Are Alexandrium catenella Blooms Spreading Offshore in Southern Chile? An In-Depth Analysis of the First PSP Outbreak in the Oceanic Coast
Abstract
1. Introduction
2. Materials and Methods
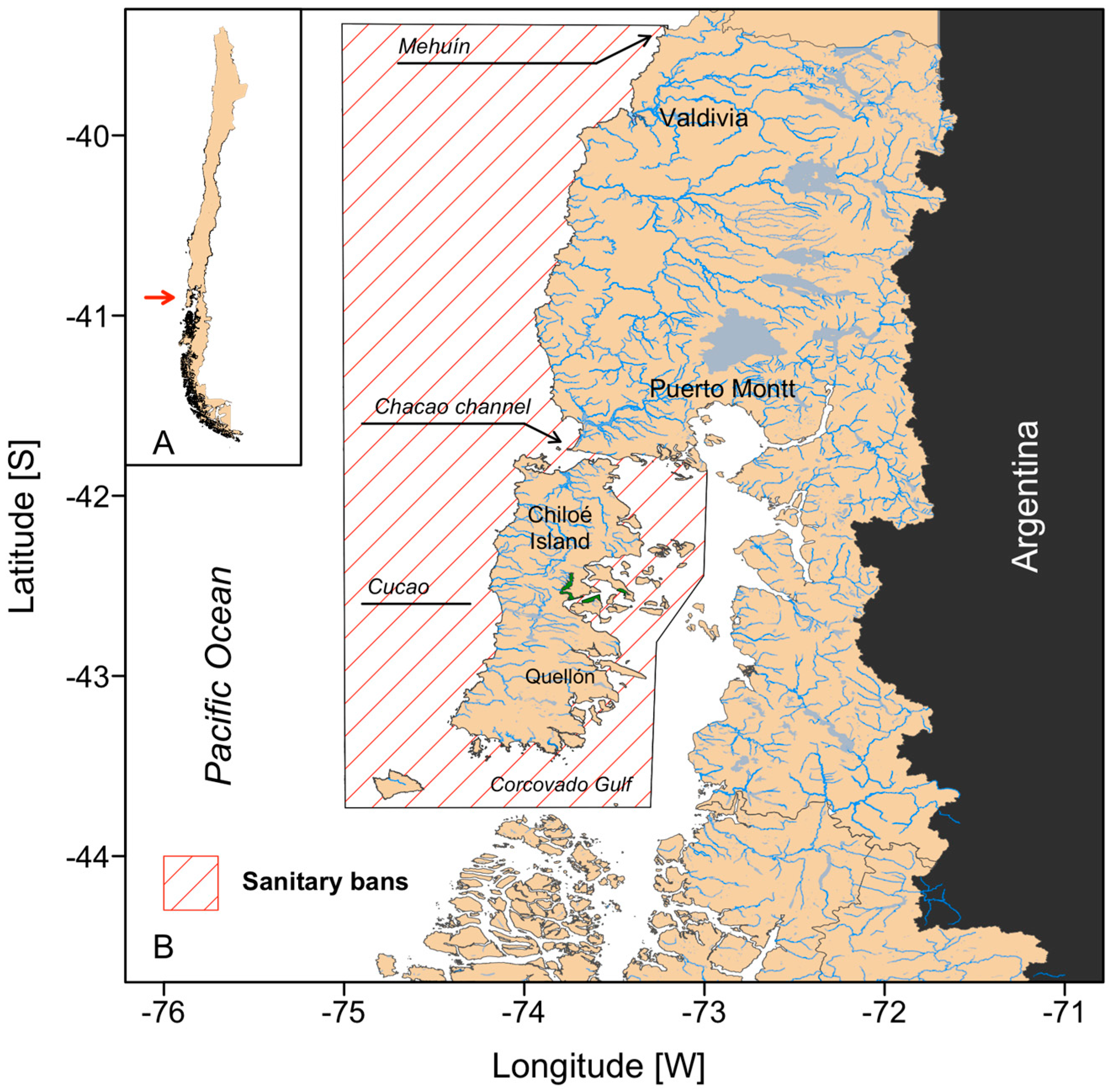
2.1. Study Area
2.2. IFOP Monitoring Programme
2.3. Wind and Ekman Transport
2.4. PST Toxicity Data
2.5. Modelling
3. Results
3.1. Alexandrium catenella Bloom Evolution
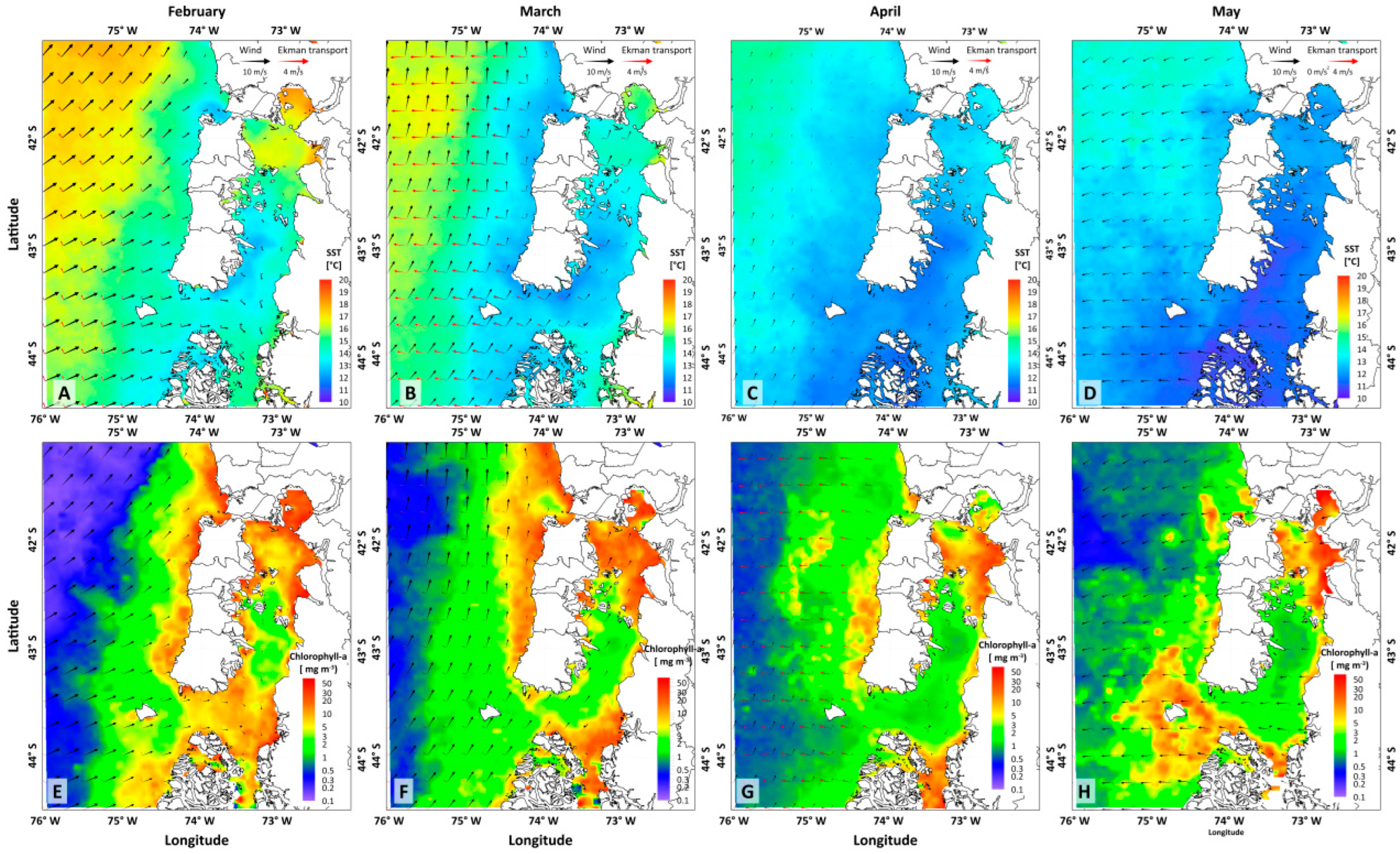
3.2. Meteorological and Oceanographic Conditions
3.3. Evolution of PSP Outbreak
3.4. Detoxification Rates
4. Discussion
4.1. Local Environmental Drivers
4.2. Accumulation-Detoxification Patterns
4.3. Socio-Economics Impacts
4.4. Future Perspectives and A. catenella Northward Expansion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hallegraeff, G.; Enevoldsen, H.; Zingone, A. Global harmful algal bloom status reporting. Harmful Algae 2021, 102, 101992. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Álvarez, G. Effects of microalgal blooms on aquaculture and fisheries. Fishes 2023, 8, 461. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef]
- Glibert, P.M.; Allen, J.I.; Artioli, Y.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal blooms distribution in response e to climate change: Projections based on model analysis. Glob. Chang. Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.M.; Alpermann, T.; Cembella, A.D.; Collos, Y.; Masseret, E.; Montesori, M. The globally distributed genus Alexandrium: Multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae 2012, 14, 10–35. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, L.; Campodonico, I.; Antunovic, M. Estudios sobre un florecimiento toxico causado por Gonyaulax catenella en Magallanes. IV. Distribución y niveles de veneno paralítico de los mariscos (noviembre de 1972-noviembre de 1973). An. Inst. Patagon. 1975, 6, 209–223. [Google Scholar]
- Molinet, C.; Lafón, A.; Lembeye, G.; Moreno, C.A. Patrones de distribución espacial y temporal de floraciones de Alexandrium catenella (Whedon & Kofoid) Balech 1985, en aguas interiores de la Patagonia noroccidental de Chile. Rev. Chil. Hist. Nat. 2003, 76, 681–698. [Google Scholar]
