Abstract
Chronic exposure to heavy metals has been widely demonstrated to induce pathological features in different tissues and, in particular, in the central nervous system. Specific neurons, including dopaminergic neurons, were observed to be more susceptible to toxic agents. Several previous studies performed on zebrafish (Danio rerio) models observed that exposure to nickel (one of the most abundant heavy metals) induces impairment of memory and anxious-like behaviors. Nevertheless, this phenotypical evidence has not been associated with dopaminergic system damage, and no reports showing the effects of nickel on dopaminergic neurons are available. In this study, we aim to analyze the precise distribution and variation in dopaminergic neurons in adult zebrafish after chronic (96 h) exposure to nickel ions dissolved in water at different sub-lethal doses (0.4 mg L−1; 2 mg L−1 and 4 mg L−1). The effects of treatment on dopaminergic neurons were evaluated by measuring transcript and protein levels of tyrosine hydroxylase (TH), described as a dopaminergic neuron marker. As shown, the expression of the th1 and th2 genes was reduced in the entire brain of zebrafish treated with nickel. Immunostaining analysis allowed us to localize TH-expressing neurons mainly in the posterior tuberculum, where they were observed to be reduced after nickel treatment in a dose-dependent fashion. Consistently, the TUNEL assay revealed a significant increase in apoptosis of TH-expressing cells after treatment with 2 mg L−1 and 4 mg L−1 of nickel. Our findings represent the first evidence of the effect of nickel on the dopaminergic system.
1. Introduction
Dopaminergic neurons play a crucial role in the central nervous system (CNS) of all vertebrates [1,2,3]. These neurons are the principal source of dopamine, an important neurotransmitter [4]. Dopamine is synthesized peripherally within the adrenal glands and, for the most part, at the central level within dopaminergic neurons [5,6,7]. The biosynthesis of dopamine is regulated by the action of the enzyme tyrosine hydroxylase (TH), which is highly expressed in dopaminergic neurons [8]. These neurons, in mammals, have been categorized into several groups: A8–A10 (diencephalon–midbrain), A11–A15 (diencephalon), A16 (olfactory bulb), and A17 (retinal) [9,10,11]. On the contrary, in teleost fishes such as zebrafish (Danio rerio), dopaminergic neurons are mainly located in the paraventricular organs and periventricular nucleus of the posterior tuberculum [12,13,14,15,16]. In zebrafish, dopamine is involved in the regulation of several functions: social interaction [17], visual sensitivity [18], learning and memory [19], addiction, and, in particular, motor activity [20,21,22].
Dopaminergic neurons were observed to be highly vulnerable to toxic agents, as evidenced by their susceptibility to environmental factors [23]. In detail, previous studies performed on zebrafish showed that exposure to heavy metals, such as cadmium [24], mercury [25], lead [26], and aluminum [27], determined highly neurotoxic effects by altering dopaminergic neuron function. In this scenario, the choice to use the zebrafish model to study the effects of heavy metal-induced neurotoxicity results in being very efficient, as shown by several previous reports [28]. The zebrafish is a type of teleost fish belonging to the Cyprinidae family and presents several advantageous characteristics, including its small size, low maintenance costs, high fecundity, ex utero reproduction, transparent offspring, and an analogous nervous system cellular physiology that is similar to that of mammals [29,30,31]. All these characteristics make it an excellent model for investigation in different fields of research, especially neurobiology [32,33,34,35].
Nickel is one of the most abundant elements in the earth’s crust [36,37]. Its concentration in the environment has increased significantly in recent years due to its growing extraction linked to industrial development [38]. Indeed, the chemical and physical characteristics make this metal widely used in medical equipment, jewelry, steel, and, in particular, electric batteries [39]. It is also used in the plating of metals and agriculture. Currently, nickel can be traced significantly in the air, soil, sediments, and water [40,41]. Many surface waters, rivers, and lakes show high levels of nickel [42,43].
Despite its functional role for plants and animals, the excessive environmental dispersion of this heavy metal, mainly due to industrial activity, could dangerously affect human and animal health [44]. The presence of nickel in aquatic environments has made it important to study its effects on aquatic organisms, particularly fish [45,46,47]. In this research, the zebrafish animal model has assumed considerable importance.
Previous behavioral studies have investigated the effects of nickel exposure on adult zebrafish animal models. The authors have observed that nickel exposure in adult zebrafish induced anxiety, altered swimming ability, and impaired memory [48]. However, no previous histopathological analysis is available to clarify whether these phenotypes were linked to the toxic effects on dopaminergic neurons.
Thus, in the present study, we aim to analyze the precise distribution and variation in dopaminergic neurons in adult zebrafish exposed to nickel ions at sub-lethal doses. To achieve this scope, we analyzed the brains of non-treated and treated zebrafish using quantitative real-time PCR and immunohistochemistry. We measured the levels of TH, which, as mentioned before, is a marker of dopaminergic neurons at both transcript and protein levels. In parallel, we also evaluated levels of apoptotic TH-expressing cells in the brains of treated and untreated zebrafish.
2. Materials and Methods
2.1. Animals
The study was carried out on adult zebrafish (Danio rerio), including males and females, 6–8 months old and 4 cm in total length, purchased from the Coral Aquarium in Bologna, Italy. Acclimatization took place in tanks containing dechlorinated tap water and distilled water (1:2) for one month at 25 °C in a 12:12 h light–dark cycle. They were fed twice a day. All procedures followed the guidelines of the European Community Council Directive and the current Italian legislation on the use and care of animals. Ethical approval of this study was obtained from the Scientific Ethics Committee of the University of Bologna (protocol no. 17/79/2014).
2.2. Nickel Exposure and Brain Dissection
Adult zebrafish were exposed to three sub-lethal concentrations of nickel ion concentrations dissolved in water (0.4 mg L−1, 2 mg L−1, and 4 mg L−1) for 96 h, according to common use in acute toxicity tests [48] and to our previous studies [49]. As previously reported [49], nickel ion concentrations were prepared from nickel chloride hexahydrate (Merck, Darmstadt, Germany) dissolved in acclimatization water. The low quantity (LQ) solution contained 0.4 mg L−1 of nickel chloride hexahydrate, corresponding to 0.1 mg L−1 of Ni2+; the medium quantity (MQ) solution contained 2 mg L−1 of nickel chloride hexahydrate, corresponding to 0.5 mg L−1 of Ni2+; and the high quantity (HQ) solution contained 4 mg L−1 of nickel chloride hexahydrate, corresponding to 1 mg L−1 of Ni2+. The concentration of 0.5 mg L−1 corresponds to the environmentally relevant nickel level previously used by Macomber and colleagues [50]. Zebrafish were anesthetized by specific anesthetic drugs (ethyl 3-aminobenzoate and methanesulfonate 0.1%, Sigma Chemicals Co., St. Louis, MO, USA), euthanized by decapitation, and their brains were dissected for the experimental procedures.
