Abstract
The feedback regulatory effects of estrogen (E2) and androgen (T) on the gonadotropin-releasing hormone (GnRH) and gonadotropin (GtH) within the brain–pituitary–gonad (BPG) axis in eels with undeveloped ovaries were investigated through in vivo studies. However, the regulatory role of the BPG axis only became apparent during ovary development in the migratory stage. To further elucidate the direct feedback regulation of the BPG axis, female Anguilla japonica underwent artificial induction of vitellogenesis, and the regulation of BPG axis tissues by GtH (human chorionic gonadotropin, hCG), E2, and T was explored through in vitro exposure. The mRNA expression levels of GnRH (mGnRH), GtH (fshb and lhb), and steroid biosynthesis enzymes (cyp11a1, hsd3b, cyp17a1, and cyp17a2) in the diencephalon, pituitary, and ovary, respectively, were determined. The results showed that the expression level of mGnRH in the diencephalon was significantly downregulated by 0.1 IU/mL hCG but upregulated by both 1 nM E2 and higher concentrations of T, suggesting a direct positive feedback regulation of E2 on mGnRH. In the pituitary, the expression levels of fshb and lhb were upregulated by E2, while fshb was suppressed by T. In the ovaries, the expression of cyp11a1 and hsd3b was upregulated by 1 nM E2, whereas T exposure resulted in an opposite effect. Cyp17a1 mRNA levels did not differ significantly with E2 treatment but were upregulated by 1 nM T. These findings suggest that low concentrations of E2 exhibited positive feedback regulation on all three levels (diencephalon, pituitary, and ovary) of the BPG axis, while T showed weaker and differential feedback regulation in BPG axis tissues. Overall, this study’s results revealed the direct feedback regulation of hCG, E2, and T on the BPG axis in eels, a phylogenetic base of teleosts.
Key Contribution:
Low concentrations of E2 showed positive feedback regulation on the three levels (diencephalon, pituitary, and ovary) of the BPG axis directly; T showed a weaker and differential feedback regulation among BPG axis tissues in Anguilla japonica, a phylogenetic base of teleosts.
1. Introduction
Anguillid eels, comprising 19 species, are widely distributed in tropical, subtropical, and temperate regions spanning 70° N to 50° S latitude. Eels hold significant commercial value, and are extensively cultured in East Asia. Eels follow a catadromous life cycle with stages including the fertilized egg, pre-leptocephalus, leptocephalus, glass eel, elver/juvenile eel, yellow eel, and silver eel. Unfortunately, the wild glass eel resources in terms of the Japanese eel (Anguilla japonica), European eel (A. anguilla), and American eel (A. rostrata) have sharply declined in the last 30 years [1]. Japanese scholars achieved the groundbreaking feat of full artificial breeding of a second generation of Japanese eel offspring in 2010 [2]. However, challenges persist, such as variations in egg quality due to exogenous hormone treatment, leading to low survival rates and high incubation costs for artificial glass eels in captivity [1]. The current yield of artificial glass eels falls short of meeting the demands of commercial culture, necessitating improvements in egg quality, optimization of environments and systems for rearing leptocephali, and the reduction of the leptocephalus stage [3]. Crucial aspects, including ovarian development and egg maturation, involving steroid hormone synthesis, nutrient accumulation, and hydration, require further research and enhancement for successful leptocephalus rearing.
Reproductive functions in vertebrates rely on the regulation of the brain–pituitary–gonad (BPG) axis. However, eels, as the phylogenetic base of teleosts, exhibit significant phylogenetic distances and substantial differences in reproductive strategy and life history compared to other teleosts [4]. Notably, eel ovary and brain aromatase show much lower enzymatic activity than that in other teleosts [5]. The whole-genome duplication event in teleosts results in two cyp19a1 genes: cyp19a1a and cyp19a1b [6]. By contrast, only one copy of cyp19a1 has been identified in eels. The tissue-specific expression of eel cyp19a1 is achieved through a tissue-specific promoter and alternative splicing, resembling patterns found in most amphibians and mammals [7].
The feedback regulatory effects of estrogen (17β--estradiol [E2]) and androgen (testosterone [T] and dihydrotestosterone [DHT]) on mGnRH and GtH levels in the BPG axis in female eels have been extensively investigated in vivo. The female eels in these studies were either silver eels or immature eels with undeveloped ovaries, as the gonadal development is inhibited during the freshwater life stage and only initiates during the migratory stage. At the GnRH level, positive E2-dependent feedback on mGnRH was observed in European eels [8], while sex steroids did not stimulate brain mGnRH in Japanese eels [9]. Concerning GtH levels, an in vivo study revealed E2-specific positive feedback on luteinizing hormone (LH) and T-specific negative feedback on follicle-stimulating hormone (FSH) in European eels [10]. Only a triple treatment with T, GnRH agonist, and dopamine-receptor antagonist (pimozide) could trigger dramatic increases in LH synthesis [11]. Both E2 and T could dose-dependently increase pituitary LH content in Japanese eels, but without any significant effect on the gonadosomatic index (GSI) [9]. Androstenedione (AD), 17α-methyltestosterone (MT), and T exhibited positive feedback effects on the BPG axis, promoting mGnRH synthesis in the brain and/or GtH (fshb or lhb) mRNA expression in the pituitary, along with a significant increase in GSI in A. japonica [12,13] and A. anguilla [14]. In summary, steroid feedback data exhibit substantial variations depending on species and reproductive cycle stage. Consequently, for fully manifesting the regulatory role of the BPG axis, eels in the process of gonadal maturation should be used to investigate the feedback regulation. A previous study showed that the expression levels of most steroidogenesis enzymes peaked in the midvitellogenic follicles of A. japonica [15]. Meanwhile, an in vitro study showed that E2 increased fshb mRNA levels but had no effect on lhb expression, while androgen (T and DHT) stimulation yielded opposite results. These data suggest that sex steroid feedback on gonadotropins operates through multiple pathways, indirectly involving cerebral control [16]. To further unveil the direct action of steroids on the BPG axis, in vitro studies as a further investigative method should be conducted.