- Hernández, C.; Díaz, P.A.; Molinet, C.; Seguel, M. Exceptional climate anomalies and northwards expansion of Paralytic Shellfish Poisoning outbreaks in Southern Chile. Harmful Algae News 2016, 54, 1–2. [Google Scholar]
- Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic Shellfish Toxins in Mesodesma donacium during an exceptional bloom of Alexandrium catenella associated to an intense mass mortality. Toxins 2019, 11, 188. [Google Scholar] [CrossRef]
- Sernapesca. Anuario Estadistico de Pesca; Servicio Nacional de Pesca: Valparaíso, Chile, 2022. [Google Scholar]
- Díaz, P.A.; Álvarez, A.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Paredes-Mella, J.; Mardones, J.I.; Norambuena, L.; Fuenzalida, G.; Labra, G.; Espinoza-González, O.; Guzmán, L. Toxic Alexandrium catenella expanding northward along the Chilean coast: New risk of paralytic shell sh poisoning off the Bío-Bío region (36° S). Mar. Pollut. Bull. 2021, 172, 112783. [Google Scholar] [CrossRef]
- Rodríguez-Villegas, C.; Pérez-Santos, I.; Lee, M.R.; Saldías, G.S.; Navarro, C.R.; Urrutia, C.; Ross, L.; Mancilla-Gutiérrez, G.; Baldrich, A.M. Deep water turbulence may drive toxic algal blooms: The case of an anoxic submarine canyon that harbors toxic dinoflagellate resting cysts. In Proceedings of the XVI Workshop of the Centre for Biotechnology and Engineering (CeBiB), Santiago, Chile, 11–13 December 2023. [Google Scholar]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Niklitschek, E.J.; Díaz, M.; Álvarez, G.; Pérez-Santos, I.; Varela, D.; Guzmán, L.; Rodríguez-Villegas, C.; et al. Modelling the spatial and temporal dynamics of paralytic shellfish toxins (PST) at different scales: Implications for research and management. Toxins 2022, 14, 786. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, L.; Pacheco, H.; Pizarro, G.; Alárcon, C. Alexandrium catenella y veneno paralizante de los mariscos en Chile. In Floraciones Algales Nocivas en el Cono Sur Americano; Sar, E.A., Ferrario, M.E., Reguera, B., Eds.; Instituto Español de Oceanografía: Madrid, Spain, 2002; Volume 11, pp. 235–255. [Google Scholar]
- Molinet, C.; Niklitschek, E.; Seguel, M.; Díaz, P. Trends of natural accumulation and detoxifcation of paralytic shellfsh poison in two bivalves from the Northwest Patagonian inland sea. Rev. Biol. Mar. Ocenog. 2010, 45, 195–204. [Google Scholar]