2.3. RNA Extraction
Total RNA was extracted from fish brains (3 fish heads for each experimental condition) by using an RNAeasy mini kit (Qiagen, Frankfurt, Germany) according to the manufacturer’s protocol. This procedure was repeated in three independent experiments. In total, for these experiments, we used 36 zebrafish.
2.4. Reverse Transcriptase PCR
Reverse transcription of mRNA into cDNA was obtained by using the Superscript III First-Strand Synthesis System kit (Invitrogen, Boston, MA, USA) according to the manufacturing procedure, as described in previous studies [51,52]. Total RNA (0.5 μg) was incubated with buffer and enzyme mix for 10 min at 25 °C, 30 min at 50 °C, and 5 min at 85 °C. Samples were then treated with RNase-H for 30 min at 37 °C and stored at −20 °C.
2.5. Quantitative Real-Time PCR
Quantitative real-time polymerase chain reaction (PCR) was performed by using the thermocycler with the MyiQ detector (Bio-Rad, Hercules, Dallas, TX, USA). cDNA was mixed with specific forward and reverse primers, SYBR-Green (Bio-Rad), and RNase-free water according to the manufacturer’s protocol [53]. The above mix was incubated for 15 min at 95 °C, then 15 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C for 40 cycles. Primer sequences used for amplification of th1 and th2 genes are listed in Table 1. Data indicate the relative increase or decrease in the levels of th1 and th2 transcripts in treated zebrafish compared to untreated controls, using ef1a to normalize the absolute quantification; the fold change was calculated using 2−∆∆Ct. Correct amplification and PCR efficiency were confirmed by melting curve analysis. Each qPCR experiment was performed on biological triplicates. In the analysis of qPCR data, each n represents the average of triplicates from a single experiment.

Table 1.
List of primer sequences for th1 and th2 gene analysis by qPCR.
2.6. Tissue Preparation
After dissection, the dorsal cranium was promptly removed, and the brain (we used 60 zebrafish in total) was directly exposed to the fixative. The heads were quickly immersed in a modified Bouin’s fixative solution, consisting of a saturated aqueous solution of picric acid and formalin (ratio 3:1), for 24 h at room temperature. The picric acid was removed by prolonged washing in 0.1 M sodium phosphate buffer (PB), pH 7.4, at room temperature. The specimens were then decalcified in 0.25 M EDTA in 0.1 M PB, pH 7.4, for 14 days at room temperature. The tissues were then dehydrated in a graded series of ethanol (70, 80, 95, and 100%, for 45 min each, at room temperature) and subsequently embedded in Paraplast Plus (Sherwood Medical, St. Louis, MO, USA; melting point 55–57 °C). Transverse microtome sections were mounted on poly-lysine slides [54]. Sections were deparaffinized in xylene, rehydrated through graded ethanol, treated with 3% H2O2 for 30 min, and rinsed in PBS (pH 7.4), followed by antigen retrieval in sodium citrate buffer (pH 6; 80 °C) for 30 min.
2.7. Immunofluorescence
After rinsing twice in 0.2% Triton PBS (PBT), non-specific binding was blocked by treating sections with 1/5 normal goat serum (Vector, Burlingame, CA, USA; cod S-1000-20) for 30 min at RT. Then, sections were incubated overnight at RT in a humidified chamber with rabbit antibody against TH (1:500, AB152; Millipore, MA, USA), able to stain both TH1 and TH2 isoforms in zebrafish tissues, as previously described [15,16,55,56,57]. The next day, the sections were washed several times in PBT and alternatively incubated with an Alexa Fluor® goat anti-rabbit 594 antibody (1:300, Invitrogen, ThermoFisher Scientific, Waltham, MA, USA) for 2 h. Tissue sections were washed in 0.2% Triton PBS, and slides were mounted with the Vectashield medium containing DAPI for nuclei counterstaining (Vector Laboratories, Burlingame, CA). Stained sections were photographed using a confocal Nikon Eclipse 90i microscope.
2.8. TUNEL Assay Combined with Immunofluorescence
After detection of TH by immunofluorescence, the signal was checked using a fluorescence microscope, and the sections were fixed for 10 min in 2% paraformaldehyde. Next, the sections were incubated with 90 µL of labeling solution plus 10 µL of enzyme solution (In Situ Cell Death Detection Kit, C10617-Roche, Basel, Switzerland) at RT for 1 h, as described in a previous study [58]. They were washed three times with PBS for 5 min and mounted. Finally, the images were examined via confocal microscopy. Stained sections were photographed using a confocal Nikon Eclipse 90i microscope.
2.9. Statistical Analysis
Data were analyzed using the GraphPad Prism 9.4.1 software (GraphPad Inc., San Diego, CA, USA); statistical comparison was performed via one-way ANOVA with multiple comparisons performed using the Tukey–Kramer post hoc test. p-values lower than 0.05 were considered statistically significant.
3. Results
3.1. Experimental Outline of the Study
To analyze the effect of nickel on dopaminergic neurons in adult zebrafish brains, as mentioned in the Material and Methods section, we used three nickel ion concentrations, which were previously observed to be non-lethal [48,49]. Next, we dissected the brains and tissues, which underwent processing to detect TH transcript and protein, respectively, by quantitative real-time PCR and/or immunohistochemistry (Figure 1).
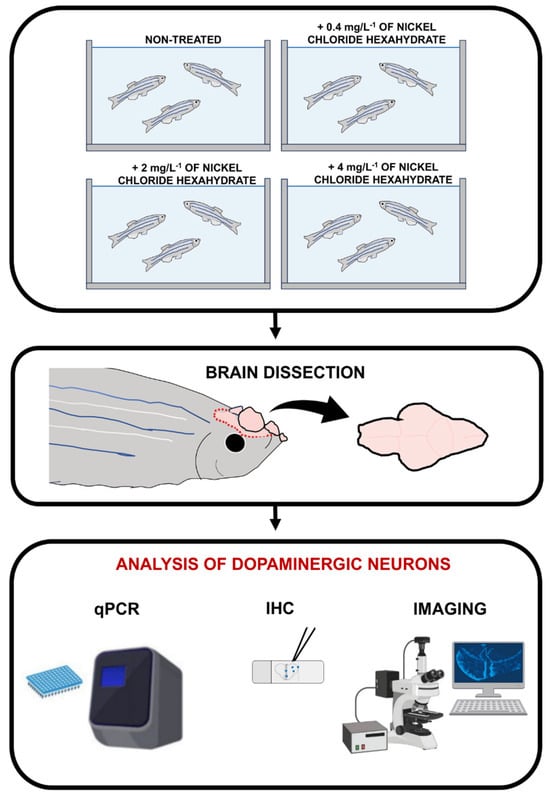
Figure 1.
