Developmental Toxicity and Teratogenic Effects of Dicarboximide Fungicide Iprodione on Zebrafish (Danio rerio) Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. ZF Brood Maintenance and Embryo Collection
2.2. ZF Embryo Development Toxicity Test
2.2.1. Embryo Exposure
2.2.2. Deformity Assessment
2.3. Cardiac Function Assessment
2.4. Body Length Measurement
2.5. Behavioral Analysis (Touch-Evoked Escape Response)
2.6. Statistical Analysis
3. Results
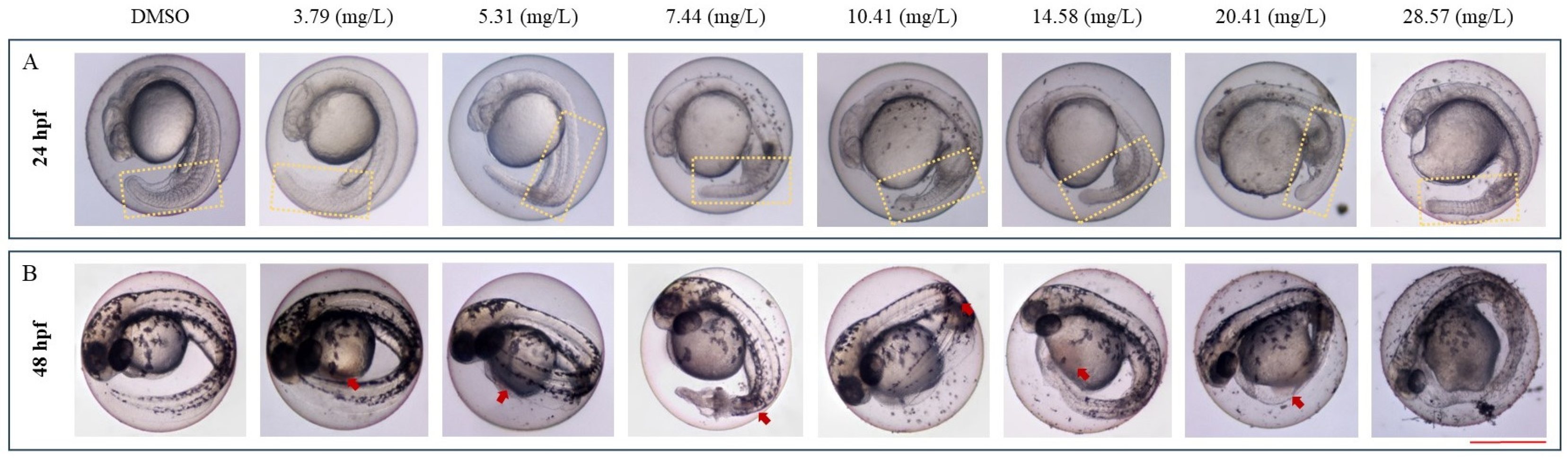
3.1. IDN Is Toxic to ZF
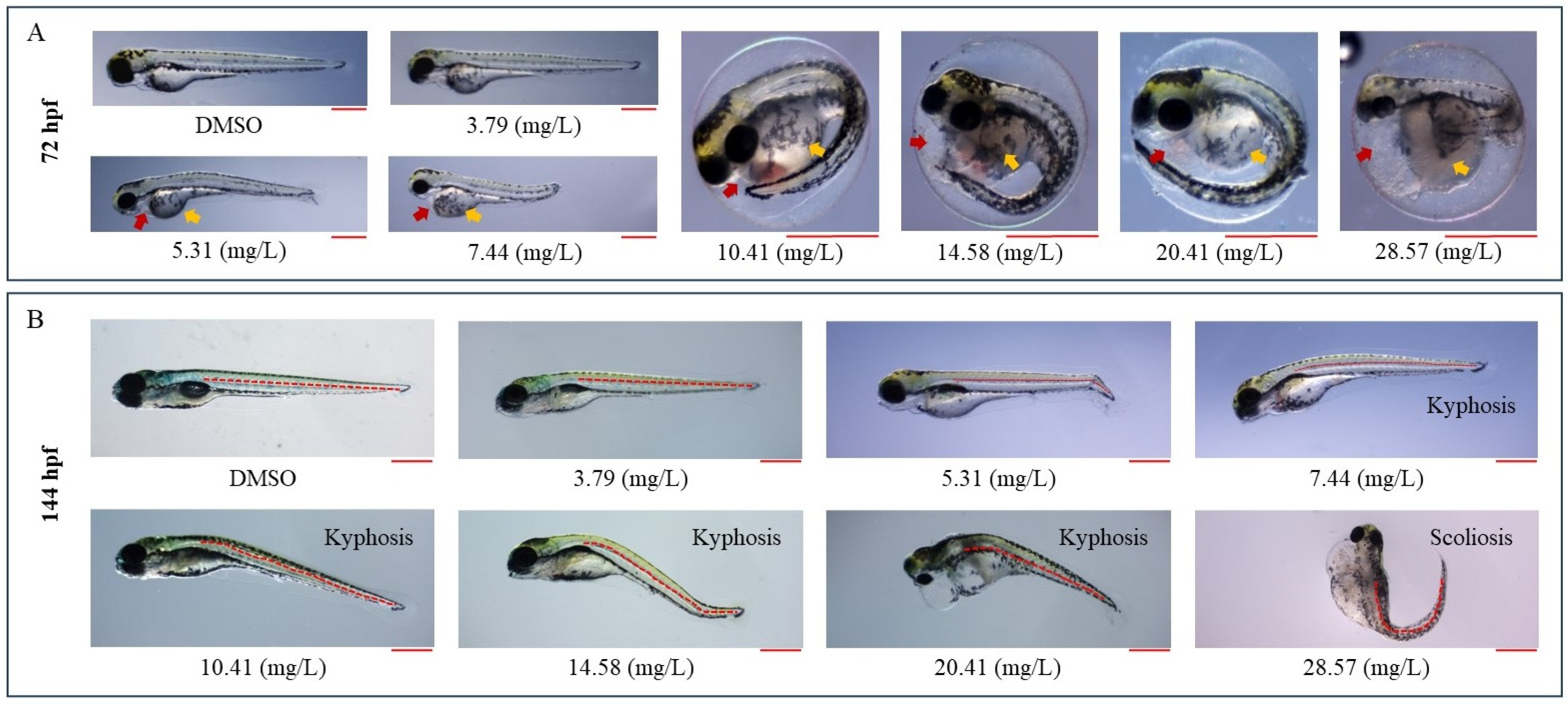
