P.C.; Choi, Y.; Haskel, D.; Laurita, G.; Wilson, S.D. Evolution of structure and magnetism across the metal-insulator transition in the pyrochlore iridate (Nd1−xCax)2Ir2O7. Phys. Rev. B 2019, 100, 054409. [Google Scholar] [CrossRef]
- Ueda, K.; Fukuda, H.; Kaneko, R.; Fujioka, J.; Tokura, Y. Evolution of possible Weyl semimetal states across the Mott transition in pyrochlore iridates induced by hole doping. Phys. Rev. B 2020, 102, 245131. [Google Scholar] [CrossRef]
- Zoghlin, E.; Porter, Z.; Britner, S.; Husremovic, S.; Choi, Y.; Haskel, D.; Laurita, G.; Wilson, S.D. Mapping the structural, magnetic and electronic behavior of (Eu1−xCax)2Ir2O7 across a metal–insulator transition. J. Phys. Condens. Matter 2020, 33, 055601. [Google Scholar] [CrossRef] [PubMed]
- Choy, J.-H.; Kim, J.-D.K.; Demazeau, G.; Jung, D.Y. LIII-Edge XANES Study on Unusually High Valent Iridium in a Perovskite Lattice. J. Phys. Chem. 1994, 98, 6258. [Google Scholar] [CrossRef]
- Choy, J.-H.; Kim, J.-D.K.; Hwang, S.-H.; Demazeau, G.; Jung, D.Y. XANES and EXAFS Studies on the Ir-O Bond Covalency in Ionic Iridium Perovskites. J. Am. Chem. Soc. 1995, 117, 8557. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, Z.-J. Polaron relaxation and variable-range-hopping conductivity in the giant-dielectric-constant material CaCu3Ti4O12. Phys. Rev. B 2004, 70, 174306. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Carlomagno, I.; Simonelli, L.; Sala, M.M.; Efimenko, A.; Meneghini, C.; Ray, S. Evolution of electronic and magnetic properties in a series of iridate double perovskites Pr2−xSrxMgIrO6 (x = 0, 0.5, 1.0). Phys. Rev. B 2019, 100, 064416. [Google Scholar] [CrossRef]