Experimental outline of the study. Adult zebrafish were treated using nickel chloride hexahydrate at different concentrations (0.4 mg L−1, 2 mg L−1, and 4 mg L−1). After treatment, zebrafish were sacrificed, and brains were dissected and processed by qPCR and/or immunohistochemistry (IHC).
3.2. Nickel Exposure Affects Brain Tyrosine Hydroxylase Genes Levels in a Dose-Dependent Fashion
Previous studies have identified two tyrosine hydroxylase genes expressed in zebrafish: th1 and th2 [59]. We analyzed the transcript level of both genes in the brains of nickel-treated and untreated zebrafish. As shown in Figure 2, qPCR analysis reveals a dose-dependent decrease of th1 and th2 expression levels in the brains of treated zebrafish compared to the control ones (Figure 2a,b).
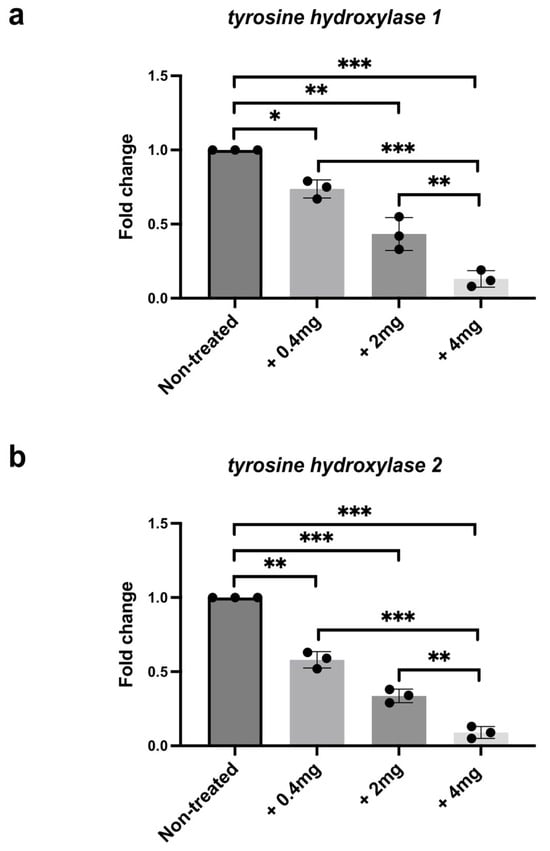
Figure 2.
qPCR gene expression analysis of (a) th1 and (b) th2 in dissected brains from nickel-treated and untreated fish. Each experiment was repeated independently three times. Statistical significance was calculated using one-way ANOVA, with multiple comparisons performed using the Tukey–Kramer post hoc test. * p < 0.01; ** p < 0.001; *** p < 0.0001. Center values denote the mean ± SD.
3.3. Treatment of Adult Zebrafish with Nickel Decreases TH-Expressing Cells in Posterior Tuberculum
In order to evaluate the precise distribution and variation in the number of dopaminergic neurons in adult zebrafish brains after nickel exposure, immunofluorescence analysis of TH protein was performed on serial sections. As mentioned in the Introduction, previous reports on zebrafish have identified that dopaminergic cells are mainly located within the posterior tuberculum; thus, we analyzed the number of TH-expressing cells in this region in both untreated control zebrafish and zebrafish treated with nickel. As expected, in the untreated zebrafish, TH was observed to be highly expressed in cells in both regions of the posterior tuberculum (TPp and PVO) (Figure 3a). Following nickel exposure, zebrafish, as shown in (Figure 3b–d), display a dramatic dose-dependent reduction in the number of cells expressing TH within the posterior tuberculum compared to untreated zebrafish. Specifically, we found that when exposed to nickel doses of 0.4 mg L−1, there was an initial decrease in TH expression within the PVO region of the tuberculum (Figure 3b). Progressively, with the increase in nickel doses, the loss of TH-expressing cells was observed to extend to the TPp region of the tuberculum (Figure 3c,d).
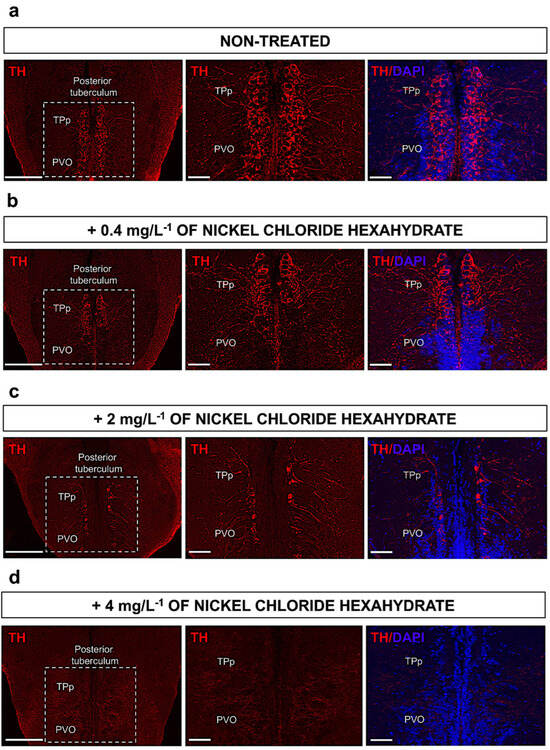
Figure 3.