In this study, artificial vitellogenesis was induced in female A. japonica by administering exogenous GtH (carp pituitary extract [CPE]) injections during the migration season. The feedback regulation on the diencephalon, pituitary, and ovary by GtH (hCG), estrogen (E2), and androgen (T), respectively, was investigated through in vitro exposure. The mRNA expression levels of key components including GnRH (mGnRH), GtH (fshb and lhb), and steroid biosynthetic enzymes (cyp11a1, hsd3b, cyp17a1, and cyp17a2) in the diencephalon, pituitary, and ovary were analyzed to elucidate the potential mechanisms of hCG, E2, and T, respectively, in regulating the feedback loop of the BPG axis. The findings of this study aim not only to provide a direct understanding of the feedback regulation of the BPG axis during vitellogenesis but also to offer scientific evidence for enhancing the artificial maturation of A. japonica.
2. Materials and Methods
2.1. Ethics Statement
All experiments in this study received approval from the Animal Experimental Committee of Jimei University. Each surgery was conducted under anesthesia induction using clove oil to reduce suffering.
2.2. Artificial Development Trial and Sampling Procedure
Three-year-old farmed female A. japonica eels (n = 30; mean body weight [BW]: 904.5 ± 121.8 g and 75.2 ± 5.5 cm; mean ± SEM) were sourced from a commercial eel farm (Fuqing, Fujiang Province, China). The eels were gradually acclimated to seawater (35 psu) for 14 days and then maintained at 21 ± 1 °C in a 500 L tank with a recirculating seawater system for a month. Twenty-six females among them were silvered. Six of these silver females were randomly sacrificed for serum E2 and T assessment and histological observation before artificial ovarian development (previtellogenic [PV] stage). Blood was sampled from the caudal vein with a disposable sterile injector. A piece of ovarian tissue was stored in 4% paraformaldehyde solution for histological observation. The rest of the silver females were weekly injected with CPE at a concentration of 10 mg/kg, facilitating artificial ovarian development to the midvitellogenic (MV) stage. Eighteen females at the MV stage (10–12 injections) were euthanized using a clove oil solution (2 mL/L) and sampled according to our previous protocol [7]. Morphometric measurements included total BW, total length, gonad weight, liver weight, and gut weight. These measurements were used to calculate GSI. GSI (%) = [gonad weight (g) × 100/BW (g)], hepatosomatic index (HSI), HSI (%) = [liver weight (g) × 100/BW (g)], and gut index (%) = [gut weight (g) × 100/BW (g)]. Following gonad weight measurement, a small ovarian tissue piece (randomly selected, n = 6), the diencephalon (tissues from three fish were pooled for one sample, n = 6), and the pituitary (tissues from three fish were pooled for one sample, n = 6) were immediately dissected and collected for in vitro incubation. Blood was also collected for serum E2 and T assessment (randomly selected, n = 6). The developmental stage of the ovaries was also confirmed by histological observation.
2.3. Serum E2 and T Assessment by Immunoenzymatic Assays
Serum E2 and T levels were determined using a Testosterone EIA Kit and an Estradiol ELISA Kit (Cayman Chemical Company, Ann Arbor, MI, USA), following the manufacturer’s instructions. Serum containing T and E2 was extracted using diethyl ether or methanol, respectively, evaporated to dryness under inert gas, and then dissolved in assay buffer. For consistency, all samples in each experiment were assayed simultaneously. Intra-assay and inter-assay coefficients of variation for T and E2 were below 15% and 12%, respectively.
2.4. Histological Observation
The fixed ovarian tissues were dehydrated, then embedded in paraffin wax, and cut serially at 5 μm. Sections were stained with Erlich’s hematoxylin and Eosin, dried, and then mounted with neutral balsam. Images were captured through a compound microscope equipped with a camera (Leica DM5500B).
2.5. In Vitro Incubation of the Ovary, Diencephalon, and Pituitary
The ovary, diencephalon, and pituitary from MV stage eels were excised, rinsed in phosphate-buffered saline, and sectioned into 0.5 mm thick slices on ice. These tissues were promptly placed in a 24-well tissue culture dish, each well containing 1 mL of L-15 medium (Gibco, Waltham, MA, USA) with 2% penicillin–streptomycin (BI, Biological Industries, Israel) and then incubated at 25 °C in a humidified atmosphere with 5% CO2. T and E2 (Sigma, St. Louis, MO, USA) stock solutions were prepared in 100% ethanol at concentrations of 33.3 mM and 166.7 mM, respectively, and diluted in L-15 medium just before use. hCG (Ningbo Second Hormone Factory, Ningbo, China) was also prepared in L-15 medium immediately before use. Following a 6 h pre-incubation, tissue fragments were treated with T (1, 10, or 100 nM), E2 (1, 10, or 100 nM), hCG (0.1, 1, or 10 IU/mL), or ethanol (0.1%, control) for 24 h. Post-incubation, tissues were preserved in RNA-later for subsequent real-time quantitative reverse transcription–polymerase chain reaction (qRT-PCR) analysis of mGnRH, fshb, lhb, cyp11a1, hsd3b, cyp17a1, and cyp17a2 mRNA (n = 6).
2.6. qRT-PCR Analysis of mGnRH, fshb, lhb, cyp11a1, hsd3B, cyp17a1, and cyp17a2 mRNA Levels
Total RNA extraction was performed using the SteadyPure™ kit (Accurate Biology, Changsha, China) for qRT-PCR analysis of mGnRH, fshb, lhb, cyp11a1, hsd3b, cyp17a1, and cyp17a2 mRNA levels. Specific primers for measuring the expression of target genes were designed and tested for specificity and efficiency (Table 1). The qRT-PCR assays were performed using a QuantStudio 1 Plus Real-time PCR Instrument (Thermo Fisher Scientific, Waltham, MA, USA) with 10 μL of PCR kit mixture that included 4.5 μL of diluted cDNA, 5 μL of SYBR GREEN 2× Premix (Accurate Biotechnology, Changsha, China), and 0.25 μL of each primer (10 μM). The protocol of the reaction, which was performed in a 96-well optical plate, involved an initial 5 min denaturation at 95 °C, followed by 40 cycles of 95 °C for 10 s, 60 °C for 15 s, and 72 °C for 15 s. A negative control (cDNA was replaced with sterile water) for each primer pair was run in duplicate on all plates. qRT-PCR assays for all target genes and the reference gene β-actin involved five biological replicates and at least three technical replicates. Amplification efficiencies of primers were determined by standard curves (Table S1, Figure S1). Amplification specificities were confirmed through melting curve analysis (Figure S2). Relative gene expression levels were normalized to that of β-actin and calculated using the 2−ΔΔCt method.