3.2. IDN Is Teratogenic to ZF
3.3. IDN Affects the Cardiac Development and Function of ZF
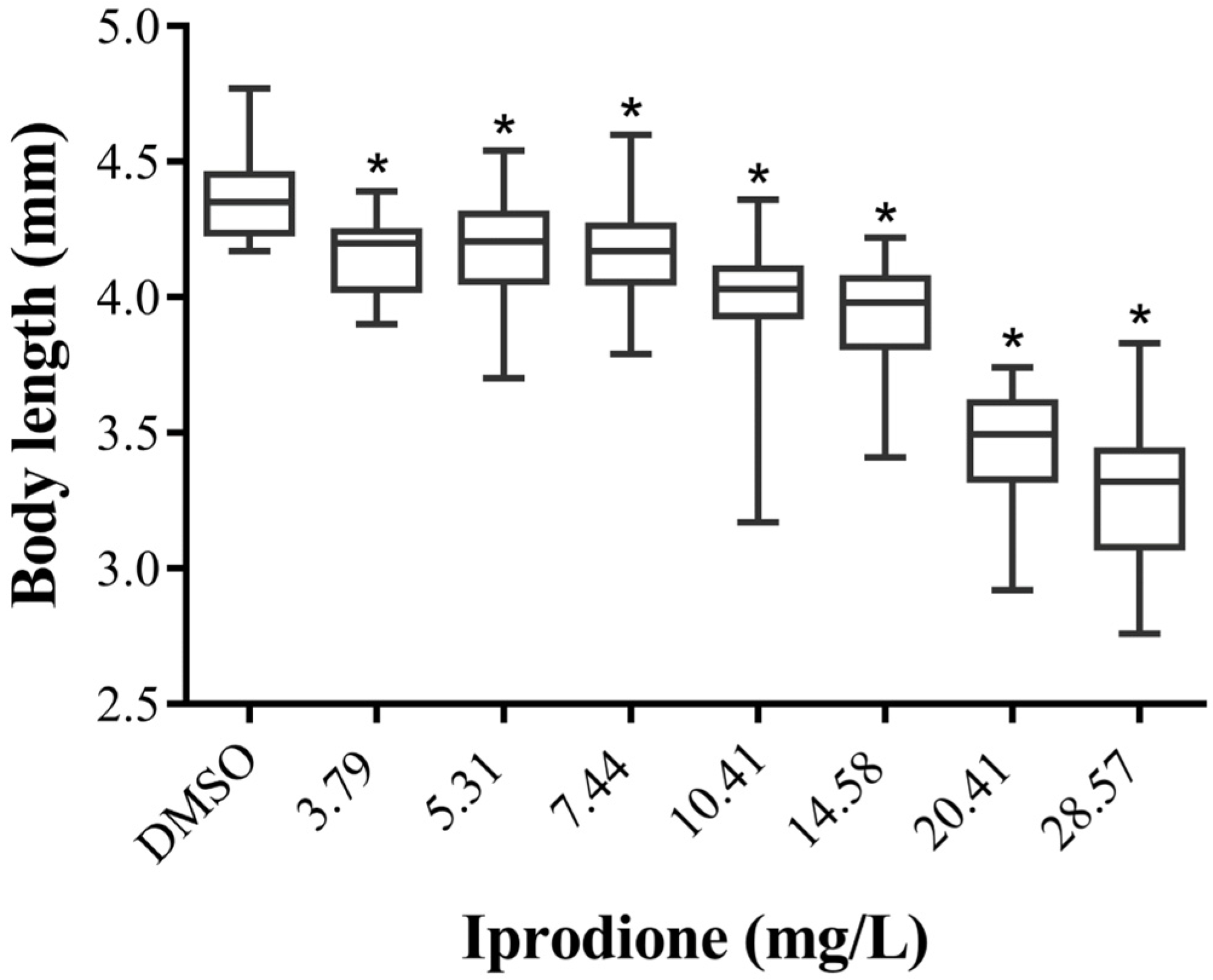
3.4. IDN Affects Normal ZF Growth
3.5. IDN Affects ZF Behavior
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahmed, A.; Shamsi, A.; Bano, B. Deciphering the toxic effects of iprodione, a fungicide and malathion, an insecticide on thiol protease inhibitor isolated from yellow Indian mustard seeds. Environ. Toxicol. Pharmacol. 2018, 61, 52–60. [Google Scholar] [CrossRef] [PubMed]
- US EPA (1998) EPA R.E.D. FACTS; EPA-738-F-98-017: IPRODIONE. Available online: https://archive.epa.gov/pesticides/reregistration/web/pdf/2335.pdf (accessed on 3 February 2023).
- Korea Crop Protection Association. Agrochemical Year Book 2023; Korea Crop Protection Association: Seoul, Republic of Korea, 2023; p. 115. [Google Scholar]
- Industry ARC. Iprodione Market-Forecast (2023–2028); Iprodione Market Size Report, 2021–2026; Industry ARC: Hyderabad, India, 2021; Available online: https://www.industryarc.com/ (accessed on 21 March 2023).