(a) Low magnification (scale bar 100 µm) of TH-expressing cells in control (non-treated) zebrafish scattered in the periventricular nucleus of the posterior tuberculum (TPp) and paraventricular organ (PVO). White rectangle is a zoom region and high magnification (scale bar 50 µm) of TH-expressing cells in posterior tuberculum and DAPI (cell nuclei). (b) Low magnification (scale bar 100 µm) of TH-expressing cells of zebrafish treated with 0.4 mg L−1 of nickel chloride hexahydrate scattered in the periventricular nucleus of the posterior tuberculum (TPp) and paraventricular organ (PVO) and high magnification (scale bar 50 µm) of zoom region of TH-expressing cells in posterior tuberculum and DAPI (cell nuclei). (c) Low magnification (scale bar 100 µm) of TH-expressing cells of zebrafish treated with 2 mg L−1 of nickel chloride hexahydrate scattered in the periventricular nucleus of the posterior tuberculum (TPp) and paraventricular organ (PVO) and high magnification (scale bar 50 µm) of zoom region of TH-expressing cells in posterior tuberculum and DAPI (cell nuclei). (d) Low magnification (scale bar 100 µm) of TH-expressing cells of zebrafish treated with 4 mg L−1 of nickel chloride hexahydrate scattered in the periventricular nucleus of the posterior tuberculum (TPp) and paraventricular organ (PVO) and high magnification (scale bar 50 µm) of zoom region of TH-expressing cells in posterior tuberculum and DAPI (cell nuclei).
Consistently, counting of TH-expressing cells, shown in Figure 4, showed a statistically significant decrease of TH-positive neurons.
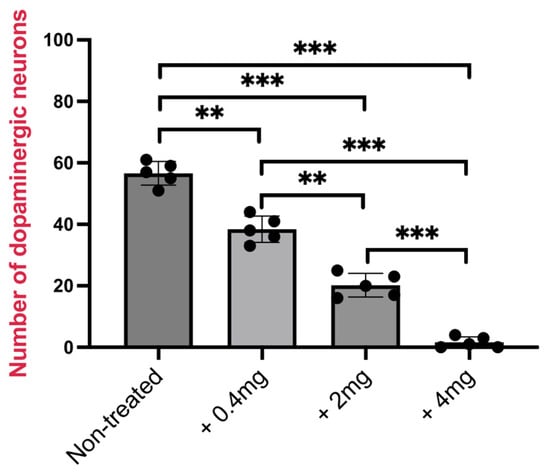
Figure 4.
Number of TH+ cells (dopaminergic neurons) in three brain sections (posterior tuberculum) from nickel-treated and untreated zebrafish (n = 5 each group). All results are represented as the means ± SD of three independent experiments (60 zebrafish in total). Statistical analysis was performed by one-way ANOVA, with multiple comparisons performed using the Tukey–Kramer post hoc test (** p < 0.001; *** p < 0.0001).
3.4. Dopaminergic Neuron Degeneration Is Associated with Increased Apoptosis in Posterior Tuberculum
To verify whether the decrease in TH+ cells in the brain of nickel-exposed zebrafish could be associated with cell death (apoptosis), we performed a TUNEL assay combined with TH immunofluorescence and DAPI staining. Consistent with previous results, we analyzed TH-expressing neurons and the TUNEL signal in the posterior tuberculum region (Figure 5a). In treated zebrafish, we found a significative increase in TH/TUNEL double-positive cells (Figure 5b); we also used DAPI to confirm the presence of cell nuclei (Figure 5b). Counting of TUNEL-positive cells reveals a significant dose-dependent increase in cell death after treatment with nickel (Figure 6).
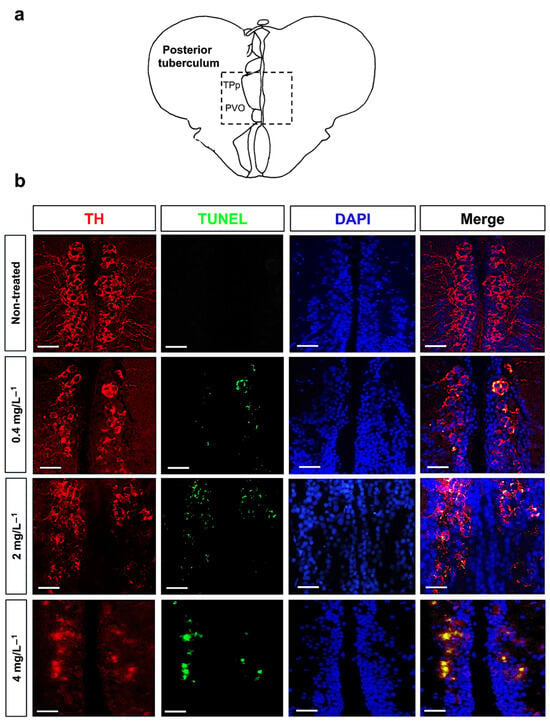
Figure 5.
(a) Representative section of posterior tuberculum region, the periventricular nucleus of the posterior tuberculum (TPp) and paraventricular organ (PVO). (b) TH-positive cells (in red), TUNEL-positive cells (in green), DAPI (blue), and merge in posterior tuberculum of nickel-treated and control (untreated) zebrafish. Confocal microscope, scale bar 50 µm.
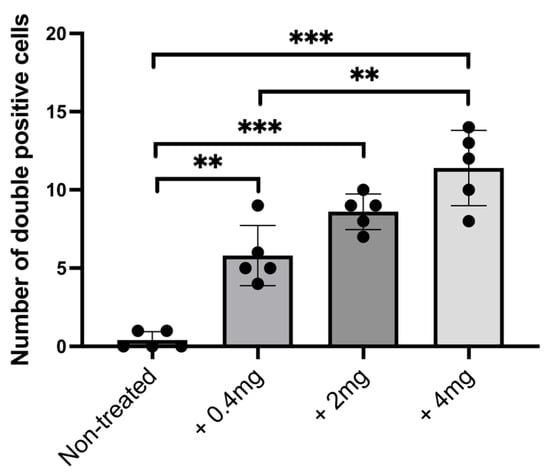
Figure 6.
Number of double-positive TH/TUNEL cells from nickel-treated and untreated zebrafish. All results are represented as the means ± SD of three independent experiments. Statistical analysis was performed by one-way ANOVA, with multiple comparisons performed using the Tukey–Kramer post hoc test (** p < 0.001; *** p < 0.0001).