- Vogl, M.; Corredor, L.T.; Dey, T.; Mørrow, R.; Scaravaggi, F.; Wolter, A.U.B.; Aswartham, S.; Wurmehl, S.; Büchner, B. Interplay of 3d- and 5d-sublattice magnetism in the double perovskite substitution series La2Zn1−xCoxIrO6. Phys. Rev. B 2018, 97, 035155. [Google Scholar] [CrossRef]
- Iakovleva, M.; Fuchs, S.; Alfonsov, A.; Grafe, H.-J.; Vogl, M.; Aswartham, S.; Wurmehl, S.; Dey, T.; Büchner, B.; Vavilova, E.; et al. Static and dynamic magnetism of the Ir-based double perovskites La2BIrO6 (B = Co, Zn) probed by magnetic resonance spectroscopies. Phys. Rev. B 2018, 98, 174401. [Google Scholar] [CrossRef]
- Cook, A.M.; Matern, S.; Hickey, C.; Aczel, A.A.; Paramekanti, A. Spin-orbit coupled jeff = 1/2 iridium moments on the geometrically frustrated fcc lattice. Phys. Rev. B 2015, 92, 020417. [Google Scholar] [CrossRef]
- Wiebe, C.R.; Greedan, J.E.; Kyriakou, P.P.; Luke, G.M.; Gardner, J.S.; Fukaya, A.; Gat-Malureanu, M.; Russo, P.L.; Savici, A.T.; Uemura, Y.J. Frustration-driven spin freezing in the S=1/2 fcc perovskite Sr2MgReO6. Phys. Rev. B 2003, 68, 134410. [Google Scholar] [CrossRef]
- Seehra, M.S.; Giebultowicz, T.M. Magnetic structures of fcc systems with nearest-neighbor and next-nearest-neighbor exchange interactions. Phys. Rev. B 1988, 38, 11898. [Google Scholar] [CrossRef]
- Lefmann, K.; Rischel, C. Quantum effects in magnetic structures on the fcc lattice. Eur. Phys. J. B 2001, 21, 313. [Google Scholar] [CrossRef]