- ODEPA. Estadísticas Silvoagropecuarias; Oficina de Estudios y Políticas Agrarias: Santiago, Chile, 2024. [Google Scholar]
- Cabello, F.C.; Godfrey, H.P. Harmful algal blooms (HABs), marine ecosystems and human health in the Chilean Patagonia. Rev. Chil. Infectología 2016, 33, 559–560. [Google Scholar] [CrossRef] [PubMed]
- Torres, C.; Estay, M. Estimación del empleo indirecto generado por la pesca, acuicultura y manufactura de recursos del mar en Chile. Rev. Álisis Económico 2023, 38, 127–150. [Google Scholar] [CrossRef]
- González-Poblete, E.; Hurtado, F.; Rojo, S.; Norambuena, C. Blue mussel aquaculture in Chile: Small or large scale industry? Aquaculture 2018, 493, 113–122. [Google Scholar] [CrossRef]
- Subpesca. Informe Sectorial Consolidado 2020–2021; Departamento de Análisis Sectorial. Subsecretaria de Pesca y Acuicultura: Valparaíso, Chile, 2021; p. 19. [Google Scholar]
- Moore, S.; Mantua, N.; Hickey, B.; Trainer, V. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae 2009, 8, 463–477. [Google Scholar] [CrossRef]
- Díaz, P.A.; Figueroa, R.I. Toxic algal bloom recurrence in the era of global change: Lessons from the Chilean Patagonian fjords. Microorganisms 2023, 11, 1874. [Google Scholar] [CrossRef]
- Garreaud, R. Record-breaking climate anomalies lead to severe drought and environmental disruption in western Patagonia in 2016. Clim. Res. 2018, 74, 217–229. [Google Scholar] [CrossRef]
- Pickard, G.L. Some physical oceanographic features of inlets of Chile. J. Fish. Res. Board Can. 1971, 28, 1077–1106. [Google Scholar] [CrossRef]
- Sauter, T. Revisiting extreme precipitation amounts over southern South America and implications for the Patagonian Icefields. Hydrol. Earth Syst. Sci. 2020, 24, 203–2016. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Garcés-Vargas, J.; Schneider, W.; Ross, L.; Parra, S.; Valle-Levinson, A. Double-diffusive layering and mixing in Patagonian fjords. Prog. Oceanogr. 2014, 129, 35–49. [Google Scholar] [CrossRef]
- Strub, P.T.; James, C.; Montecino, V.; Rutllant, J.A.; Blanco, J.L. Ocean circulation along the southern Chile transition region (38°–46° S): Mean, seasonal and interannual variability, with a focus on 2014–2016. Prog. Oceanogr. 2019, 172, 159–198. [Google Scholar] [CrossRef] [PubMed]