4. Discussion
CNS neurons represent the main cell type that shows high susceptibility to the action of exogenous chemical substances [60]. This especially occurs with heavy metals, such as iron, mercury, arsenic, and cadmium, which can affect neuronal function and survival through several mechanisms, including oxidative stress [61,62,63]. The study of neuropathological features associated with heavy metal toxicity found enormous advantages in the use of the zebrafish model [64]. With particular reference to nickel toxicity, previous studies performed on adult zebrafish observed that nickel exposure is associated with the development of anxious-like behavior and impaired exploratory activity and memory, most likely through activation of a special mechanism of iron-induced cell death called ferroptosis [65]. Considering that no previous evidence has ever elucidated nickel-induced dopaminergic neurotoxicity, neither in adults nor in embryos, our results are a very first. In particular, in the present study, we analyzed dopaminergic cell distribution after the exposure of adult zebrafish to sub-lethal doses of nickel. First, by using the qPCR experimental approach, we found a dose-dependent decrease in th1 and th2 transcripts. Our results show that the applied nickel exposures equally affected both genes that code for TH. Next, by performing an immunofluorescence analysis, we also showed that nickel can induce a dose-dependent decrease in TH protein in the posterior tuberculum (PT). Therefore, the decrease in the expression of TH is due to a downregulation that acts directly on the transcription of th1 and th2. As mentioned before, the PT in zebrafish has been reported to represent the dopaminergic neurons in the substantia nigra pars compacta of mammals. Locus coeruleus was not analyzed in this study as this TH+ region is the regional expression of noradrenergic catecholamines, which is beyond the scope of our study. In agreement with previous studies [66,67,68], the progressive reduction in TH-positive cells within the posterior tuberculum seems to be associated with increased apoptosis in animals treated with mercury or cadmium. In our model, we also showed a significant increase in apoptotic cells by using the TUNEL assay. In agreement with our data, a previous histopathological analysis of brain tissues in rainbow trout after exposure to nickel [69] showed that this agent is neurotoxic for fish. In addition, recent investigations from our laboratory also observed a dose-dependent alteration of olfactory sensory epithelium after nickel exposure in adult zebrafish, most likely due to increased cell death [49]. However, the impact on olfactory neurons varied among the several olfactory cell types, and this cannot completely explain the marked neurobehavioral alterations previously described, as the olfactory system has a secondary control on cognitive functions [70]. The histochemical dose-dependent decrease in TH levels in this study is a consequence of two events induced by nickel: the downregulation of transcription for the two genes responsible for coding TH and the activation of an apoptotic mechanism.
In adult teleost fish, it has been widely described that the dopaminergic system controls neurobehavioral features, such as anxiety and memory. Indeed, knock-out models for the dopamine transporter (DAT) show a higher pharmacological-induced anxiety [71]. Similarly, dopaminergic circuity has been of great interest in studies about neurocognitive disorders, as these neurons seem to be responsible for cognition, memory, and learning abilities.
Exposure to metal has been widely associated with neurodevelopmental and neurodegenerative disorders. In zebrafish larvae, exposure to neurotoxins has been applied to induce a phenotype that mimics Parkinson’s disease (PD) [28]. The epidemiological studies have not yet evidenced factors surely responsible for the increase in cases of Alzheimer‘s disease that have been recorded in recent times. Prolonged exposure to heavy metals is considered a possible factor responsible for the increase in neurodegenerative diseases [72]. Indeed, in several zebrafish models generated by using genetics and/or treating zebrafish with specific drugs to mimic human PD, the authors demonstrated the loss of dopaminergic neurons [73,74,75,76].
In our study on the action of heavy metals in zebrafish, nickel was found to affect dopaminergic neurons. All the different nickel-induced damage ends up altering the behavior of fish. They become more exposed to the actions of predators and hindered in the search for food and partners and avoidance of unfavorable conditions. Further studies on fish may facilitate an understanding of the action of heavy metals on the aquatic environment. However, it should be kept in mind that the consequent reduction in the fish population has a negative environmental and socio-economic impact.
5. Conclusions
This work reports on the variation in the anatomical distribution of dopaminergic neurons in adult zebrafish brains exposed to nickel. It is interesting that TH decreases in a dose-dependent manner in the posterior tuberculum. This fact further reinforces the thesis that zebrafish are excellent animal models to be used in neurobiology research. In addition, our results highlighted that nickel exposure induced a specific cell death of dopaminergic neurons. We hope that our work will inspire further experimental genetic and molecular studies that could clarify the specific mechanisms through which nickel exerts its toxicity on the dopaminergic system.
Author Contributions
Conceptualization, P.C. and S.R.; methodology, S.R., M.L. and P.C.; software, P.C.; validation, S.R.; formal analysis, S.R. and P.C.; resources, P.C.; data curation, S.R. and V.F.; writing—original draft preparation, P.C., S.R., V.F. and M.L.; review and editing, P.C. and V.F.; visualization, P.C.; project administration, P.C.; funding acquisition, P.C. and V.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by national public funds (grant numbers Franceschini-RFO2022 and Cacialli-RFO2023) from the Italian Ministry of University and Research (MIUR).