Table 1.
Primer sequences for the seven genes and the reference gene.
2.7. Statistical Analysis
Data are presented as mean ± SEM. Groups were compared using one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. All statistical analyses were performed using IBM SPSS Statistics 22.0 software (New York, NY, USA), with p-values < 0.05 considered statistically significant.
3. Results
3.1. Reproductive Status of the MV Stage Female A. Japonica
After 10–12 weeks of intraperitoneal injections of CPE, significant increases in BW and ovarian growth were observed. The GSI, HSI, and gut indexes were 21.86 ± 1.43%, 0.18 ± 0.01%, and 0.16 ± 0.02%, respectively (n = 18). Serum E2 levels in sampled MV stage eels were significantly higher (p < 0.01) compared to those in PV stage eels, whereas serum T levels showed no significant change (n = 6; Figure 1). Follicle growth was accompanied by enlargement of vitellogenic oocytes, which were filled with oil droplets. The yolk granular layer was observed in the peripheral ooplasm after weekly injection of CPE (Figure 2).
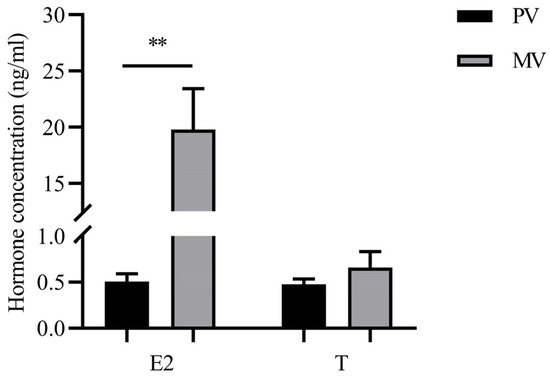
Figure 1.
Serum levels of 17β-estrogen (E2) and testosterone (T) in the previtellogenic (PV) stage and midvitellogenic (MV) stage of A. japonica. Data are presented as mean ± SEM (n = 6). Asterisks across the columns represent statistical significance between PV and MV (**, p < 0.01).
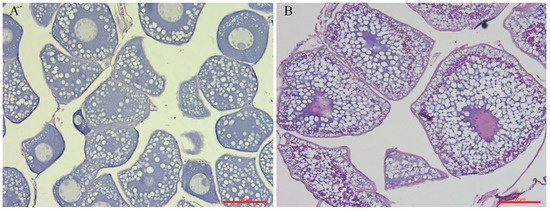
Figure 2.
Histological sections from female A. japonica showing previtellogenic (PV) (A) stage and midvitellogenic (MV) stage (B) ovaries. Scale bars = 100 μm.
3.2. E2, T, and hCG Regulated mGnRH mRNA Levels in the Diencephalon In Vitro
mGnRH expression in the diencephalon was significantly downregulated (p < 0.05) by 0.1 IU/mL hCG compared with the control group. However, higher concentrations of hCG did not alter mGnRH expression (Figure 3A). mGnRH expression was significantly (p < 0.05) increased by E2 at concentrations of 100 nM (Figure 3B). Additionally, mGnRH expression was dose-dependently upregulated by T (Figure 3C).
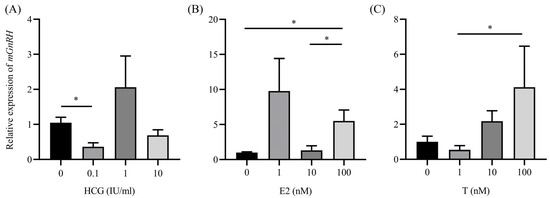
Figure 3.
Changes in mGnRH expression in the diencephalon stimulated by hCG (A), E2 (B), or T (C) in vitro. Data are presented as mean ± SEM (n = 6). Groups are compared using one-way analysis of variance, followed by Tukey’s multiple comparison test. Asterisks across the columns represent statistical significance (*, p < 0.05).
3.3. E2 and T Regulated fshb and lhb mRNA Levels in the Pituitary In Vitro
The expression of fshb in the pituitary was significantly upregulated (p < 0.05), and peaked at 10 nM E2 (Figure 4A). By contrast, fshb expression significantly decreased (p < 0.01) under the stimulation of 1 nM and 10 nM T (Figure 4B). The change in lhb expression exhibited the same pattern as that in fshb expression in response to E2 (Figure 4C), but no significant change was shown with increasing concentrations of T (Figure 4D).
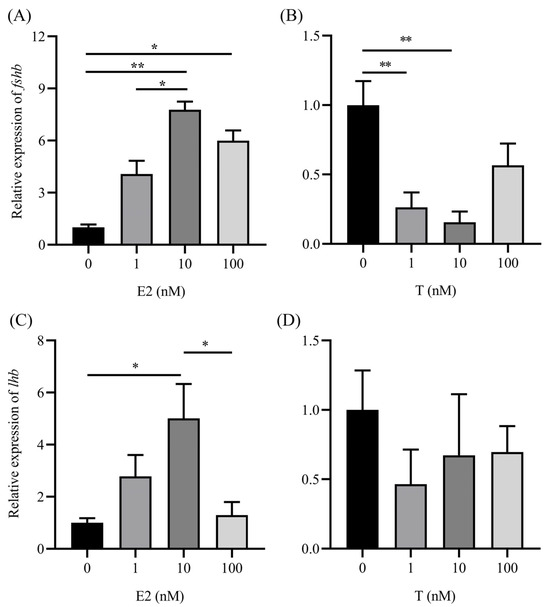
Figure 4.