- Stensvand, A.; Christiansen, A. Investigation on fungicide residues in greenhouse-grown strawberries. J. Agric. Food Chem. 2000, 48, 917–920. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Xu, G.; Wang, F.; Sun, H.; Li, Y. Iprodione residues and dissipation rates in tobacco leaves and soil. Bull. Environ. Contam. Toxicol. 2012, 89, 877–881. [Google Scholar] [CrossRef] [PubMed]
- Loutfy, N.; Malhat, F.; Kamel, E.; Saber, A. Residual pattern and dietary intake of iprodione on grapes under Egyptian field conditions: A prelude to risk assessment profile. Hum. Ecol. Risk Assess. Int. J. 2015, 21, 265–279. [Google Scholar] [CrossRef]
- Campos, M.; Perruchon, C.; Karas, P.; Karavasilis, D.; Diez, M.; Karpouzas, D. Bioaugmentation and rhizosphere-assisted biodegradation as strategies for optimization of the dissipation capacity of biobeds. J. Environ. Manag. 2017, 187, 103–110. [Google Scholar] [CrossRef]
- Vidal, J.M.; García, M.G.; Galera, M.M.; Frenich, A.G. Comparison of multicomponent determination of iprodione, procymidone and chlorothalonil by partial least squares modelling using spectrophotometric and high-performance liquid chromatography data. Anal. Lett. 1997, 30, 2409–2432. [Google Scholar] [CrossRef]
- Katsoula, A.; Vasileiadis, S.; Sapountzi, M.; Karpouzas, D.G. The response of soil and phyllosphere microbial communities to repeated application of the fungicide iprodione: Accelerated biodegradation or toxicity? FEMS Microbiol. Ecol. 2020, 96, fiaa056. [Google Scholar] [CrossRef]
- Radice, S.; Ferraris, M.; Marabini, L.; Grande, S.; Chiesara, E. Effect of iprodione, a dicarboximide fungicide, on primary cultured rainbow trout (Oncorhynchus mykiss) hepatocytes. Aquat. Toxicol. 2001, 54, 51–58. [Google Scholar] [CrossRef]
- EFSA. Peer Review of the Pesticide Risk Assessment of the Active Substance Iprodione; EFSA: Parma, Italy, 2016.
- Durand, P.; Martin, G.; Blondet, A.; Gilleron, J.; Carette, D.; Janczarski, S.; Christin, E.; Pointis, G.; Perrard, M.-H. Effects of low doses of carbendazim or iprodione either separately or in mixture on the pubertal rat seminiferous epithelium: An ex vivo study. Toxicol. Vitr. 2017, 45, 366–373. [Google Scholar] [CrossRef]
- Blystone, C.R.; Lambright, C.S.; Cardon, M.C.; Furr, J.; Rider, C.V.; Hartig, P.C.; Wilson, V.S.; Gray, L.E., Jr. Cumulative and antagonistic effects of a mixture of the antiandrogens vinclozolin and iprodione in the pubertal male rat. Toxicol. Sci. 2009, 111, 179–188. [Google Scholar] [CrossRef]
- Maskey, E.; Crotty, H.; Wooten, T.; Khan, I.A. Disruption of oocyte maturation by selected environmental chemicals in zebrafish. Toxicol. Vitr. 2019, 54, 123–129. [Google Scholar] [CrossRef]
- Wei, Y.; Meng, Y.; Huang, Y.; Liu, Z.; Zhong, K.; Ma, J.; Zhang, W.; Li, Y.; Lu, H. Development toxicity and cardiotoxicity in zebrafish from exposure to iprodione. Chemosphere 2021, 263, 127860. [Google Scholar] [CrossRef]
- Hu, W.; Chen, G.; Yuan, W.; Guo, C.; Liu, F.; Zhang, S.; Cao, Z. Iprodione induces hepatotoxicity in zebrafish by mediating ROS generation and upregulating p53 signalling pathway. Ecotoxicol. Environ. Saf. 2024, 270, 115911. [Google Scholar] [CrossRef]