Institutional Review Board Statement
All procedures followed the guidelines of the European Community Council Directive and the current Italian legislation on the use and care of animals. Ethical approval of this study was obtained from the Scientific Ethics Committee of the University of Bologna (protocol no. 17/79/2014).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated in this study are available from the corresponding authors upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ljungberg, T.; Ungerstedt, U. Sensory inattention produced by 6-hydroxydopamine-induced degeneration of ascending dopamine neurons in the brain. Exp. Neurol. 1976, 53, 585–600. [Google Scholar] [CrossRef]
- Chinta, S.J.; Andersen, J.K. Dopaminergic neurons. Int. J. Biochem. Cell Biol. 2005, 37, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Vernier, P. The evolution of dopamine systems in chordates. Front. Neuroanat. 2011, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Butcher, L.L.; Talbot, K.; Bilezikjian, L. Acetylcholinesterase neurons in dopamine-containing regions of the brain. J. Neural Transm. 1975, 37, 127–153. [Google Scholar] [CrossRef]
- Schultz, W. Dopamine neurons and their role in reward mechanisms. Curr. Opin. Neurobiol. 1997, 7, 191–197. [Google Scholar] [CrossRef]
- Ekstrom, P.; Honkanen, T.; Steinbusch, H.W. Distribution of dopamine-immunoreactive neuronal perikarya and fibres in the brain of a teleost, Gasterosteus aculeatus L. comparison with tyrosine hydroxylase- and dopamine-beta-hydroxylase-immunoreactive neurons. J. Chem. Neuroanat. 1990, 3, 233–260. [Google Scholar]
- Hollerman, J.R.; Grace, A.A. The effects of dopamine-depleting brain lesions on the electrophysiological activity of rat substantia nigra dopamine neurons. Brain Res. 1990, 533, 203–212. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Brownstein, M.; Palkovits, M.; Kizer, S.; Axelrod, J. Tyrosine hydroxylase and dopamine-beta-hydroxylase: Distribution in the individual rat hypothalamic nuclei. J. Neurochem. 1974, 23, 869–871. [Google Scholar] [CrossRef] [PubMed]
- Buijs, R.M.; Geffard, M.; Pool, C.W.; Hoorneman, E.M. The dopaminergic innervation of the supraoptic and paraventricular nucleus. A light and electron microscopical study. Brain Res. 1984, 323, 65–72. [Google Scholar] [CrossRef]
- Bayer, S.A.; Wills, K.V.; Triarhou, L.C.; Ghetti, B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp. Brain Res. 1995, 105, 191–199. [Google Scholar] [CrossRef]
- Gomez Ramos, B.; Ohnmacht, J.; De Lange, N.; Valceschini, E.; Ginolhac, A.; Catillon, M.; Ferrante, D.; Rakovic, A.; Halder, R.; Massart, F.; et al. Multiomics analysis identifies novel facilitators of human dopaminergic neuron differentiation. EMBO Rep. 2024, 25, 254–285. [Google Scholar] [CrossRef]
- Holzschuh, J.; Ryu, S.; Aberger, F.; Driever, W. Dopamine transporter expression distinguishes dopaminergic neurons from other catecholaminergic neurons in the developing zebrafish embryo. Mech. Dev. 2001, 101, 237–243. [Google Scholar] [CrossRef]
- Schweitzer, J.; Driever, W. Development of the dopamine systems in zebrafish. Adv. Exp. Med. Biol. 2009, 651, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Rink, E.; Wullimann, M.F. Development of the catecholaminergic system in the early zebrafish brain: An immunohistochemical study. Brain Res. Dev. Brain Res. 2002, 137, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Nyuzuki, H.; Ito, S.; Nagasaki, K.; Nitta, Y.; Matsui, N.; Saitoh, A.; Matsui, H. Degeneration of dopaminergic neurons and impaired intracellular trafficking in Atp13a2 deficient zebrafish. IBRO Rep. 2020, 9, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Sugie, A. An optimized method for counting dopaminergic neurons in zebrafish. PLoS ONE 2017, 12, e0184363. [Google Scholar] [CrossRef] [PubMed]
- Scerbina, T.; Chatterjee, D.; Gerlai, R. Dopamine receptor antagonism disrupts social preference in zebrafish: A strain comparison study. Amino Acids 2012, 43, 2059–2072. [Google Scholar] [CrossRef]
- Li, L.; Dowling, J.E. Effects of dopamine depletion on visual sensitivity of zebrafish. J. Neurosci. 2000, 20, 1893–1903. [Google Scholar] [CrossRef]
- Cleal, M.; Fontana, B.D.; Double, M.; Mezabrovschi, R.; Parcell, L.; Redhead, E.; Parker, M.O. Dopaminergic modulation of working memory and cognitive flexibility in a zebrafish model of aging-related cognitive decline. Neurobiol. Aging 2021, 102, 1–16. [Google Scholar] [CrossRef]
- Barrios, J.P.; Wang, W.C.; England, R.; Reifenberg, E.; Douglass, A.D. Hypothalamic Dopamine Neurons Control Sensorimotor Behavior by Modulating Brainstem Premotor Nuclei in Zebrafish. Curr. Biol. 2020, 30, 4606–4618. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.; Ryan, J.; Noble, S.; Yu, M.; Yilbas, A.E.; Ekker, M. Impaired dopaminergic neuron development and locomotor function in zebrafish with loss of pink1 function. Eur. J. Neurosci. 2010, 31, 623–633. [Google Scholar] [CrossRef]
- Zhao, T.; Zondervan-van der Linde, H.; Severijnen, L.A.; Oostra, B.A.; Willemsen, R.; Bonifati, V. Dopaminergic neuronal loss and dopamine-dependent locomotor defects in Fbxo7-deficient zebrafish. PLoS ONE 2012, 7, e48911. [Google Scholar] [CrossRef] [PubMed]
- Darna, M.; Beckmann, J.S.; Gipson, C.D.; Bardo, M.T.; Dwoskin, L.P. Effect of environmental enrichment on dopamine and serotonin transporters and glutamate neurotransmission in medial prefrontal and orbitofrontal cortex. Brain Res. 2015, 1599, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Hu, J.; Liu, D.; Yin, J.; Chen, M.; Zhou, L.; Yin, H. Cadmium chloride-induced transgenerational neurotoxicity in zebrafish development. Environ. Toxicol. Pharmacol. 2021, 81, 103545. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.S.; Noble, S.; Godoy, R.; Ekker, M.; Chan, H.M. Delayed effects of methylmercury on the mitochondria of dopaminergic neurons and developmental toxicity in zebrafish larvae (Danio rerio). Aquat. Toxicol. 2016, 175, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bui Thi, N.H.; Nguyen Thi, N.A.; Audira, G.; Siregar, P.; Liang, S.T.; Huang, J.C.; Hsiao, C.D. Chronic Exposure to Low Concentration Lead Chloride-Induced Anxiety and Loss of Aggression and Memory in Zebrafish. Int. J. Mol. Sci. 2020, 21, 1844. [Google Scholar] [CrossRef] [PubMed]