Changes in the expression of fshb (A,B) and lhb (C,D) in the pituitary stimulated by E2 (A,C) or T (B,D) in vitro. Data are presented as mean ± SEM (n = 6). Groups are compared using one-way analysis of variance, followed by Tukey’s multiple comparison test. Asterisks across the columns represent statistical significance (*, p < 0.05; **, p < 0.01).
3.4. E2 and T Regulated cyp11a1, hsd3B, cyp17a1, and cyp17a2 mRNA Levels in the Ovary In Vitro
In the ovaries, cyp11a1 expression was significantly upregulated (p < 0.05) by 1 nM E2, and decreased with increasing E2 concentrations (Figure 5A). Conversely, cyp11a1 expression was significantly reduced (p < 0.05) after 1 nM T stimulation, but increased with higher T concentrations (Figure 5B). hsd3b expression was significantly altered (p < 0.05) by 1 nM E2 compared with the control group (Figure 5C). Conversely, hsd3b expression was significantly (p < 0.05 or 0.01) downregulated by 1 nM or 10 nM T, respectively, compared with the control group (Figure 5D). cyp17a1 mRNA levels did not change significantly with E2 treatment (Figure 5E). cyp17a1 expression in the ovaries was significantly upregulated (p < 0.05) by 1 nM T, then decreased with higher T concentrations (Figure 5F). cyp17a2 expression did not change significantly with either E2 or T at all tested concentrations (Figure 5G,H). Overall, cyp11a1 and hsd3b expression in the ovaries was more sensitive to E2 and T than cyp17a1 and cyp17a2.
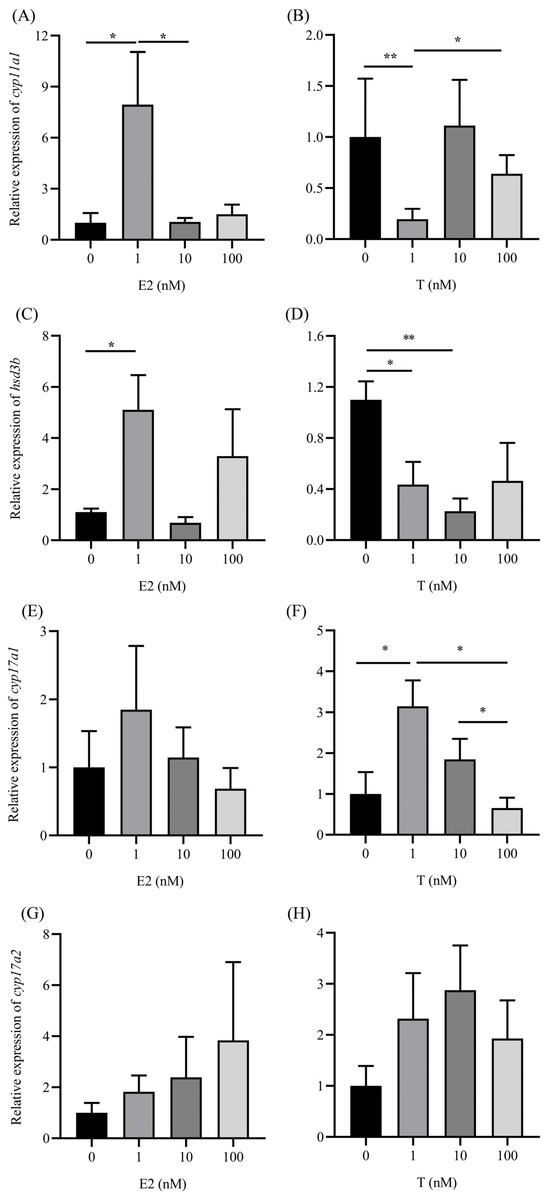
Figure 5.
Changes in cyp11a1 (A,B), hsd3b (C,D), cyp17a1 (E,F), and cyp17a2 (G,H) expression in the ovaries stimulated by E2 or T in vitro. Data are presented as mean ± SEM (n = 6). Groups are compared using one-way analysis of variance, followed by Tukey’s multiple comparison test. Asterisks across the columns represent statistical significance (*, p < 0.05; **, p < 0.01).
4. Discussion
In the present study, the expression of genes involved in the BPG axis showed a significant change to lower concentrations of exposure hormones, while converse results were found in the highest-concentration group (Figure 3A, Figure 4B,C, and Figure 5). A dose-dependent effect was not found in these groups. The exposure hormone concentrations in this study were also used in the in vitro study of eels and other teleosts [17,18]. It seems that the lowest concentration of E2 showed a positive feedback effect, and the highest concentration, which reached nearly their maximum serum levels (Figure 1), showed a rare biological effect in the present study.
4.1. E2, T, and hCG Regulated mGnRH mRNA Expression in the Diencephalon
mGnRH expression in the diencephalon was downregulated by 0.1 IU/mL hCG, while it remained unchanged with other tested concentrations of hCG (Figure 3A). It appears that hCG does not exhibit positive feedback regulation directly upstream of the BPG axis. Our previous study demonstrated that hCG did not upregulate the expression of fshr or lhr in the diencephalon in vitro [7]. Hence, the lack of positive feedback regulation by hCG may be attributed to the failure to activate its receptor (LHR) expression. Another possible explanation could be that the feedback regulation of hCG on the GnRH system may be, at least in part, mediated indirectly by GnRH receptors. GnRH mRNA levels were not significantly affected by hCG treatment in rat testes. By contrast, GnRH-R mRNA levels markedly decreased in male rat testes and other non-pituitary target cells containing LH receptors after hCG injection [19]. Additionally, hCG injections for 39 days resulted in the potentiation of the GTH response to luteinizing hormone-releasing hormone (LHRH-A) in mature male goldfish indirectly through the stimulation of endogenous T secretion [20]. However, further research is needed for direct evidence in female animals.