- Hassan, M.A.; El Bohy, K.M.; El Sharkawy, N.I.; Imam, T.S.; El-Metwally, A.E.; Hamed Arisha, A.; Mohammed, H.A.; Abd-Elhakim, Y.M. Iprodione and chlorpyrifos induce testicular damage, oxidative stress, apoptosis and suppression of steroidogenic-and spermatogenic-related genes in immature male albino rats. Andrologia 2021, 53, e13978. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; El Sharkawy, N.I.; Gharib, H.S.; Hassan, M.A.; Metwally, M.M.; Elbohi, K.M.; Hassan, B.A.; Mohammed, A.T. Neurobehavioral responses and toxic brain reactions of juvenile rats exposed to iprodione and chlorpyrifos, alone and in a mixture. Toxics 2023, 11, 431. [Google Scholar] [CrossRef]
- He, J.-H.; Gao, J.-M.; Huang, C.-J.; Li, C.-Q. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicol. Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef]
- Nagel, R. DarT: The embryo test with the Zebrafish Danio rerio—A general model in ecotoxicology and toxicology. Altex 2002, 19, 38–48. [Google Scholar]
- Lammer, E.; Carr, G.; Wendler, K.; Rawlings, J.; Belanger, S.; Braunbeck, T. Is the fish embryo toxicity test (FET) with the zebrafish (Danio rerio) a potential alternative for the fish acute toxicity test? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2009, 149, 196–209. [Google Scholar] [CrossRef]
- Kimmel, C.B.; Ballard, W.W.; Kimmel, S.R.; Ullmann, B.; Schilling, T.F. Stages of embryonic development of the zebrafish. Dev. Dyn. 1995, 203, 253–310. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Kim, J.; Oh, J.-A.; Yoon, C.-Y.; Park, H.-H. Transcriptome-based identification of genes responding to the organophosphate pesticide phosmet in Danio rerio. Genes 2021, 12, 1738. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Kim, J.; Oh, J.-A.; Yoon, C.-Y.; Park, H.-H. Developmental toxic effects of thiram on developing zebrafish (Danio rerio) embryos. Toxics 2022, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Vasamsetti, B.M.K.; Kim, N.-S.; Chon, K.; Park, H.-H. Teratogenic and developmental toxic effects of etridiazole on zebrafish (Danio rerio) embryos. Appl. Biol. Chem. 2020, 63, 80. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Kim, N.S.; Chon, K.; Park, H.-H. Developmental toxic effects of phosmet on zebrafish (Danio rerio) embryos. Korean J. Pestic. Sci. 2020, 24, 343–351. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD: Paris, France, 2013. [Google Scholar]
- Wu, A.; Yu, Q.; Lu, H.; Lou, Z.; Zhao, Y.; Luo, T.; Fu, Z.; Jin, Y. Developmental toxicity of procymidone to larval zebrafish based on physiological and transcriptomic analysis. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2021, 248, 109081. [Google Scholar] [CrossRef]
- Sant, K.E.; Timme-Laragy, A.R. Zebrafish as a model for toxicological perturbation of yolk and nutrition in the early embryo. Curr. Environ. Health Rep. 2018, 5, 125–133. [Google Scholar] [CrossRef]
- Schoots, A.F.; Stikkelbroeck, J.J.; Bekhuis, J.F.; Denucé, J.M. Hatching in teleostean fishes: Fine structural changes in the egg envelope during enzymatic breakdown in vivo and in vitro. J. Ultrastruct. Res. 1982, 80, 185–196. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a model vertebrate for investigating chemical toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Amindari, A.; Butcher, J.T.; Althani, A.; Yacoub, M. Heart function and hemodynamic analysis for zebrafish embryos. Dev. Dyn. 2017, 246, 868–880. [Google Scholar] [CrossRef]