- Saldías, G.S.; Sobarzo, M.; Quiñones, R. Freshwater structure and its seasonal variability off western Patagonia. Prog. Oceanogr. 2019, 174, 143–153. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Díaz, P.A.; Silva, N.; Garreaud, R.; Montero, P.; Henríquez-Castillo, C.; Barrera, F.; Linford, P.; Amaya, C.; Contreras, S.; et al. Oceanography time series reveals annual asynchrony input between oceanic and estuarine waters in Patagonian fjords. Sci. Total Environ. 2021, 798, 149241. [Google Scholar] [CrossRef] [PubMed]
- Sievers, A.H.; Silva, N. Water masses and circulation in austral Chilean channels and fjords. In Progress in the Oceanographic Knowledge of Chilean Inner Waters, from Puerto Montt to Cape Horn; Silva, N., Palma, S., Eds.; Comité Oceanográfico Nacional-Pontificia Universidad Católica de Valparaíso: Valparaíso, Chile, 2008; pp. 53–58. [Google Scholar]
- Calvete, C.; Sobarzo, M. Quantification of the surface brackish water layer and frontal zones in southern Chilean fjords between Boca del Guafo (43°30′ S) and Estero Elefantes (46°30′ S). Cont. Shelf. Res. 2011, 31, 162–171. [Google Scholar] [CrossRef]
- Pérez-Santos, I.; Seguel, R.; Schneider, W.; Linford, P.; Donoso, D.; Navarro, E.; Amaya-Cárcamo, C.; Pinilla, E.; Daneri, G. Synoptic-scale variability of surface winds and ocean response to atmospheric forcing in the eastern austral Pacific Ocean. Ocean Sci. Discuss. 2019, 15, 1247–1266. [Google Scholar] [CrossRef]
- Crawford, D.W.; Montero, P.; Daneri, G. Blooms of Alexandrium catenella in coastal waters of Chilean Patagonia: Is subantarctic surface water involved? Front. Mar. Sci. 2021, 8, 612628. [Google Scholar] [CrossRef]
- Aiken, C. Barotropic tides of the Chilean Inland Sea and their sensitivity to basin geometry. J. Geophys. Res. 2008, 113, C8024. [Google Scholar] [CrossRef]
- Lindahl, O. A dividable hose for phytoplankton sampling. In Report of the Working Group on Phytoplankton and Management of Their Effects; C.M.1986/L: 1926, annex 3: 1-3; International Council for the Exploration of the Sea: Hirtshals, Denmark, 1986. [Google Scholar]