mGnRH expression was evaluated in response to E2 and T, with T showing a less significant effect than E2 (Figure 3B,C). Prior research indicated that gonadal hormones, including estrogens and androgens, positively affect GnRH during experimental maturation [21]. In undeveloped ovaries, a combination of E2 and T or E2 alone increased mGnRH synthesis in the diencephalon and mesencephalon of A. anguilla, whereas T alone had no effect [8]. By contrast, neither E2 nor T stimulated mGnRH content in the brain of A. japonica [9]. Recent studies have identified estrogen receptor 1 (ESR1)-positive neurons in the preoptic area projecting to GnRH1 neurons in medaka [22], and have shown that GnRH1, GnRH2, and GnRH3 neuronal populations in tilapia express various ESR subtypes [23]. Two estrogen response elements (EREs) were found in the salmon mGnRH gene [24], suggesting that E2 might directly act on the mGnRH gene in teleost fish. E2, rather than DHT, has also been observed to stimulate GnRH release in frogs in vitro [25], indicating a growing acceptance of E2’s direct positive feedback on mGnRH.
The data on androgen feedback regulation of GnRH show considerable variability, depending on the investigation methods and the reproductive cycle stage. Our previous study demonstrated that the expression of esra, fshr, and lhr was significantly upregulated by T, similarly to ara [7]. A prior in vivo study also revealed that androgens (AD and MT) upregulated mGnRH expression, but the expression of ara was not evaluated, suggesting that androgens’ positive feedback might predominantly occur through indirect effects [13]. Previous research showed that T, by converting to E2, could enhance pituitary responsiveness to exogenous LHRH-A in female goldfish in vivo [20].
4.2. E2 and T Regulated fshb and lhb mRNA Expression in Pituitary
E2 was found to upregulate the expression levels of fshb and lhb. It stimulated an increase in LH content (A. japonica) [9] and lhb mRNA (A. anguilla) in vivo, as well as fshb mRNA levels in vitro [15] in the pituitary. However, E2 had no effect on lhb expression in A. anguilla in vitro [15]. Previous studies revealed that E2 or T injections for 6 or 8 weeks did not significantly induce ovarian development in A. japonica [9]. Given that gonadal development in eels begins at the migratory stage, we believe that supplementing MV stage eels provides a more comprehensive understanding of the feedback regulatory role of the BPG axis in this study. Earlier research suggested that positive steroid feedback is mediated not only directly but also indirectly in model fish [26]. E2’s stimulation of pituitary GTH II is thought to act through the GnRH pathway in European silver eels [8]. The positive feedback regulation of E2 in fish seems to be multi-pathway.
In the current study, testosterone (T) was observed to downregulate the expression of fshb, while having no effect on lhb expression levels. T treatment led to a slight decrease in fshb mRNA levels in vitro [10]. Our in vivo study similarly found a reduction in fshb expression, correlating with significant ovarian development due to androgens [13]. This consistency between in vitro and in vivo results may be attributed to the lesser role of GnRH in FSH regulation, as noted in both teleosts [27] and mammals [28], and to the fact that FSH regulation is influenced not only by steroids but also by gonadal activin in A. japonica [29,30].
Contrarily, T did not influence lhb expression in this study. Although there was no increase in ovarian ars expression, AD and MT upregulated lhb expression in vivo [13]. Studies on silver eels at the onset of ovarian development revealed that T and DHT enhanced LH levels [9], yet neither ara nor arb expression saw an increase [31]. MT, convertible by aromatase to 17-methylestradiol-17β, can bind to the ovarian estradiol receptor [32], suggesting that T exerts an indirect positive feedback regulation on lhb expression. Both E2 and T enhanced the GTH response to LHRH-A in female goldfish, while nonaromatizable androgens (DHT and 11-KT) had no effect [19]. However, DHT, a nonaromatizable androgen, also increased LH content [9], and T was found to elevate lhb expression in A. anguilla in vitro [10], indicating the possibility of alternative androgen feedback mechanisms.
4.3. E2 and T Regulated cyp11a1, hsd3B, cyp17a1, and cyp17a2 mRNA Expression in Ovary
Regarding the expression of cyp11a1 and hsd3b in the ovaries, these were more responsive to E2 and T than cyp17a1 and cyp17a2 in this study. The steroidogenic enzyme genes cyp11a1, cyp17, and cyp19a1a are crucial in the steroid biosynthesis pathway, representing key regulatory stages [33,34]. Our prior research showed fluctuating mRNA levels of enzymes (cyp11a1, hsd3b, cyp17a1, cyp17a2, cyp19a1, hsd17b1, hsd17b12, and hsd20b) during follicle development in artificially treated A. japonica with weekly CPE and hCG injections [34]. Steroidogenesis in eels, as in other teleosts, is stringently regulated by gonadotropins (CPE/hCG) [34].
Cholesterol is converted into pregnenolone by the cholesterol side-chain cleavage enzyme, P450scc or cyp11a1. This step is the slowest and controls the rate of steroid hormone synthesis [34]. A previous study suggested that P450scc’s expression is relatively insensitive to low to medium circulating E2 levels (1 ng/mL), although a reduction in mRNA levels was observed with high-dose implants (90 ng/mL) [17] and was also found in the present study. The present study indicated that positive feedback modulation at low E2 concentrations (1 nM corresponding to 0.27 ng/mL) could increase the conversion rate at this step, with E2 concentrations lower than those in serum.
In the ovaries, the expression level of hsd3b is upregulated by E2 but downregulated by T. Its expression level is significantly higher than those of cyp11a1, cyp17a1, and cyp17a2 during ovarian development [15], suggesting hsd3b as a primary target for steroid biosynthesis feedback regulation. Previous research has shown that mammalian hsd3b’s transcriptional activity is influenced by various signaling and regulatory pathways, including ERα and AR [35]. Whether a similar phenomenon occurs in other teleosts is yet to be determined [33]. In rainbow trout, hsd3b mRNA levels significantly decreased with 1 and 10 µg E2/g BW implants in previtellogenic ovarian follicles [17]. The discrepancy in hsd3b mRNA levels between rainbow trout and this study might be due to different ovarian development stages. Low E2 concentrations had a positive feedback effect on hsd3b, similar to cyp11a1. Feedback regulation of E2 may be divergent and requires further study in teleosts, a phylogenetic base of vertebrates.