- de Vries, M.A.; Mclaughlin, A.C.; Bos, J.-W.G. Valence Bond Glass on an fcc Lattice in the Double Perovskite Ba2YMoO6. Phys. Rev. Lett. 2010, 104, 177202. [Google Scholar] [CrossRef] [PubMed]
- Aharen, T.; Greedan, J.E.; Bridges, C.A.; Aczel, A.A.; Rodriguez, J.; MacDougall, G.; Luke, G.M.; Imai, T.; Michaelis, V.K.; Kroeker, S.; et al. Magnetic properties of the geometrically frustrated S=½ antiferromagnets, La2LiMoO6 and Ba2YMoO6, with the B-site ordered double perovskite structure: Evidence for a collective spin-singlet ground state. Phys. Rev. B 2010, 81, 224409. [Google Scholar] [CrossRef]
- Ishii, K.; Jarrige, I.; Yoshida, M.; Ikeuchi, K.; Mizuki, J.; Ohashi, K.; Takayama, T.; Matsuno, J.; Takagi, H. Momentum-resolved electronic excitations in the Mott insulator Sr2IrO4 studied by resonant inelastic x-ray scattering. Phys. Rev. B 2011, 83, 115121. [Google Scholar] [CrossRef]
- Nag, A.; Bhowal, S.; Chakraborty, A.; Sala, M.M.; Efimenko, A.; Bert, F.; Biswas, P.K.; Hillier, A.D.; Itoh, M.; Kaushik, S.D.; et al. Origin of magnetic moments and presence of spin-orbit singlets in Ba2YIrO6. Phys. Rev. B 2018, 98, 014431. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Clancy, J.P.; Gretarsson, H.; Cao, G.; Singh, Y.; Kim, J.; Upton, M.H.; Casa, D.; Gog, T. Probing electronic excitations in iridates with resonant inelastic x-ray scattering and emission spectroscopy techniques. arXiv 2018, arXiv:1805.03612. [Google Scholar] [CrossRef]