- ICES. Report of the Working Group on Exceptional Algal Blooms; 17–19 March 1986, ICES C.M. 1986/L.: 26; International Council for the Exploration of the Sea: Hirtshals, Denmark, 1986; p. 31. [Google Scholar]
- Lovegrove, T. An improved form of sedimentation apparatus for use with an inverted microscope. J. Cons. Int. Explor. Mer. 1960, 25, 279–284. [Google Scholar] [CrossRef]
- Utermöhl, H. Zur Vervollkomnung der quantitativen phytoplankton-Methodik. Mitt. Int. Ver. Limnol. 1958, 9, 1–38. [Google Scholar]
- Hersbach, H.; Bell, B.; Berrisford, P.; Hirahara, S.; Horányi, S.; Muñoz-Sabater, J.; Nicolas, J.; Peubey, C.; Radu, R.; Schepers, D.; et al. The ERA5 global reanalysis. Q. J. R. Meteorol. Soc. 2020, 146, 1999–2049. [Google Scholar] [CrossRef]
- Bakun, A.; Parrish, R.H. Turbulence, transport, and pelagic fish in the California and Peru Current Systems. CalCOFI Rep. 1982, 23, 99–112. [Google Scholar]
- Mendo, J.; Pizarro, L.; Castillo, S. Monthly turbulence and Ekman transport indexes, 1953 to 1985, based on local wind records from Trujillo and Callao, Peru. In The Peruvian Anchoveta and Its Upwelling Ecosystem: Three Decades of Changes; ICLARM Studies and Reviews; Pauly, D., Tsukayama, I., Eds.; WorldFish: Penang, Malaysia, 1987; Volume 46, pp. 75–88. [Google Scholar]
- Parrish, R.H.; Bakun, A.; Husby, D.; Nelson, C.S. Comparative climatology of selected environmental processes in relation to eastern boundary current pelagic fish reproduction. FAO Fish. Rep. 1982, 291, 731–778. [Google Scholar]
- AOAC. Official Method 49.10.03 for the analysis of PSP toxins in shellfish. J. AOAC Int. 2005, 88, 1714. [Google Scholar]
- Elzhov, T.V.; Mullen, K.M.; Spiess, A.-N.; Bolker, B. minpack.lm: R Interface to the Levenberg-Marquardt Nonlinear Least-Squares Algorithm Found in MINPACK, Plus Support for Bounds. R Package Version 1.2-4. 2023. Available online: https://CRAN.R-project.org/package=minpack.lm (accessed on 1 March 2024).
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical-Theoretic Approach; Springer: Amsterdam, The Netherlands, 2002; p. 496. [Google Scholar]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; ISBN 3-900051-07-0. Available online: http://www.r-project.org/ (accessed on 1 March 2024).