This study also found that mRNA levels of ovarian 17a-hydroxylase/17,20-lyase (P450c17a1) were upregulated by T. However, cyp17a1 expression did not significantly change with E2 treatment. A similar result was observed in Gobiocypris rarus, where cyp17a1 transcript level exhibited a highly significant increase in the ovary of female G. rarus when exposed to 100 ng/L MT for 7 days but not when exposed to EE2. MT exposure also significantly reduced cyp17a1 gene methylation levels in G. rarus, suggesting a negative correlation between cyp17a1 gene methylation and mRNA expression in response to MT and EE2 [36]. Several potential cis-elements, including a CRE binding site, were found in cyp17a1’s promoter regions, but ARE and ERE were absent [36]. A previous study indicated that T elevated the mRNA levels of fshr and lhr rather than ar in the explant ovary [7]. This suggests that T’s upregulation of cypl7a1 expression might be attributed to CRE binding protein (CREBP). cAMP responsive element modulator (CREM) has been shown to decrease the methylation levels of the cyp17a1 gene [37]. Increasing evidence indicates that cyp17 activation by cAMP occurs through CREs in its promoter in humans [38,39], bovines [40], and mice [37].
5. Conclusions
Our study revealed the direct feedback regulation of hCG, E2, and T in the BPG axis in eels (Figure 6). Low concentrations of hCG negatively regulated mGnRH expression, while low concentrations of E2 positively regulated the BPG axis at three levels (diencephalon, pituitary, and ovary). T exhibited a weaker regulation effect on mGnRH and a contrasting effect on FSH. Taken together, considering the results of previous in vivo [8,9,10,12,13,14] and in vitro [7] studies, the results of the present study suggest that estrogen showed a direct positive feedback regulation in the BPG axis, and androgens’ positive feedback in the diencephalon and pituitary might predominantly occur through ESR, FSHR, or LHR indirectly in vivo. This study also highlighted the differential effect of T on enzymes during sex steroid hormone synthesis. The mechanisms of regulation involved, such as the structural basis of tissue-specific promoters and tissue-specific methylation levels, warrant further investigation. To the best of our knowledge, this is the first study to describe the direct feedback regulation of E2 and T at three levels of the BPG axis in eels. The findings enhance our understanding of the direct feedback regulation of hCG, E2, and T in the BPG axis in eels.
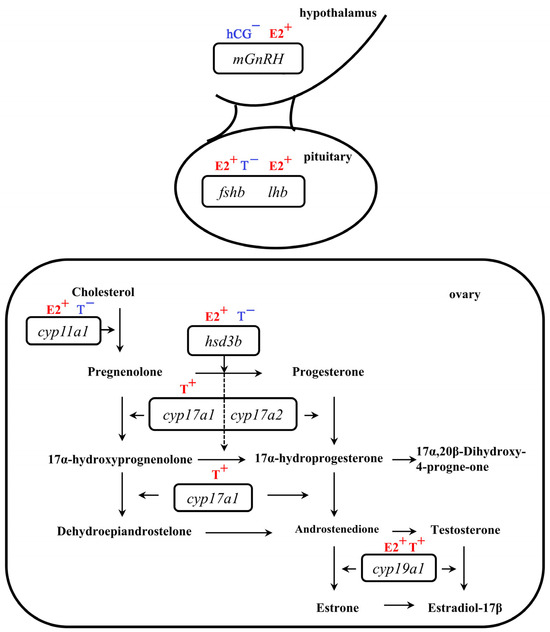
Figure 6.
Schema of the putative role of hCG, E2, and T in direct feedback regulation at different levels in the brain–pituitary–gonad (BPG) axis of eels. (+, positive feedback regulation; −, negative feedback regulation).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9070265/s1, Figure S1: Standard curves of the primers. Figure S2: Melting curves of the primers. Table S1: Amplification efficiency-values and R2-values of primers used in the study.
Author Contributions
Conceptualization, X.L.; investigation, X.L., S.P., Z.B., L.C. and H.H.; data curation, X.L., S.P., Z.B. and L.C.; validation, Y.J. and Y.W.; writing—original draft, X.L.; writing—review and editing, Y.J. and Y.W.; supervision, Y.W.; funding acquisition, X.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Natural Science Foundation of Xiamen, China (grant number 3502Z202373029); the Natural Science Foundation of Fujian Province of China (grant number 2023J01144); the National Natural Science Foundation of China (grant number 41806193); and the Educational Research Project for Young and Middle-aged Teachers of Fujian Provincial Department of Education, China (grant number JAT220168).
Institutional Review Board Statement
This study was approved by the Animal Experimental Committee of Jimei University (Approval Code: Animal Ethics No. 2046; Approval Date: 01/01/2020-31/12/2024).
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
3β-HSD/hsd3b, 3β-hydroxysteroid dehydrogenase; AD, androstenedione; BPG, brain–pituitary–gonad; BW, body weight; CPE, carp pituitary extract; cyp19a1, P450 aromatase; DHT, dihydrotestosterone; E2, 17β-estradiol; ESR, estrogen receptor; FSH/fshb, follicle-stimulating hormone; GSI, gonadosomatic index; GtH, gonadotropins; hCG, human chorionic gonadotropin; HIS, hepatosomatic index; LH/lhb, luteinizing hormone; lhr, luteinizing hormone receptor; LHRH, luteinizing hormone-releasing hormone; mGnRH, mammal gonadotropin-releasing hormone; MT, 17α-methyltestosterone; MV, midvitellogenic stage; P450scc/cyp11a1, P450 side-chain cleavage; P450c17/cyp17a1/cyp17a2, 17α-hydroxylase/C17-20 lyase; PV, previtellogenic stage; T, testosterone.