- Planas, J.V.; Palstra, A.P.; Magnoni, L.J. Physiological Adaptations to Swimming in Fish; Frontiers Media SA: Lausanne, Switzerland, 2017. [Google Scholar] [CrossRef][Green Version]
- Lauder, G.V. Patterns of evolution in the feeding mechanism of actinopterygian fishes. Am. Zool. 1982, 22, 275–285. [Google Scholar] [CrossRef]
- Park, W.; An, G.; Lim, W.; Song, G. Exposure to iprodione induces ROS production and mitochondrial dysfunction in porcine trophectoderm and uterine luminal epithelial cells, leading to implantation defects during early pregnancy. Chemosphere 2022, 307, 135894. [Google Scholar] [CrossRef]
- Islam, M.T. Oxidative stress and mitochondrial dysfunction-linked neurodegenerative disorders. Neurol. Res. 2017, 39, 73–82. [Google Scholar] [CrossRef]
- Oswald, M.C.; Garnham, N.; Sweeney, S.T.; Landgraf, M. Regulation of neuronal development and function by ROS. FEBS Lett. 2018, 592, 679–691. [Google Scholar] [CrossRef]
- Chen, X.-F.; Lin, Z.-C.; Qi, Z.; Cai, Z.; Chen, Z.-F. Effects of pollutant toxicity on the eyes of aquatic life monitored by visual dysfunction in zebrafish: A review. Environ. Chem. Lett. 2023, 21, 1177–1201. [Google Scholar] [CrossRef]
- Schonthaler, H.B.; Lampert, J.M.; von Lintig, J.; Schwarz, H.; Geisler, R.; Neuhauss, S.C. A mutation in the silver gene leads to defects in melanosome biogenesis and alterations in the visual system in the zebrafish mutant fading vision. Dev. Biol. 2005, 284, 421–436. [Google Scholar] [CrossRef]
- Zhao, S.; Rizzolo, L.J.; Barnstable, C.J. Differentiation and transdifferentiation of the retinal pigment epithelium. Int. Rev. Cytol. 1997, 171, 225–266. [Google Scholar] [CrossRef]
- Neuhauss, S.C.; Biehlmaier, O.; Seeliger, M.W.; Das, T.; Kohler, K.; Harris, W.A.; Baier, H. Genetic disorders of vision revealed by a behavioral screen of 400 essential loci in zebrafish. J. Neurosci. 1999, 19, 8603–8615. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Yoon, C.-Y.; Kim, J.; Choi, J.-Y.; Hwang, S.; Park, K.-H. Transcriptome profiling of etridiazole-exposed zebrafish (Danio rerio) embryos reveals pathways associated with cardiac and ocular toxicities. Int. J. Mol. Sci. 2023, 24, 15067. [Google Scholar] [CrossRef]
- Vasamsetti, B.M.K.; Chon, K.; Choi, J.-Y.; Kim, J.; Yoon, C.-Y. Transcriptome analysis of thiram-treated zebrafish (Danio rerio) embryos reveals disruption of reproduction signaling pathways. Biology 2023, 12, 156. [Google Scholar] [CrossRef]
- Díaz-Martín, R.D.; Carvajal-Peraza, A.; Yáñez-Rivera, B.; Betancourt-Lozano, M. Short exposure to glyphosate induces locomotor, craniofacial, and bone disorders in zebrafish (Danio rerio) embryos. Environ. Toxicol. Pharmacol. 2021, 87, 103700. [Google Scholar] [CrossRef]
- Merola, C.; Fabrello, J.; Matozzo, V.; Faggio, C.; Iannetta, A.; Tinelli, A.; Crescenzo, G.; Amorena, M.; Perugini, M. Dinitroaniline herbicide pendimethalin affects development and induces biochemical and histological alterations in zebrafish early-life stages. Sci. Total Environ. 2022, 828, 154414. [Google Scholar] [CrossRef]
- Staal, Y.C.; Meijer, J.; van der Kris, R.J.; de Bruijn, A.C.; Boersma, A.Y.; Gremmer, E.R.; Zwart, E.P.; Beekhof, P.K.; Slob, W.; van der Ven, L.T. Head skeleton malformations in zebrafish (Danio rerio) to assess adverse effects of mixtures of compounds. Arch. Toxicol. 2018, 92, 3549–3564. [Google Scholar] [CrossRef]