- Díaz, P.A.; Pérez-Santos, I.; Basti, L.; Garreaud, R.; Pinilla, E.; Barrera, F.; Tello, A.; Schwerter, C.; Arenas-Uribe, S.; Soto-Riquelme, C.; et al. The impact of local and climate change drivers on the formation, dynamics, and potential recurrence of a massive fish-killing microalgal bloom in Patagonian fjord. Sci. Total Environ. 2023, 865, 161288. [Google Scholar] [CrossRef]
- León-Muñoz, J.; Urbina, M.A.; Garreaud, R.; Iriarte, J.L. Hydroclimatic conditions trigger record harmful algal bloom in western Patagonia (summer 2016). Sci. Rep. 2018, 8, 1330. [Google Scholar] [CrossRef]
- Díaz, P.A.; Pérez-Santos, I.; Schwerter, C.; Rosales, S.A.; Álvarez, G.; Villanueva, F.; Urrutia, P.; Basti, L. High-Biomass Harmful Algal Blooms (HB-HAB) in the Chilean Fjords System: Prorocentrum micans. Harmful Algae News 2023, 73, 8–9. [Google Scholar]
- Mancilla-Gutiérrez, G.; Díaz, P.A.; Pérez-Santos, I.; Pujol, C.; Schwerter, C.; Altamirano, R.; Arenas-Uribe, S.; Navarro, P.; Saldías, G.S. High-Biomass Harmful Algal Blooms (HB-HAB) in the Chilean Fjords System: Lepidodinium chlorophorum. Harmful Algae News 2022, 71, 5–6. [Google Scholar]
- Mardones, J.I.; Bolch, C.; Guzmán, L.; Paredes, J.; Varela, D.; Hallegraeff, G.M. Role of resting cysts in Chilean Alexandrium catenella dinoflagellate blooms revisited. Harmful Algae 2016, 55, 238–249. [Google Scholar] [CrossRef]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Díaz, M.; Labra, G.; Figueroa, R. Coupling planktonic and benthic shifts during a bloom of Alexandrium catenella in southern Chile: Implications for bloom dynamics and recurrence. Harmful Algae 2014, 40, 9–22. [Google Scholar] [CrossRef]
- Rodríguez-Villegas, C.; Figueroa, R.I.; Baldrich, A.; Pérez-Santos, I.; Díaz, M.; Tomasetti, S.J.; Seguel, M.; Álvarez, G.; Salgado, P.; Díaz, P.A. Small and patchy is enough: An example about how toxic HAB events can spread through low resting cyst loads. Harmful Algae 2023, 129, 102495. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Molinet, C.; Seguel, M.; Díaz, M.; Labra, G.; Figueroa, R.I. Species diversity and abundance of dinoflagellate resting cysts seven months after a bloom of Alexandrium catenella in two contrasting coastal systems of the Chilean Inland Sea. Eur. J. Phycol. 2018, 53, 410–421. [Google Scholar] [CrossRef]
- Rodríguez-Villegas, C.; Figueroa, R.I.; Pérez-Santos, I.; Molinet, C.; Saldías, G.S.; Rosales, S.A.; Álvarez, G.; Linford, P.; Díaz, P.A. Continental shelf off northern Chilean Patagonia: A potential risk zone for the onset of Alexandrium catenella toxic bloom? Mar. Pollut. Bull. 2022, 184, 114103. [Google Scholar] [CrossRef]
- Anderson, D.M.; Stock, C.; Keafer, B.; Nelson, A.; McGillicuddy, D.; Keller, M.; Thompson, B.; Matrai, P.; Martin, J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep Sea Res. II 2005, 52, 2522–2542. [Google Scholar] [CrossRef]
- Figueroa, R.I.; Estrada, M.; Garcés, E. Life histories of microalgal species causing harmful blooms: Haploids, diploids and the relevance of benthic stages. Harmful Algae 2018, 73, 44–57. [Google Scholar] [CrossRef]
- Shumway, S. Phycotoxin-related shellfish poisoning: Bivalve molluscs are not the only vectors. Rev. Fish. Sci. 1995, 3, 1–31. [Google Scholar] [CrossRef]
- Compagnon, D.; Lembeye, G.; Marcos, N.; Ruiz-Tagle, N.; Lagos, N. Accumulation of paralitic shellfish poisoning toxins in the bivalve Aulacomya ater and two carnivorous gastropods Concholepas concholepas and Argobuccinum ranelliformes during an Alexandrium catenella bloom in southern Chile. J. Shellfish Res. 1998, 17, 67–73. [Google Scholar]
- Hamann, L.; Blanke, A. Suspension feeders: Diversity, principles of particle separation and biomimetic potential. J. R. Soc. Interface 2022, 19, 20210741. [Google Scholar] [CrossRef]