- Capriello, T.; Di Meglio, G.; De Maio, A.; Scudiero, R.; Bianchi, A.R.; Trifuoggi, M.; Toscanesi, M.; Giarra, A.; Ferrandino, I. Aluminium exposure leads to neurodegeneration and alters the expression of marker genes involved to parkinsonism in zebrafish brain. Chemosphere 2022, 307 Pt 1, 135752. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.; Planchart, A. The neurological toxicity of heavy metals: A fish perspective. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2018, 208, 12–19. [Google Scholar] [CrossRef]
- Li, Y.F.; Cheng, Y.B.; Tian, Z.L.; Liu, N.G. Research Progress of Zebrafish Model in Toxicology and Its Application Prospects in Forensic Science. Fa Yi Xue Za Zhi 2021, 37, 867–872. [Google Scholar] [CrossRef]
- Nowik, N.; Podlasz, P.; Jakimiuk, A.; Kasica, N.; Sienkiewicz, W.; Kaleczyc, J. Zebrafish: An animal model for research in veterinary medicine. Pol. J. Vet. Sci. 2015, 18, 663–674. [Google Scholar] [CrossRef]
- Choi, T.Y.; Choi, T.I.; Lee, Y.R.; Choe, S.K.; Kim, C.H. Zebrafish as an animal model for biomedical research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Engert, F.; Wilson, S.W. Zebrafish neurobiology: From development to circuit function and behaviour. Dev. Neurobiol. 2012, 72, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Nikolaou, N.; Meyer, M.P. Neurobiology: Imaging prey capture circuits in zebrafish. Curr. Biol. 2015, 25, R273–R275. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Key, B.; Devine, C.A. Zebrafish as an experimental model: Strategies for developmental and molecular neurobiology studies. Methods Cell Sci. 2003, 25, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Ricci, S.; Frabetti, F.; Ferrando, S.; Franceschini, V. Exposure of Zebrafish Embryos to Urea Affects Gene Expression in Neuronal Cells. Environments 2024, 11, 41. [Google Scholar] [CrossRef]
- Hernandez, E.; Obrist-Farner, J.; Brenner, M.; Kenney, W.F.; Curtis, J.H.; Duarte, E. Natural and anthropogenic sources of lead, zinc, and nickel in sediments of Lake Izabal, Guatemala. J. Environ. Sci. 2020, 96, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Esmaeilbeigi, M.; Kazemi, A.; Sharafi, S.; Sahebi, Z.; Asl, A.G. Spatial health risk assessments of nickel in the groundwater sources of a mining-impacted area. Sci. Rep. 2024, 14, 11017. [Google Scholar] [CrossRef] [PubMed]
- Genchi, G.; Carocci, A.; Lauria, G.; Sinicropi, M.S.; Catalano, A. Nickel: Human Health and Environmental Toxicology. Int. J. Environ. Res. Public Health 2020, 17. [Google Scholar] [CrossRef]
- Han, X.X.; Li, J.; Oner, I.H.; Zhao, B.; Leimkuhler, S.; Hildebrandt, P.; Weidinger, I.M. Nickel electrodes as a cheap and versatile platform for studying structure and function of immobilized redox proteins. Anal. Chim. Acta 2016, 941, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Es-Sette, B.; Ajdor, Y.; Zidane, F.; Fakhraddine, A.; Foutlane, A. Conceptuel model of transport of trace metals (chromium and nickel) in the Sebou River-Morocco. Environ. Technol. 2005, 26, 831–841. [Google Scholar] [CrossRef]
- Wang, X.; Wei, D.; Ma, Y.; McLaughlin, M.J. Soil ecological criteria for nickel as a function of soil properties. Environ. Sci. Pollut. Res. Int. 2018, 25, 2137–2146. [Google Scholar] [CrossRef]
- Benvenuti, T.; Rodrigues, M.; Arenzon, A.; Bernardes, A.M.; Zoppas-Ferreira, J. Toxicity effects of nickel electroplating effluents treated by photoelectrooxidation in the industries of the Sinos River Basin. Braz. J. Biol. 2015, 75 (Suppl. 2), 17–24. [Google Scholar] [CrossRef]
- Kastratovic, V.; Bigovic, M.; Jacimovic, Z.; Kosovic, M.; Durovic, D.; Krivokapic, S. Levels and distribution of cobalt and nickel in the aquatic macrophytes found in Skadar Lake, Montenegro. Environ. Sci. Pollut. Res. Int. 2018, 25, 26823–26830. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.K.; Al-Mutairi, K.A. Comparative Study of Potentially Toxic Nickel and Their Potential Human Health Risks in Seafood (Fish and Mollusks) from Peninsular Malaysia. Biology 2022, 11, 376. [Google Scholar] [CrossRef] [PubMed]
- Ptashynski, M.D.; Klaverkamp, J.F. Accumulation and distribution of dietary nickel in lake whitefish (Coregonus clupeaformis). Aquat. Toxicol. 2002, 58, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Ptashynski, M.D.; Pedlar, R.M.; Evans, R.E.; Wautier, K.G.; Baron, C.L.; Klaverkamp, J.F. Accumulation, distribution and toxicology of dietary nickel in lake whitefish (Coregonus clupeaformis) and lake trout (Salvelinus namaycush). Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2001, 130, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Zhao, L.J.; Sun, B.S.; Zhong, R.G. Determination of lead, cadmium, copper, and nickel in the tonghui river of beijing, china, by cloud point extraction-high resolution continuum source graphite furnace atomic absorption spectrometry. J. Environ. Qual. 2013, 42, 1752–1762. [Google Scholar] [CrossRef]
- Nabinger, D.D.; Altenhofen, S.; Bitencourt, P.E.R.; Nery, L.R.; Leite, C.E.; Vianna, M.; Bonan, C.D. Nickel exposure alters behavioral parameters in larval and adult zebrafish. Sci. Total Environ. 2018, 624, 1623–1633. [Google Scholar] [CrossRef] [PubMed]
- Lazzari, M.; Bettini, S.; Milani, L.; Maurizii, M.G.; Franceschini, V. Differential nickel-induced responses of olfactory sensory neuron populations in zebrafish. Aquat. Toxicol. 2019, 206, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Macomber, L.; Hausinger, R.P. Mechanisms of nickel toxicity in microorganisms. Metallomics 2011, 3, 1153–1162. [Google Scholar] [CrossRef]
- Cacialli, P.; Mailhe, M.P.; Wagner, I.; Merkler, D.; Golub, R.; Bertrand, J.Y. Synergistic prostaglandin E synthesis by myeloid and endothelial cells promotes fetal hematopoietic stem cell expansion in vertebrates. EMBO J. 2022, 41, e108536. [Google Scholar] [CrossRef] [PubMed]