- Aczel, A.A.; Clancy, J.P.; Chen, Q.; Zhou, H.D.; Reig-i-Plessis, D.; MacDougall, G.J.; Ruff, J.P.C.; Upton, M.H.; Islam, Z.; Williams, T.J.; et al. Revisiting the Kitaev material candidacy of Ir4+ double perovskite iridates. Phys. Rev. B 2019, 99, 134417. [Google Scholar] [CrossRef]
- Liu, X.; Katukuri, V.M.; Hozoi, L.; Yin, W.G.; Dean, M.P.M.; Upton, M.H.; Kim, J.; Casa, D.; Said, A.; Gog, T.; et al. Testing the Validity of the Strong Spin-Orbit-Coupling Limit for Octahedrally Coordinated Iridate Compounds in a Model System Sr3CuIrO6. Phys. Rev. Lett. 2012, 109, 157401. [Google Scholar] [CrossRef]
- Hozoi, L.; Gretarsson, H.; Clancy, J.P.; Jeon, B.-G.; Lee, B.; Kim, K.H.; Yushankhai, V.; Fulde, P.; Casa, D.; Gog, T.; et al. Longer-range lattice anisotropy strongly competing with spin-orbit interactions in pyrochlore iridates. Phys. Rev. B 2014, 89, 115111. [Google Scholar] [CrossRef]
- Sala, M.M.; Ohgushi, K.; Al-Zein, A.; Hirata, Y.; Monaco, G.; Krisch, M. CaIrO3: A Spin-Orbit Mott Insulator Beyond the jeff = 1/2 Ground State. Phys. Rev. Lett. 2014, 112, 176402. [Google Scholar] [CrossRef]
- Lu, X.; Olalde-Velasco, P.; Huang, Y.; Bisogni, V.; Pelliciari, J.; Fatale, S.; Dantz, M.; Vale, J.G.; Hunter, E.C.; Chang, J.; et al. Dispersive magnetic and electronic excitations in iridate perovskites probed by oxygen K-edge resonant inelastic x-ray scattering. Phys. Rev. B 2018, 97, 041102(R). [Google Scholar] [CrossRef]
- Gretarsson, H.; Clancy, J.P.; Liu, X.; Hill, J.P.; Bozin, E.; Singh, Y.; Manni, S.; Gegenwart, P.; Kim, J.; Said, A.H.; et al. Crystal-Field Splitting and Correlation Effect on the Electronic Structure of A2IrO3. Phys. Rev. Lett. 2013, 110, 076402. [Google Scholar] [CrossRef]