- Ben-Gigirey, B.; Rossignoli, A.E.; Riobó, P.; Rodríguez, F. First report of paralytic shellfish toxins in marine invertebrates and fish in Spain. Toxins 2020, 12, 723. [Google Scholar] [CrossRef]
- Roje-Busatt, R.; Ujević, I. PSP toxins profile in ascidian Microcosmus vulgaris (Heller, 1877) after human poisoning in Croatia (Adriatic Sea). Toxicon 2014, 79, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Bricelj, V.M.; Shumway, S. Paralytic shellfish toxins in bivalve molluscs: Occurrence, transfer kinetics, and biotransformation. Rev. Fish. Sci. 1998, 6, 315–383. [Google Scholar] [CrossRef]
- Blanco, J.; Moroño, A.; Franco, J.; Reyero, M.I. PSP detoxification kinetics in the mussel Mytilus galloprovincialis. One- and two- comportment models and the effect of some environmental variables. Mar. Ecol. Prog. Ser. 1997, 158, 165–175. [Google Scholar] [CrossRef]
- Blanco, J.; Reyero, M.; Franco, J. Kinetics of accumulation and transformation of paralytic shellfish toxins in the blue mussel Mytilus galloprovincialis. Toxicon 2003, 42, 777–784. [Google Scholar] [CrossRef]
- Bustos, B.; Román, A. A sea uprooted: Islandness and political identity on Chiloé Island, Chile. Isl. Stud. J. 2019, 14, 97–114. [Google Scholar] [CrossRef]
- Rincón, P.; Villagrán, L.; Fuenzalida, B.; Martínez, V.; Muñoz, C.; Neira, M.; Neira, Á.; Orellana, M. Efectos psicosociales de un desastre socioambiental: La ‘marea roja’ en Chiloé, Chile. Rev. Estud. Latinoam. Sobre Reducción Riesgo Desastres REDER 2023, 7, 156–167. [Google Scholar] [CrossRef]
- Véliz, A.; Retamal, A. The red tide phenomenon in the south of Chile and its impact on the psychosocial well-being of the inhabitants of a fishing community in Los Lagos region. Rev. Notas Históricas Y Geográficas 2019, 23, 236–257. [Google Scholar]
- Mardones, J.I.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the environmental processes responsible for the world’s largest farmed fish-killing harmful algal bloom: Chile, 2016. Sci. Total Environ. 2021, 766, 144383. [Google Scholar] [CrossRef]
- Anderson, R.; Villarreal, R. Economic Impact of the 2016 Red Tide over the Exporting Sector of Chile’s Tenth Region; MPRA Paper 106764; University Library of Munich: Munich, Germany, 2020. [Google Scholar]
- Azócar, P.; Sir, H. Infrastructure and dignity: Notes on the becoming of the Chilean revolt. South Atl. Q. 2023, 122, 861–868. [Google Scholar]
- Arriagada, N. Adaptive Capacity in Social-Environmental Crisis: The Case of the Red Tide/Salmon Farming Conflict in Chiloé (Chile); University of British Columbia: Vancouver, BC, Canada, 2019. [Google Scholar]
- Herrera, M. Controversias socioambientales al sur de Chile: El caso de la crisis de la marea roja en la Isla Grande de Chiloé. Región Y Soc. 2020, 32, e1343. [Google Scholar] [CrossRef]
- Mascareño, A.; Cordero, R.; Azócar, G.; Billi, M.; Henríquez, P.A.; Ruz, G.A. Controversies in social-ecological systems: Lessons from a major red tide crisis on Chiloe Island, Chile. Ecol. Soc. 2018, 23, 15. [Google Scholar] [CrossRef]
- Valdebenito, J. Crisis socioecológica y comunicación durante la Marea Roja de Chiloé (2016). Texto Livre Ling. E Tecnol. 2021, 14, e26231. [Google Scholar] [CrossRef]
- Mardones, J.I.; Holland, D.S.; Anderson, L.; Le Bihan, V.; Gianella, F.; Clément, A.; Davidson, K.; Sakamoto, S.; Yoshida, T.; Trainer, V.L. Estimating and mitigating the economic costs of harmful algal blooms on commercial and recreational shellfish harvesters. PICES Sci. Rep. 2020, 59, 66–83. [Google Scholar]