- Çelik, E.S.; Kaya, H.; Yılmaz, S. Effects of phosalone on mineral contents and spinal deformities in common carp (Cyprinus carpio, L. 1758). Turk. J. Fish. Aquat. Sci. 2012, 12, 259–264. [Google Scholar] [CrossRef]
- Muramoto, S. Vertebral column damage and decrease of calcium concentration in fish exposed experimentally to cadmium. Environ. Pollut. Ser. A Ecol. Biol. 1981, 24, 125–133. [Google Scholar] [CrossRef]

| Concentration (mg/L) | Mortality (%) (n = 6) | |||
|---|---|---|---|---|
| 24 hpf | 48 hpf | 72 hpf | 96 hpf | |
| DMSO | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| 3.79 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| 5.31 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| 7.43 | 0.00 (0.00–0.00) | 0.00 (0.00–1.25) | 0.00 (0.00–1.25) | 0.00 (0.00–1.25) |
| 10.41 | 0.00 (0.00–5.00) | 5.00 (0.00–5.00) | 5.00 (3.75–5.00) * | 5.00 (3.75–5.00) * |
| 14.57 | 0.00 (0.00–6.25) | 15.00 (5.00–21.25) * | 17.50 (8.75–21.25) * | 17.50 (8.75–21.25) * |
| 20.40 | 7.50 (5.00–22.50) * | 42.50 (23.75–46.25) * | 42.50 (36.25–61.25) * | 45.00 (36.25–61.25) * |
| 28.57 | 17.50 (5.00–26.25) * | 62.50 (42.50–70.00) * | 70.00 (56.25–75.00) * | 67.50 (60.00–75.00) * |
| 40.00 | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * |
| LC50 (Mean ± SD) | 29.38 ± 0.38 | 24.53 ± 4.07 | 23.05 ± 3.81 | 23.05 ± 3.84 |
| Negative control | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) |
| Positive control | 38.93 (33.81–51.72) * | 60.94 (58.88–65.73) * | 78.03 (70.03–84.90) * | 80.15 (77.68–82.70) * |
| Iprodione (mg/L) | Abnormal Somite (%) | Delayed Retina Pigment (%) | Hyperemia (%) | Abnormal Tail Blood Flow (%) | |||
| 24 hpf | DMSO | 0.00 (0.00–0.00) | |||||
| 3.79 | 0.00 (0.00–0.00) | ||||||
| 5.31 | 2.50 (0.00–10.00) | ||||||
| 7.43 | 2.50 (0.00–15.00) | ||||||
| 10.41 | 27.50 (0.00–37.50) | ||||||
| 14.57 | 39.45 (0.00–58.75) | ||||||
| 20.40 | 44.74 (0.00–60.53) | ||||||
| 28.57 | 100 (66.08–100.00) * | ||||||
| 40.00 | - | ||||||
| 48 hpf | DMSO | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | |||
| 3.79 | 12.50 (0.00–36.25) | 0.00 (0.00–2.50) | 0.00 (0.00–0.00) | ||||
| 5.31 | 12.50 (0.00–25.00) | 5.00 (0.00–16.25) | 0.00 (0.00–12.50) | ||||
| 7.43 | 18.29 (0.00–37.50) | 17.50 (10.00–25.79) * | 10.40 (0.00–22.50) | ||||
| 10.41 | 60.53 (49.47–75.99) * | 69.34 (56.58–75.99) * | 73.68 (63.75–100.00) * | ||||
| 14.57 | 81.14 (77.96–88.96) * | 94.45 (77.63–100.00) * | 83.75 (73.36–100.00) * | ||||
| 20.40 | 95.84 (90.68–100.00) * | 95.46 (85.32–100.00) * | 95.00 (85.46–100.00) * | ||||
| 28.57 | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | ||||
| 40.00 | - | - | - | ||||
| Iprodione (mg/L) | Pericardial Edema (%) | Yolk Sac Edema (%) | Unhatched Egg (%) | ||||
| 72 hpf | DMSO | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 32.50 (13.75–35.00) | |||
| 3.79 | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 37.50 (20.00–56.25) | ||||
| 5.31 | 25.00 (0.00–35.00) | 17.50 (0.00–22.50) | 27.50 (20.00–91.25) | ||||
| 7.43 | 38.56 (13.75–45.00) * | 27.50 (0.00–36.78) | 62.50 (48.03–100.00) * | ||||
| 10.41 | 68.42 (53.95–81.05) * | 52.64 (18.42–75.99) * | 100.00 (87.04–100.00) * | ||||
| 14.57 | 94.59 (90.31–100.00) * | 87.29 (42.54–100.00) * | 100.00 (100.00–100.00) * | ||||
| 20.40 | 100.00 (69.70–100.00) * | 100.00(69.70–100.00) * | 100.00 (100.00–100.00) * | ||||