- Paramekanti, A.; Singh, D.J.; Yuan, B.; Casa, D.; Said, A.; Kim, Y.-J.; Christianson, A.D. Spin-orbit coupled systems in the atomic limit: Rhenates, osmates, iridates. Phys. Rev. B 2018, 97, 235119. [Google Scholar] [CrossRef]
- Takegami, D.; Kasinathan, D.; Wolff, K.K.; Altendorf, S.G.; Chang, C.F.; Hoefer, K.; Melendez-Sans, A.; Utsumi, Y.; Meneghin, F.; Ha, T.D.; et al. Charge-transfer energy in iridates: A hard x-ray photoelectron spectroscopy study. Phys. Rev. B 2020, 102, 045119. [Google Scholar] [CrossRef]
- Nag, A.; Bhowal, S.; Sala, M.M.; Efimenko, A.; Dasgupta, I.; Ray, S. Hopping-Induced Ground-State Magnetism in 6H Perovskite Iridates. Phys. Rev. Lett. 2019, 123, 017201. [Google Scholar] [CrossRef]
- Revelli, A.; Sala, M.M.; Monaco, S.G.; Becker, P.; Bohatý, L.; Hermanns, M.; Koethe, T.C.; Fröhlich, T.; Warzanowski, P.; Lorenz, T.; et al. Resonant inelastic x-ray incarnation of Young’s double-slit experiment. Sci. Adv. 2019, 5, eaav4020. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, J.R. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Newville, M. EXAFS analysis using FEFF and FEFFIT. J. Synchrotron Rad. 2001, 8, 322. [Google Scholar] [CrossRef] [PubMed]
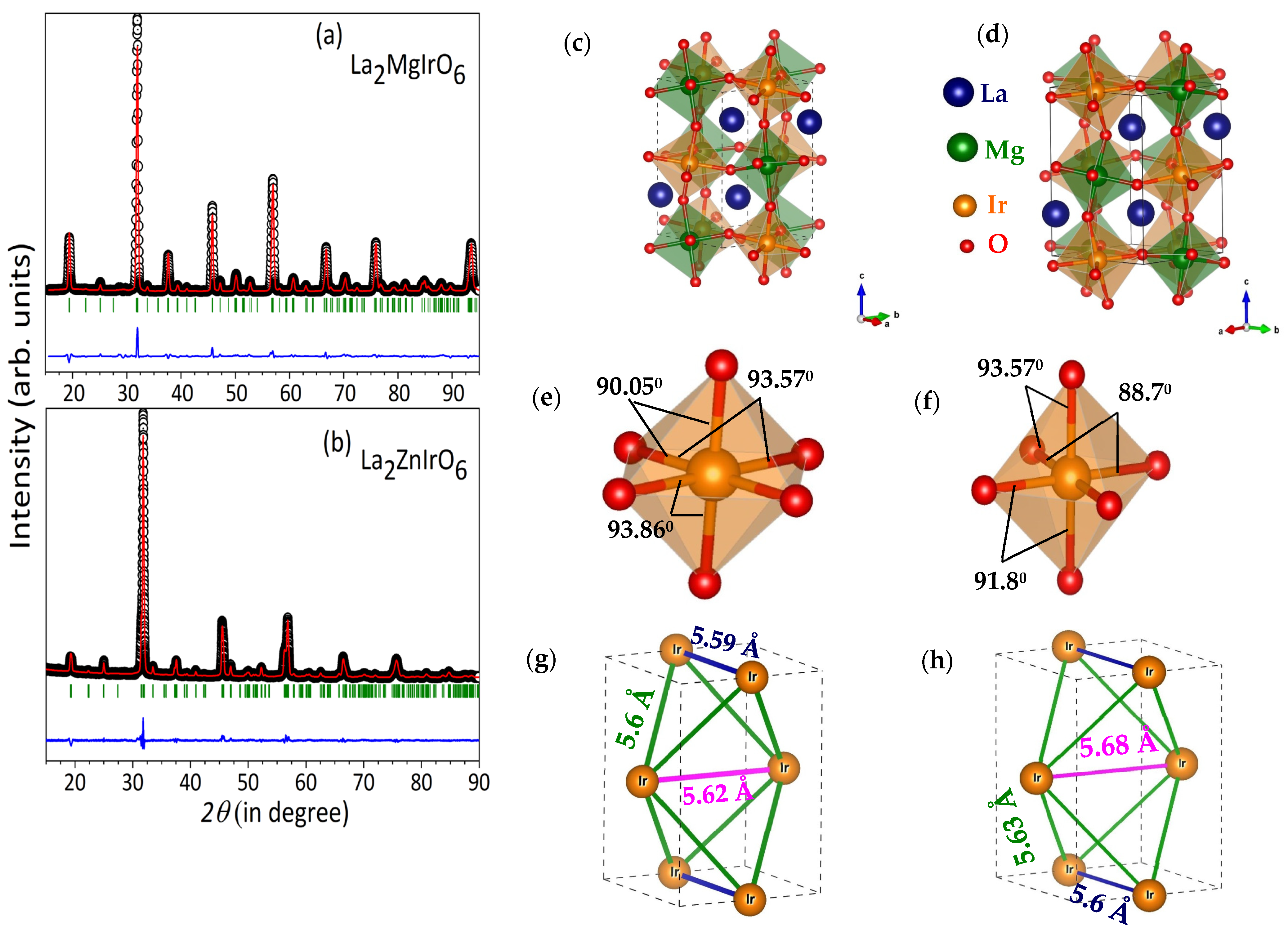
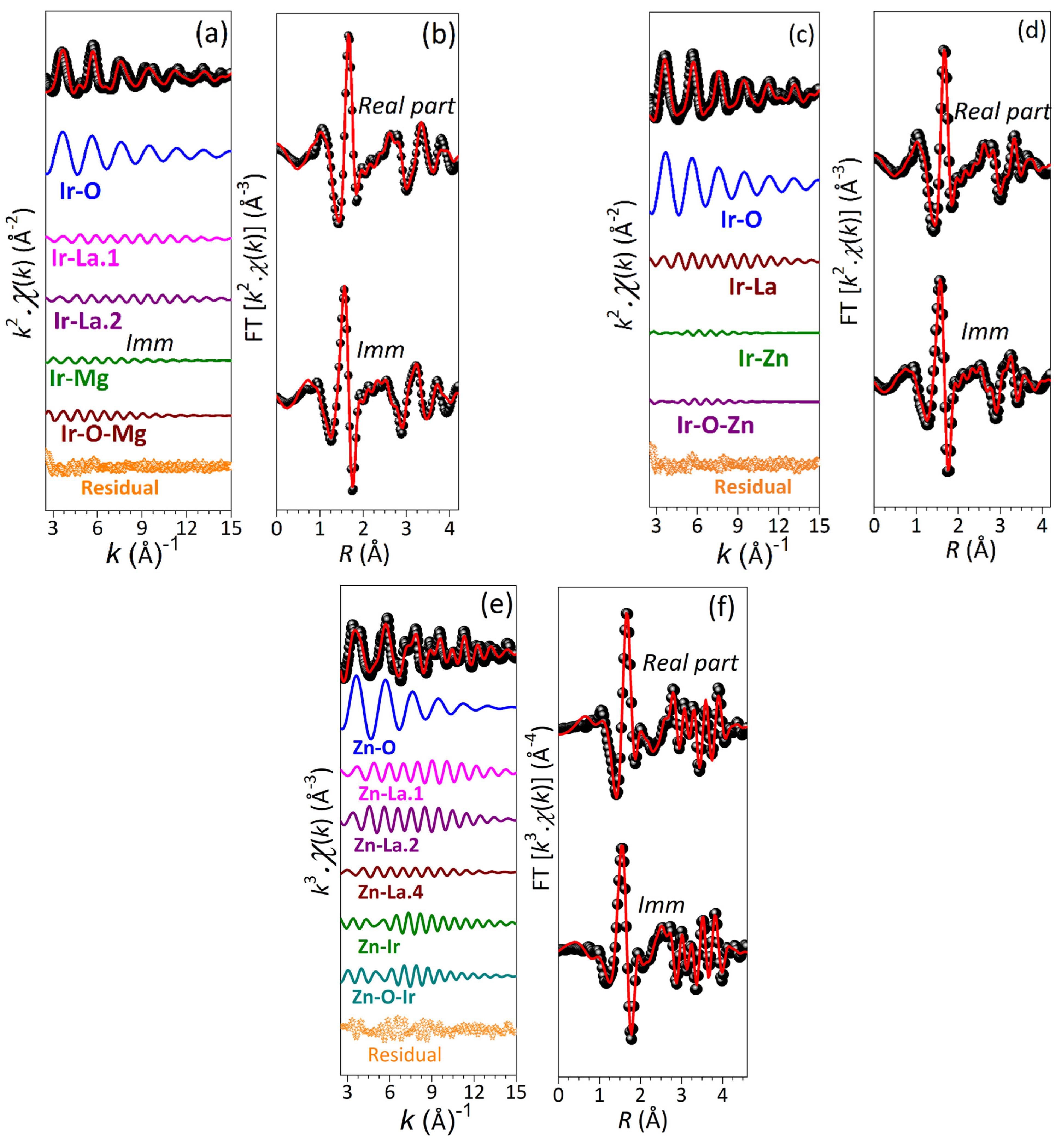

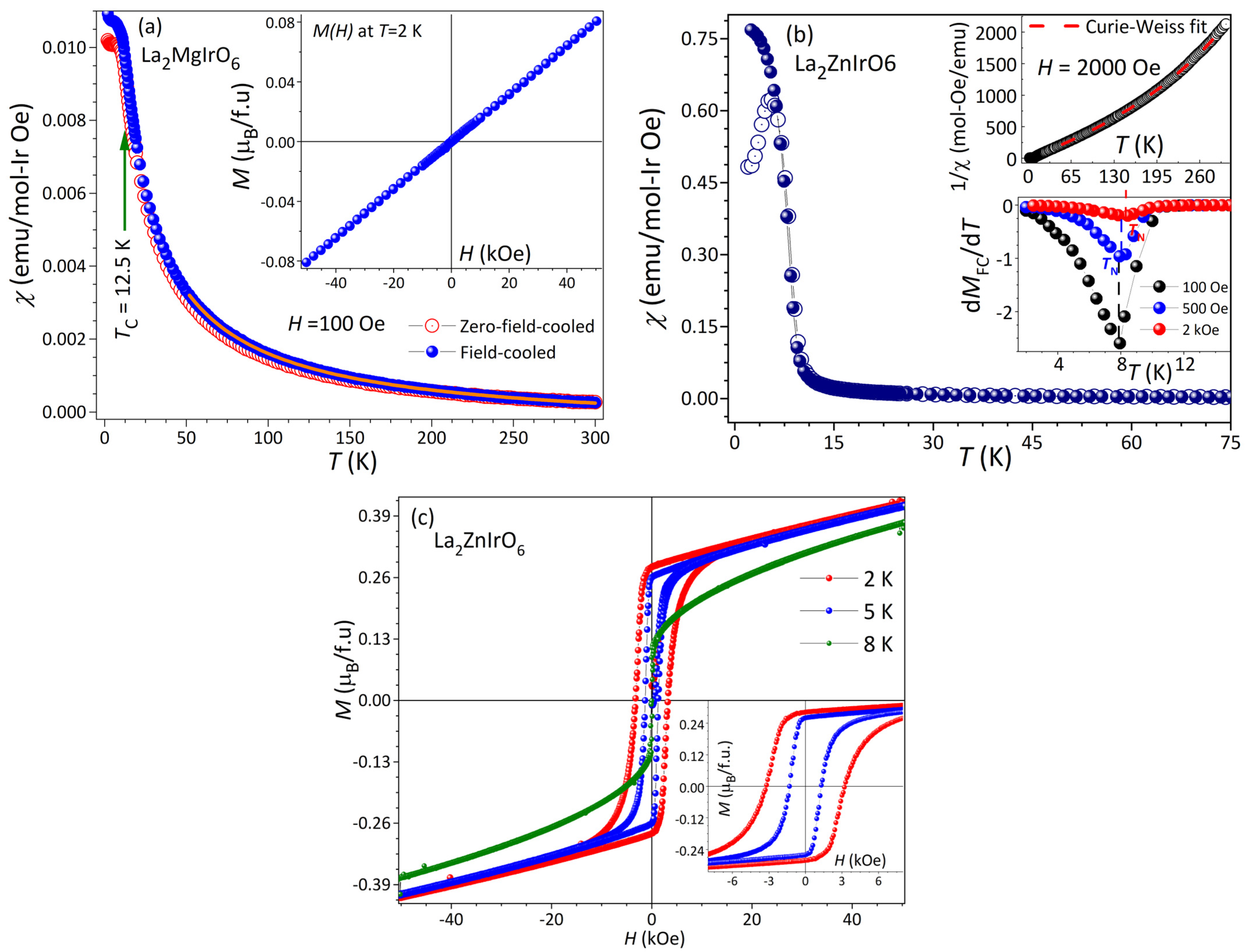
| Sample | Atom | Occupancy | x | y | z |
|---|---|---|---|---|---|
| La2MgIrO6 | La | 1 | 0.4999(1) | 0.5349(1) | 0.2495(2) |
| Mg1/Ir1 | 0.9188(2)/0.0812 | 0.5 | 0 | 0 | |