- Marín, A.; Lizana, G.; Valdivieso, D.; Díaz, P.A. Red Tide Adaptation and Response Network (REARMAR): Bridging local, scientific and policy knowledge for small-scale benthic fisheries in the northern Chilean Patagonia. Harmful Algae News 2022, 70, 10–11. [Google Scholar]
- Keafer, B.A.; Churchill, J.H.; McGillicuddy, D.J., Jr.; Anderson, D.M. Bloom development and transport of toxic Alexandrium fundyense populations within a coastal plume in the Gulf of Maine. Deep Sea Res. II 2005, 52, 2674–2697. [Google Scholar] [CrossRef]
- McGillicuddy, D.; Signell, R.; Stock, C.; Keafer, B.; Keller, M.; Hetland, R.; Anderson, D. A mechanism for offshore initiation of harmful algal blooms in the coastal Gulf of Maine. J. Plankton Res. 2003, 25, 1131–1138. [Google Scholar] [CrossRef]
- Anderson, D.M.; Fachon, E.; Pickart, R.S.; Lin, P.; Fisher, A.D.; Richlen, M.L.; Uva, V.; Brosnahan, M.L.; McRaven, L.; Bahr, F.; et al. Evidence for massive and recurrent toxic blooms of Alexandrium catenella in the Alaskan Arctic. Proc. Natl. Acad. Sci. USA 2021, 118, e2107387118. [Google Scholar] [CrossRef]
- Anderson, D.M.; Couture, D.A.; Kleindinst, J.L.; Keafer, B.A.; McGuillicuddy, D.J.J.; Martin, J.L.; Richlen, M.L.; Hickey, J.M.; Solow, A.R. Understanding interannual, decadal level variability in paralytic shellfish poisoning toxicity in the Gulf of Maine: The HAB Index. Deep Sea Res. II 2014, 103, 264–276. [Google Scholar] [CrossRef] [PubMed]





| Model | ΔAICc | AICcw | ||||
|---|---|---|---|---|---|---|
| Surf Clam | Giant Barnacle | Red Sea Squirt | Surf Clam | Giant Barnacle | Red Sea Squirt | |
| Null | 252.4 | 44.76 | 83.73 | 0 | 0 | 0 |
| Equation (1) | 13.22 | 8.84 | 13.63 | 0.001 | 0.011 | 0.001 |
| Equation (2) | 4.37 | 5.61 | 3.69 | 0.067 | 0.056 | 0.109 |
| Equation (3) | 0 | 8.72 | 0 | 0.593 | 0.012 | 0.691 |
| Equation (4) | 1.12 | 0 | 2.49 | 0.339 | 0.921 | 0.199 |
| Species | Best Model | Maximum PST (PSTmax) | Baseline PST (PSTbl) | Instantaneous Decay Rate (k) | Power Time Dependence Parameter (m) |
|---|---|---|---|---|---|
| Surf clam | Equation (3) | 7784 (170) | 195 (47) | −0.07 (0.055) | n.a. |
| Giant barnacle | Equation (4) | 602 (72) | 50 (17) | −4.7·10−6 (3.15·10−5) | 3.7 (2.12) |
| Red sea squirt | Equation (3) | 813 (53) | 57 (13) | −0.05 | n.a. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz, P.A.; Rosales, S.A.; Molinet, C.; Niklitschek, E.J.; Marín, A.; Varela, D.; Seguel, M.; Díaz, M.; Figueroa, R.I.; Basti, L.; et al. Are Alexandrium catenella Blooms Spreading Offshore in Southern Chile? An In-Depth Analysis of the First PSP Outbreak in the Oceanic Coast. Fishes 2024, 9, 340. https://doi.org/10.3390/fishes9090340
Díaz PA, Rosales SA, Molinet C, Niklitschek EJ, Marín A, Varela D, Seguel M, Díaz M, Figueroa RI, Basti L, et al. Are Alexandrium catenella Blooms Spreading Offshore in Southern Chile? An In-Depth Analysis of the First PSP Outbreak in the Oceanic Coast. Fishes. 2024; 9(9):340. https://doi.org/10.3390/fishes9090340
Chicago/Turabian StyleDíaz, Patricio A., Sergio A. Rosales, Carlos Molinet, Edwin J. Niklitschek, Andrés Marín, Daniel Varela, Miriam Seguel, Manuel Díaz, Rosa I. Figueroa, Leila Basti, and et al. 2024. "Are Alexandrium catenella Blooms Spreading Offshore in Southern Chile? An In-Depth Analysis of the First PSP Outbreak in the Oceanic Coast" Fishes 9, no. 9: 340. https://doi.org/10.3390/fishes9090340
APA StyleDíaz, P. A., Rosales, S. A., Molinet, C., Niklitschek, E. J., Marín, A., Varela, D., Seguel, M., Díaz, M., Figueroa, R. I., Basti, L., Hernández, C., Carbonell, P., Cantarero, B., & Álvarez, G. (2024). Are Alexandrium catenella Blooms Spreading Offshore in Southern Chile? An In-Depth Analysis of the First PSP Outbreak in the Oceanic Coast. Fishes, 9(9), 340. https://doi.org/10.3390/fishes9090340