References
- Dekker, W.; Casselman, J.M.; Jellyman, D.J.; Tatsukawa, K. Resources. In Eel Biology; Aida, K., Tsukamoto, K., Yamauchi, K., Eds.; Springer: Tokyo, Japan, 2003; pp. 237–298. [Google Scholar]
- Masuda, Y.; Imaizumi, H.; Oda, K.; Hashimoto, H.; Usuki, H.; Teruya, K. Artificial completion of the Japanese eel, Anguilla japonica, life cycle: Challenge to mass production. Bull. Fish. Res. Agency 2012, 35, 111–117. [Google Scholar]
- Okamura, A.; Horie, N.; Mikawa, N.; Yamada, Y.; Tsukamoto, K. Recent advances in artificial production of glass eels for conservation of anguillid eel populations. Ecol. Freshw. Fish 2014, 23, 95–110. [Google Scholar] [CrossRef]
- Asturiano, J.F. Improvements on the Reproductive Control of the European Eel. In Reproduction in Aquatic Animals: From Basic Biology to Aquaculture Technology; Yoshida, M., Asturiano, J.F., Eds.; Springer: Singapore, 2020; pp. 293–320. [Google Scholar]
- Jeng, S.R.; Dufour, S.; Chang, C.F. Differential expression of neural and gonadal aromatase enzymatic activities in relation to gonadal development in Japanese eel, Anguilla japonica. J. Exp. Zool. A Comp. Exp. Biol. 2005, 303, 802–812. [Google Scholar] [CrossRef] [PubMed]
- Tchoudakova, A.; Callard, G.V. Identification of multiple CYP19 genes encoding different cytochrome P450 aromatase isozymes in brain and ovary. Endocrinology 1998, 139, 2179–2189. [Google Scholar] [CrossRef]
- Lai, X.J.; Peng, S.; Liu, L.P.; Zou, Z.H.; Cao, L.; Wang, Y.L. Tissue-specific promoters regulate the transcription of cyp19a1 in the brain-pituitary-gonad axis of Japanese eel (Anguilla japonica). J. Steroid Biochem. Mol. Biol. 2023, 232, 106334. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Le Belle, N.; King, J.A.; Millar, R.P.; Dufour, S. Differential regulation of the two forms of gonadotropin-releasing hormone (mGnRH and cGnRH-II) by sex steroids in the European female silver eel (Anguilla anguilla). Neuroendocrinology 1995, 61, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.R.; Chen, G.R.; Lai, J.Y.; Huang, Y.S.; Dufour, S.; Chang, C.F. Regulation of pituitary gonadotropin II and growth hormone content by sex steroids and pituitary extract in the aquacultured Japanese eel, Anguilla japonica. Aquaculture 2002, 209, 319–332. [Google Scholar] [CrossRef]
- Schmitz, M.; Aroua, S.; Vidal, B.; Le Belle, N.; Elie, P.; Dufour, S. Differential regulation of luteinizing hormone and follicle-stimulating hormone expression during ovarian development and under sexual steroid feedback in the European eel. Neuroendocrinology 2005, 81, 107–119. [Google Scholar] [CrossRef] [PubMed]
- Vidal, B.; Pasqualini, C.; Le Belle, N.; Holland, M.C.; Sbaihi, M.; Vernier, P.; Zohar, Y.; Dufour, S. Dopamine inhibits luteinizing hormone synthesis and release in the juvenile European eel: A neuroendocrine lock for the onset of puberty. Biol. Reprod. 2014, 71, 1491–1500. [Google Scholar] [CrossRef]
- Lin, H.R.; Zhang, M.L.; Zhang, S.M.; Van Der Kraak, G.; Peter, R.E. Stimulation of pituitary gonadotropin and ovarian development by chronic administration of testosterone in female Japanese silver eel, Anguilla japonica. Aquaculture 1991, 96, 87–95. [Google Scholar] [CrossRef]
- Lai, X.J.; Li, Z.Q.; Xie, Y.J.; Chen, S.X.; Wang, Y.L. Androstenedione and 17α-methyltestosterone induce early ovary development of Anguilla japonica. Theriogenology 2018, 120, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Querat, B.; Hardy, D.; Fontaine, Y.A. Regulation of the type-II gonadotropin a and b subunit mRNA by oestradiol and testosterone in the European eel. J. Mol. Endocrinol. 1991, 7, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.J.; Peng, S.; Wang, Y.L. Dynamic transcriptome analysis of ovarian follicles in artificial maturing Japanese eel (Anguilla japonica). Theriogenology 2022, 180, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Aroua, S.; Weltzien, F.A.; Le Belle, N.; Dufour, S. Development of real-time RT-PCR assays for eel gonadotropins and their application to the comparison of in vivo and in vitro effects of sex steroids. Gen. Comp. Endocrinol. 2007, 153, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, I.; Kusakabe, M.; Young, G. Differential suppressive effects of low physiological doses of estradiol-17β in vivo on levels of mRNAs encoding steroidogenic acute regulatory protein and three steroidogenic enzymes in previtellogenic ovarian follicles of rainbow trout. Gen. Comp. Endocrinol. 2009, 3, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Montero, M.; Le Belle, N.; Vidal, B.; Dufour, S. Primary cultures of dispersed pituitary cells from estradiol-pretreated female silver eels (Anguilla Anguilla L.): Immunocytochemical characterization of gonadotropic cells and stimulation of gonadotropin release. Gen. Comp. Endocrinol. 1996, 104, 103–115. [Google Scholar] [CrossRef] [PubMed]
- Botté, M.C.; Lerrant, Y.; Lozach, A.; Bérault, A.; Counis, R.; Kottler, M.L. LH down-regulates gonadotropin-releasing hormone (GnRH) receptor, but not GnRH, mRNA levels in the rat testis. J. Endocrinol. 1999, 162, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.L.; Peter, R.E.; Sloley, B.D. Testosterone and estradiol potentiate the serum gonadotropin response to gonadotropin-releasing hormone in goldfish. Biol. Reprod. 1991, 44, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Dufour, S.; Montero, M.; Le Belle, N.; Bassompierre, M.; King, J.A.; Millar, R.P.; Peter, R.E.; Fontaine, Y.A. Differential distribution and response to experimental sexual maturation of two forms of brain gonadotropin-releasing hormone (GnRH) in the European eel, Anguilla anguilla. Fish Physiol. Biochem. 1993, 11, 99–106. [Google Scholar] [CrossRef]