| Ir2/Mg2 | 0.9188 */0.0812 | 0 | 0.5 | 0 | |
| O1 | 1 | 0.2109(4) | 0.1985(2) | −0.0425(1) | |
| O2 | 1 | 0.2698(4) | 0.7278(5) | −0.0502(1) | |
| O3 | 1 | 0.4267(5) | −0.0168(2) | 0.2576(4) | |
| La2ZnIrO6 | La | 1 | 0.5019(2) | 0.5457(3) | 0.2481(5) |
| Zn1/Ir1 | 0.8965(6)/0.1035 | 0.5 | 0 | 0 | |
| Ir2/Z2 | 0.8965 */0.1035 | 0 | 0.5 | 0 | |
| O1 | 1 | 0.2153(6) | 0.2002(4) | −0.0341(3) | |
| O2 | 1 | 0.3016(5) | 0.7325(4) | −0.0522(5) | |
| O3 | 1 | 0.4063(5) | −0.0223(5) | 0.2364(6) |
| Type of Distortion | Connectivity | La2MgIrO6 | La2ZnIrO6 |
|---|---|---|---|
| Tilting | Ir-O3-Mg/Zn | 156.00(1)° | 149.50(1)° |
| Ir-O2-Mg/Zn | 155.47(5)° | 151.86(2)° | |
| Ir-O1-Mg/Zn | 152.10(6)° | 155.56(4)° | |
| Rotational | O1-Ir-O3 | 89.94(6)° | 93.57(2)° |
| O1-Ir-O2 | 93.57(4)° | 91.27(4)° | |
| O2-Ir-O3 | 86.13(5)° | 88.17(6)° |
| Sample with Absorption Edge | Shell | N | σ2(102 Å2) | R(Å) |
|---|---|---|---|---|
| La2ZnIrO6 (Ir-L3 edge) | Ir-O | 6.0 * | 0.21(3) | 2.009(4) |
| Ir-La1 | 4.0 * | 0.43(5) | 3.25(2) | |
| Ir-La2 | 4.0 * | 0.43 * | 3.43(3) | |
| Ir-Zn/Ir (SS) | 5.3(2)/0.7 | 1.68(4) | 4.01(2) | |
| Ir-O-Zn/Ir (MS) | 10.7 */1.3 | 1.68 * | 4.07(3) | |
| La2MgIrO6 (Ir-L3 edge) | Ir-O | 6.0 * | 0.22(3) | 1.995(5) |
| Ir-La1 | 4.0 * | 0.75(5) | 3.27(6) | |
| Ir-La2 | 4.0 * | 0.75 * | 3.43(2) | |
| Ir-Mg/Ir (SS) | 5.2(2)/0.8 | 0.55(3) | 3.98(2) | |
| Ir-O-Mg/Ir (MS) | 10.4 */1.6 | 0.55 * | 4.02(2) | |
| La2ZnIrO6 (Zn-K edge) | Zn-O | 6.0 | 0.77(2) | 2.078(4) |
| Zn-La1 | 2.0 * | 0.66(5) | 3.26(5) | |