| 28.57 | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | ||||
| 40.00 | - | - | - | ||||
| 96 hpf | DMSO | 0.00 (0.00–0.00) | |||||
| 3.79 | 0.00 (0.00–0.00) | ||||||
| 5.31 | 0.00 (0.00–17.50) | ||||||
| 7.43 | 2.50 (0.00–100.00) | ||||||
| 10.41 | 36.06 (21.06–100.00) * | ||||||
| 14.57 | 100.00 (87.65–100.00) * | ||||||
| 20.40 | 100.00 (100.00–100.00) * | ||||||
| 28.57 | 100.00 (100.00–100.00) * | ||||||
| 40.00 | - | ||||||
| Iprodione (mg/L) | Abnormal Swim Bladder (%) | Spine Curve (%) | Abnormal Touch Response (%) | ||||
| 144 hpf | DMSO | 0.00 (0.00–0.00) | 0.00 (0.00–0.00) | 0.00 (0.00–10.00) | |||
| 3.79 | 60.00 (35.00–85.00) * | 0.00 (0.00–5.00) | 10.00 (0.00–10.00) | ||||
| 5.31 | 100.00 (80.00–100.00) * | 10.00 (0.00–20.00) | 5.00 (0.00–12.50) | ||||
| 7.43 | 100.00 (95.00–100.00) * | 20.00 (0.00–40.00) | 10.00 (7.50–10.00) | ||||
| 10.41 | 100.00 (100.00–100.00) * | 50.00 (40.00–65.00) * | 55.00 (27.50–82.50) * | ||||
| 14.57 | 100.00 (100.00–100.00) * | 60.00 (60.00–80.00) * | 90.00 (67.50–90.00) * | ||||
| 20.40 | 100.00 (100.00–100.00) * | 80.00 (75.00–100.00) * | 100.00 (100.00–100.00) * | ||||
| 28.57 | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | 100.00 (100.00–100.00) * | ||||
| 40.00 | - | - | |||||
| Deformity | Time (hpf) | LC50 (mg/L) | EC50 (mg/L) | Teratogenic Index (96 hpf LC50/EC50) |
|---|---|---|---|---|
| Mortality | 24 | 29.38 ± 0.38 | - | - |
| Mortality | 48 | 24.53 ± 4.07 | - | - |
| Mortality | 72 | 23.05 ± 3.81 | - | - |
| Mortality | 96 | 23.05 ± 3.84 | - | - |
| Abnormal somite | 24 | - | 21.42 ± 6.00 | 1.20 ± 0.47 |
| Delayed retina pigment | 48 | - | 9.05 ± 1.39 | 2.61 ± 0.40 |
| Hyperemia | 48 | - | 9.66 ± 0.94 | 2.41 ± 0.28 |
| Abnormal tail blood flow | 48 | - | 9.69 ± 0.55 | 2.39 ± 0.14 |
| Unhatched egg | 72 | - | 6.57 ± 2.29 | 4.05 ± 1.61 |
| Pericardial edema | 72 | - | 8.94 ± 1.42 | 2.64 ± 0.42 |
| Yolk sac edema | 72 | - | 11.91 ± 4.58 | 2.19 ± 0.69 |
| Unhatched egg | 96 | - | 9.70 ± 2.95 | 2.65 ± 0.91 |
| Abnormal swim bladder | 144 | - | 3.44 ± 0.74 | 7.15 ± 2.14 |
| Spine curve | 144 | - | 9.97 ± 1.07 | 2.34 ± 0.31 |
| Abnormal touch response | 144 | - | 10.64 ± 1.76 | 2.22 ± 0.33 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoon, C.-Y.; Chon, K.; Vasamsetti, B.M.K.; Hwang, S.; Park, K.-H.; Kyung, K.S. Developmental Toxicity and Teratogenic Effects of Dicarboximide Fungicide Iprodione on Zebrafish (Danio rerio) Embryos. Fishes 2024, 9, 425. https://doi.org/10.3390/fishes9110425
Yoon C-Y, Chon K, Vasamsetti BMK, Hwang S, Park K-H, Kyung KS. Developmental Toxicity and Teratogenic Effects of Dicarboximide Fungicide Iprodione on Zebrafish (Danio rerio) Embryos. Fishes. 2024; 9(11):425. https://doi.org/10.3390/fishes9110425
Chicago/Turabian StyleYoon, Chang-Young, Kyongmi Chon, Bala Murali Krishna Vasamsetti, Sojeong Hwang, Kyeong-Hun Park, and Kee Sung Kyung. 2024. "Developmental Toxicity and Teratogenic Effects of Dicarboximide Fungicide Iprodione on Zebrafish (Danio rerio) Embryos" Fishes 9, no. 11: 425. https://doi.org/10.3390/fishes9110425
APA StyleYoon, C.-Y., Chon, K., Vasamsetti, B. M. K., Hwang, S., Park, K.-H., & Kyung, K. S. (2024). Developmental Toxicity and Teratogenic Effects of Dicarboximide Fungicide Iprodione on Zebrafish (Danio rerio) Embryos. Fishes, 9(11), 425. https://doi.org/10.3390/fishes9110425