- Zempo, B.; Karigo, T.; Kanda, S.; Akazome, Y.; Oka, Y. Morphological analysis of the axonal projections of EGFP-labeled Esr1-expressing neurons in transgenic female medaka. Endocrinology 2018, 159, 1228–1241. [Google Scholar] [CrossRef]
- Ogawa, S.; Parhar, I.S. Single-cell gene profiling reveals social status-dependent modulation of nuclear hormone receptors in GnRH neurons in a male cichlid fish. Int. J. Mol. Sci. 2020, 21, 2724. [Google Scholar] [CrossRef] [PubMed]
- Klungland, H.; Andersen, O.; Kisen, G.; Aleström, P.; Tora, L. Estrogen receptor binds to the salmon GnRH gene in a region with long palindromic sequences. Mol. Cell. Endocrinol. 1993, 95, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.S.; Lunden, J.B.; Jones, J.T. Effects of steroid hormones on spermatogenesis and GnRH release in male Leopard frogs, Rana pipiens. Gen. Comp. Endocrinol. 2003, 134, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Trudeau, V.L. Neuroendocrine control of reproduction in teleost fish: Concepts and controversies. Annu. Rev. Anim. Biosci. 2022, 10, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Kanda, S.; Abe, T.; Oka, Y. Evolution of the hypothalamic-pituitary-gonadal axis regulation in vertebrates revealed by knockout medaka. Endocrinology 2016, 157, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Burger, L.L.; Haisenleder, D.J.; Dalkin, A.C.; Marshall, J.C. Regulation of gonadotropin subunit gene transcription. J. Mol. Endocrinol. 2004, 33, 559–584. [Google Scholar] [CrossRef] [PubMed]
- Miura, C.; Miura, T.; Kudo, N.; Yamashita, M.; Yamauchi, K. cDNA cloning of a stage-specific gene expressed during HCG-induced spermatogenesis in the Japanese eel. Dev. Growth Differ. 1999, 41, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Aroua, S.; Maugars, G.; Jeng, S.R.; Chang, C.F.; Weltzien, F.A.; Rousseau, K.; Dufour, S. Pituitary gonadotropins FSH and LH are oppositely regulated by the activin/follistatin system in a basal teleost, the eel. Gen. Comp. Endocrinol. 2012, 175, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Jeng, S.R.; Pasquier, J.; Yueh, W.S.; Chen, G.R.; Lee, Y.H.; Dufour, S.; Chang, C.F. Differential regulation of the expression of cytochrome P450 aromatase, estrogen and androgen receptor subtypes in the brain-pituitary-ovarian axis of the Japanese eel (Anguilla japonica) reveals steroid dependent and independent mechanisms. Gen. Comp. Endocrinol. 2012, 175, 163–172. [Google Scholar] [CrossRef]
- Hornung, M.W.; Jensen, K.M.; Korte, J.J.; Kahl, M.D.; Durhan, E.J.; Denny, J.S.; Henry, T.R.; Ankley, G.T. Mechanistic basis for estrogenic effects in fathead minnow (Pimephales promelas) following exposure to the androgen 17α-methyltestosterone: Conversion of 17α-methyltestosterone to 17α-methylestradiol. Aquat. Toxicol. 2004, 66, 15–23. [Google Scholar] [CrossRef]
- Tokarz, J.; Möller, G.; Hrabě de Angelis, M.; Adamski, J. Steroids in teleost fishes: A functional point of view. Steroids 2015, 103, 123–144. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, A.; Senthilkumaran, B. Steroidogenesis and its regulation in teleost-a review. Fish Physiol. Biochem. 2020, 46, 803–818. [Google Scholar] [CrossRef] [PubMed]
- Mullany, L.K.; Hanse, E.A.; Romano, A.; Blomquist, C.H.; Mason, I.; Delvoux, B.; Anttila, C.; Albrecht, J.H. Cyclin D1 regulates hepatic estrogen and androgen metabolism. Am. J. Physiol. 2010, 6, G884–G895. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, S.; Liu, S.; Zhang, Y.; Yuan, C.; Wang, Z. DNA methylation in the 5′ flanking region of cytochrome P450 17 in adult rare minnow Gobiocypris rarus—Tissue difference and effects of 17α-ethinylestradiol and 17α-methyltestoterone exposures. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2014, 162, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Košir, R.; Zmrzljak, U.P.; Bele, T.; Acimovic, J.; Perse, M.; Majdic, G.; Prehn, C.; Adamski, J.; Rozman, D. Circadian expression of steroidogenic cytochromes P450 in the mouse adrenal gland—Involvement of cAMP-responsive element modulator in epigenetic regulation of Cyp17a1. FEBS J. 2012, 279, 1584–1593. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, H.; Hum, D.W.; Staels, B.; Miller, W.L. Transcription of the human genes for cytochrome P450scc and P450c17 is regulated differently in human adrenal NCI-H295 cells than in mouse adrenal Y1 cells. J. Clin. Endocrinol. Metab. 1997, 82, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Sewer, M.B.; Li, D.; Dammer, E.B.; Jagarlapudi, S.; Lucki, N. Multiple signaling pathways coordinate CYP17 gene expression in the human adrenal cortex. Acta Chim. Slov. 2008, 55, 53–57. [Google Scholar]
- Lund, J.; Ahlgren, R.; Wu, D.H.; Kagimoto, M.; Simpson, E.R.; Waterman, M.R. Transcriptional regulation of the bovine CYP17 (P-450 (17) alpha) gene. Identification of two cAMP regulatory regions lacking the consensus cAMP-responsive element (CRE). J. Biol. Chem. 1990, 265, 3304–3312. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).