| Zn-La2 | 4.0 * | 0.97(7) | 3.44(4) | |
| Zn-La3 | 2.0 * | 0.97 * | 3.72(6) | |
| Zn-Ir/Zn | 5.4(2)/0.6 | 0.81(6) | 3.98(2) | |
| Zn-O-Ir/Zn | 10.8 */1.2 | 0.81 * | 4.04(6) |
| H (Oe) | TZFC(K) | TN(K) | µeff(µB/Ir4+) | ΘCW(K) | T(K) | MS (µB/f.u.) | ||
|---|---|---|---|---|---|---|---|---|
| 100 | 8.5 | 7.9 | 1.63 | −7.03 | 2 | 0.292 | ||
| χ vs. T | 500 | 6.15 | 8.1 | 1.47 | −2.12 | M vs. H | 5 | 0.278 |
| 2000 | 5.3 | 8.45 | 1.43 | 0.2 | 8 | 0.209 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bandyopadhyay, A.; Pandey, D.K.; Meneghini, C.; Efimenko, A.; Sala, M.M.; Ray, S. Exploring Low Energy Excitations in the d5 Iridate Double Perovskites La2BIrO6 (B = Zn, Mg). Condens. Matter 2025, 10, 53. https://doi.org/10.3390/condmat10040053
Bandyopadhyay A, Pandey DK, Meneghini C, Efimenko A, Sala MM, Ray S. Exploring Low Energy Excitations in the d5 Iridate Double Perovskites La2BIrO6 (B = Zn, Mg). Condensed Matter. 2025; 10(4):53. https://doi.org/10.3390/condmat10040053
Chicago/Turabian StyleBandyopadhyay, Abhisek, Dheeraj Kumar Pandey, Carlo Meneghini, Anna Efimenko, Marco Moretti Sala, and Sugata Ray. 2025. "Exploring Low Energy Excitations in the d5 Iridate Double Perovskites La2BIrO6 (B = Zn, Mg)" Condensed Matter 10, no. 4: 53. https://doi.org/10.3390/condmat10040053
APA StyleBandyopadhyay, A., Pandey, D. K., Meneghini, C., Efimenko, A., Sala, M. M., & Ray, S. (2025). Exploring Low Energy Excitations in the d5 Iridate Double Perovskites La2BIrO6 (B = Zn, Mg). Condensed Matter, 10(4), 53. https://doi.org/10.3390/condmat10040053