- Mahony, C.B.; Cacialli, P.; Pasche, C.; Monteiro, R.; Savvides, S.N.; Bertrand, J.Y. Hapln1b, a central organizer of the ECM, modulates kit signaling to control developmental hematopoiesis in zebrafish. Blood Adv. 2021, 5, 4935–4948. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Dogan, S.; Linnerz, T.; Pasche, C.; Bertrand, J.Y. Minichromosome maintenance protein 10 (mcm10) regulates hematopoietic stem cell emergence in the zebrafish embryo. Stem Cell Rep. 2023, 18, 1534–1546. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P. Expression of Nerve Growth Factor and Its Receptor TrkA in the Reproductive System of Adult Zebrafish. Vet. Sci. 2022, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Ruuskanen, J.O.; Wullimann, M.F.; Vernier, P. Two tyrosine hydroxylase genes in vertebrates New dopaminergic territories revealed in the zebrafish brain. Mol. Cell Neurosci. 2010, 43, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Caldwell, L.J.; Davies, N.O.; Cavone, L.; Mysiak, K.S.; Semenova, S.A.; Panula, P.; Armstrong, J.D.; Becker, C.G.; Becker, T. Regeneration of Dopaminergic Neurons in Adult Zebrafish Depends on Immune System Activation and Differs for Distinct Populations. J. Neurosci. 2019, 39, 4694–4713. [Google Scholar] [CrossRef] [PubMed]
- Omar, N.A.; Kumar, J.; Teoh, S.L. Parkinson’s disease model in zebrafish using intraperitoneal MPTP injection. Front. Neurosci. 2023, 17, 1236049. [Google Scholar] [CrossRef] [PubMed]
- Cacialli, P.; Mahony, C.B.; Petzold, T.; Bordignon, P.; Rougemont, A.L.; Bertrand, J.Y. A connexin/ifi30 pathway bridges HSCs with their niche to dampen oxidative stress. Nat. Commun. 2021, 12, 4484. [Google Scholar] [CrossRef]
- Filippi, A.; Mahler, J.; Schweitzer, J.; Driever, W. Expression of the paralogous tyrosine hydroxylase encoding genes th1 and th2 reveals the full complement of dopaminergic and noradrenergic neurons in zebrafish larval and juvenile brain. J. Comp. Neurol. 2010, 518, 423–438. [Google Scholar] [CrossRef]
- Fukusumi, H.; Handa, Y.; Shofuda, T.; Kanemura, Y. Evaluation of the susceptibility of neurons and neural stem/progenitor cells derived from human induced pluripotent stem cells to anticancer drugs. J. Pharmacol. Sci. 2019, 140, 331–336. [Google Scholar] [CrossRef]
- Althomali, R.H.; Abbood, M.A.; Saleh, E.A.; Djuraeva, L.; Abdullaeva, B.S.; Habash, R.T.; Alhassan, M.S.; Alawady, A.H.R.; Alsaalamy, A.H.; Najafi, M.L. Exposure to heavy metals and neurocognitive function in adults: A systematic review. Environ. Sci. Eur. 2024, 36, 18. [Google Scholar] [CrossRef]
- Vellingiri, B.; Suriyanarayanan, A.; Selvaraj, P.; Abraham, K.S.; Pasha, M.Y.; Winster, H.; Gopalakrishnan, A.V.; Singaravelu, G.; Reddy, J.K.; Ayyadurai, N.; et al. Role of heavy metals (copper (Cu), arsenic (As), cadmium (Cd), iron (Fe) and lithium (Li)) induced neurotoxicity. Chemosphere 2022, 301, 134625. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Deng, P.; Li, G.Y.; Liu, H.L.; Zuo, J.L.; Cui, W.W.; Zhang, H.X.; Chen, X.; Yao, J.J.; Peng, X.T.; et al. Neurotoxicity of Combined Exposure to the Heavy Metals (Pb and As) in Zebrafish. Toxics 2024, 12, 282. [Google Scholar] [CrossRef] [PubMed]
- Paduraru, E.; Iacob, D.; Rarinca, V.; Plavan, G.; Ureche, D.; Jijie, R.; Nicoara, M. Zebrafish as a Potential Model for Neurodegenerative Diseases: A Focus on Toxic Metals Implications. Int. J. Mol. Sci. 2023, 24, 3428. [Google Scholar] [CrossRef] [PubMed]
- Kacprzak, V.; Patel, N.A.; Riley, E.; Yu, L.; Yeh, J.J.; Zhdanova, I.V. Dopaminergic control of anxiety in young and aged zebrafish. Pharmacol. Biochem. Behav. 2017, 157, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129285. [Google Scholar] [CrossRef] [PubMed]
- Dare, E.; Fetissov, S.; Hokfelt, T.; Hall, H.; Ogren, S.O.; Ceccatelli, S. Effects of prenatal exposure to methylmercury on dopamine-mediated locomotor activity and dopamine D2 receptor binding. Naunyn Schmiedebergs Arch. Pharmacol. 2003, 367, 500–508. [Google Scholar] [CrossRef]
- Glazer, L.; Brennan, C.H. Developmental Exposure to Low Concentrations of Methylmercury Causes Increase in Anxiety-Related Behaviour and Locomotor Impairments in Zebrafish. Int. J. Mol. Sci. 2021, 22, 10961. [Google Scholar] [CrossRef]
- Topal, A.; Atamanalp, M.; Oruc, E.; Halici, M.B.; Sisecioglu, M.; Erol, H.S.; Gergit, A.; Yilmaz, B. Neurotoxic effects of nickel chloride in the rainbow trout brain: Assessment of c-Fos activity, antioxidant responses, acetylcholinesterase activity, and histopathological changes. Fish. Physiol. Biochem. 2015, 41, 625–634. [Google Scholar] [CrossRef]
- Calvo-Ochoa, E.; Byrd-Jacobs, C.A. The Olfactory System of Zebrafish as a Model for the Study of Neurotoxicity and Injury: Implications for Neuroplasticity and Disease. Int. J. Mol. Sci. 2019, 20, 1639. [Google Scholar] [CrossRef]
- Wasel, O.; Freeman, J.L. Chemical and Genetic Zebrafish Models to Define Mechanisms of and Treatments for Dopaminergic Neurodegeneration. Int. J. Mol. Sci. 2020, 21, 5981. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Calabro, R.S.; D’Aleo, G.; Marra, A.; Sessa, E.; Bua, D.G.; Potorti, A.G.; Dugo, G.; Bramanti, P.; et al. Heavy metals and neurodegenerative diseases: An observational study. Biol. Trace Elem. Res. 2014, 161, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.L.; Outeiro, T.F.; Ferreira, J.J. Zebrafish as an Animal Model for Drug Discovery in Parkinson’s Disease and Other Movement Disorders: A Systematic Review. Front. Neurol. 2018, 9, 347. [Google Scholar] [CrossRef] [PubMed]
- Najib, N.H.M.; Nies, Y.H.; Abd Halim, S.A.S.; Yahaya, M.F.; Das, S.; Lim, W.L.; Teoh, S.L. Modeling Parkinson’s Disease in Zebrafish. CNS Neurol. Disord. Drug Targets 2020, 19, 386–399. [Google Scholar] [CrossRef]
- Bretaud, S.; Lee, S.; Guo, S. Sensitivity of zebrafish to environmental toxins implicated in Parkinson’s disease. Neurotoxicol. Teratol. 2004, 26, 857–864. [Google Scholar] [CrossRef]
- Lulla, A.; Barnhill, L.; Bitan, G.; Ivanova, M.I.; Nguyen, B.; O’Donnell, K.; Stahl, M.C.; Yamashiro, C.; Klarner, F.G.; Schrader, T.; et al. Neurotoxicity of the Parkinson Disease-Associated Pesticide Ziram Is Synuclein-Dependent in Zebrafish Embryos. Environ. Health Perspect. 2016, 124, 1766–1775. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).