Functional Adaptations of Hemocytes of Aplysia depilans (Gmelin, 1791) and Their Putative Role in Neuronal Regeneration
Abstract
1. Introduction
2. Materials and Methods
2.1. Samples and Tissue Preparation
2.2. Histology
2.3. Immunofluorescence
2.4. Cell Sizes
2.5. Quantitative Analysis
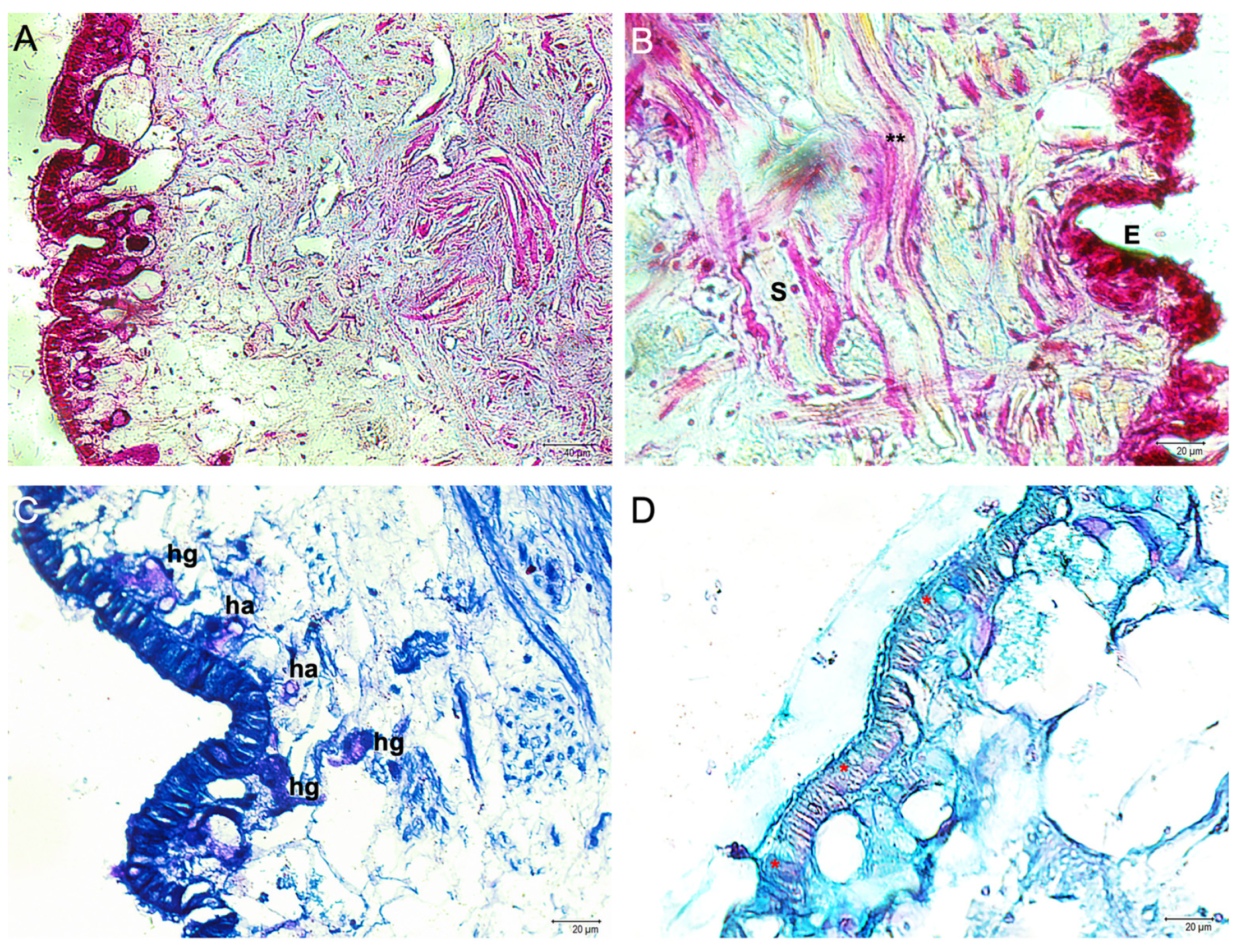
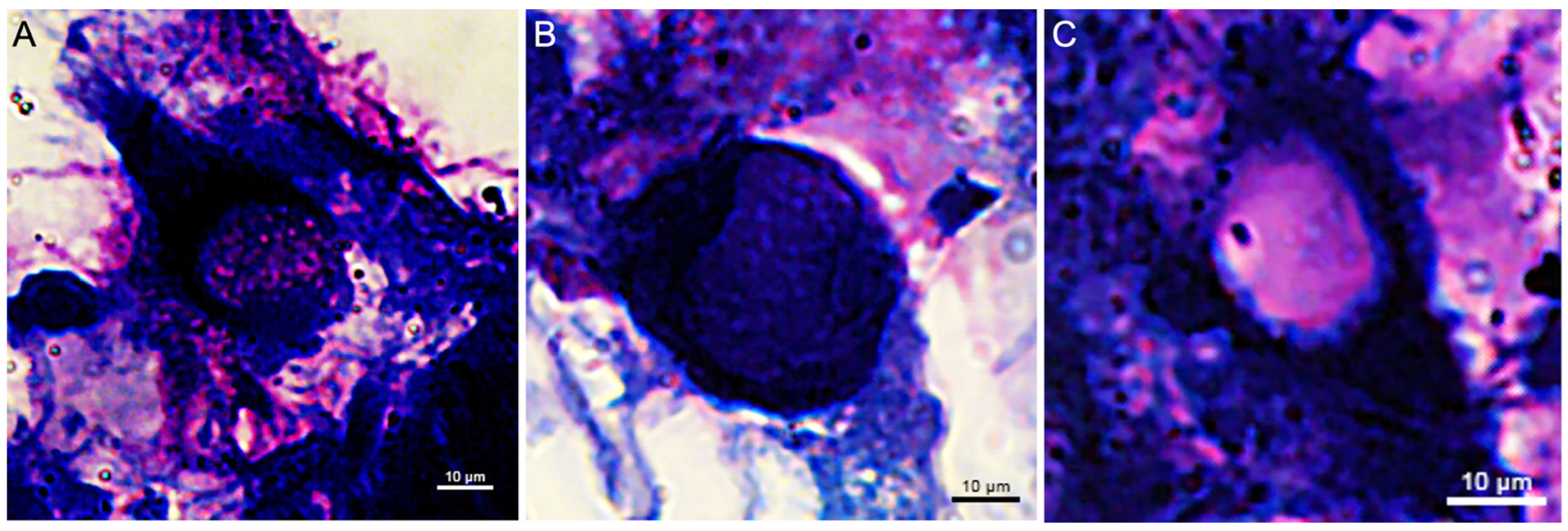
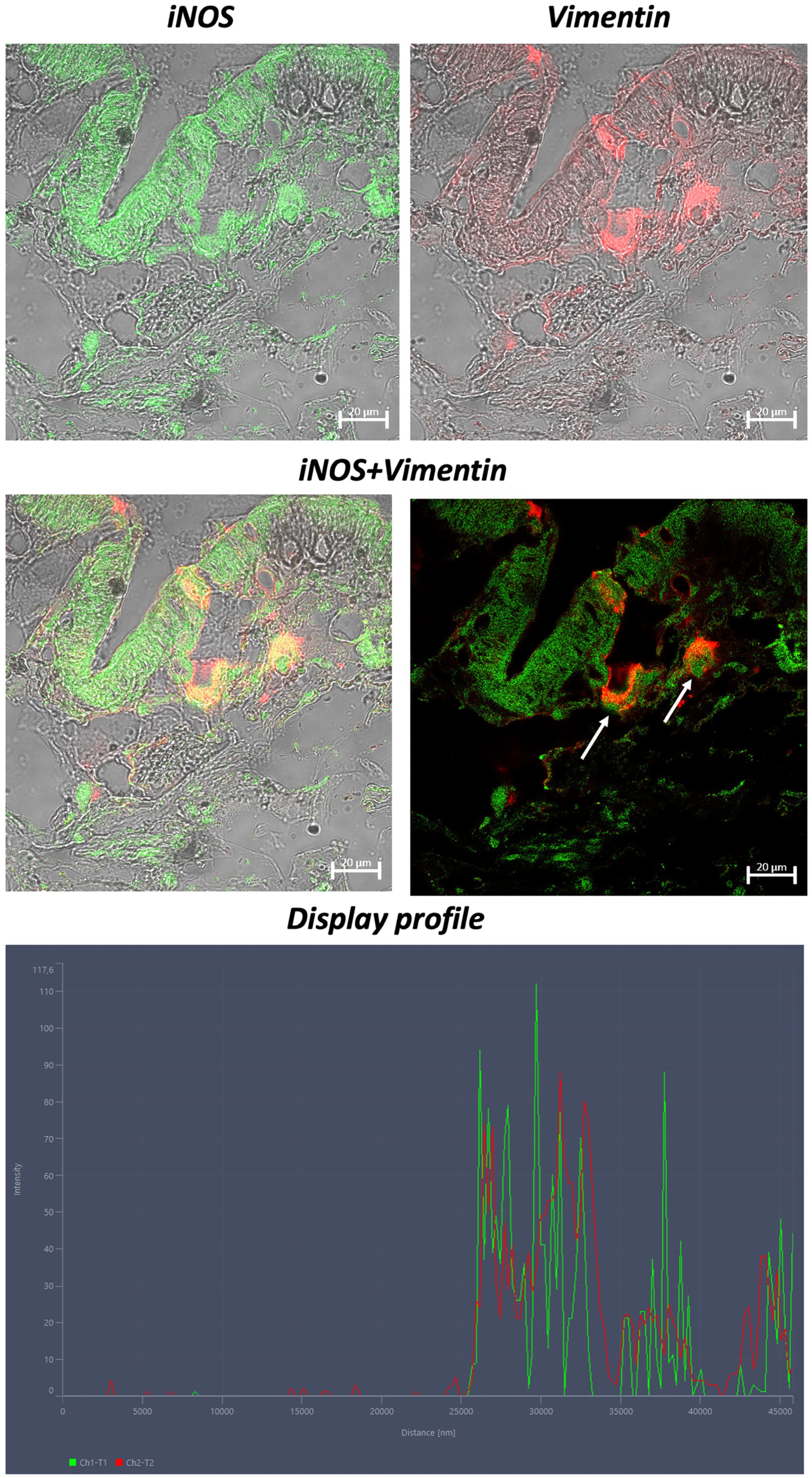
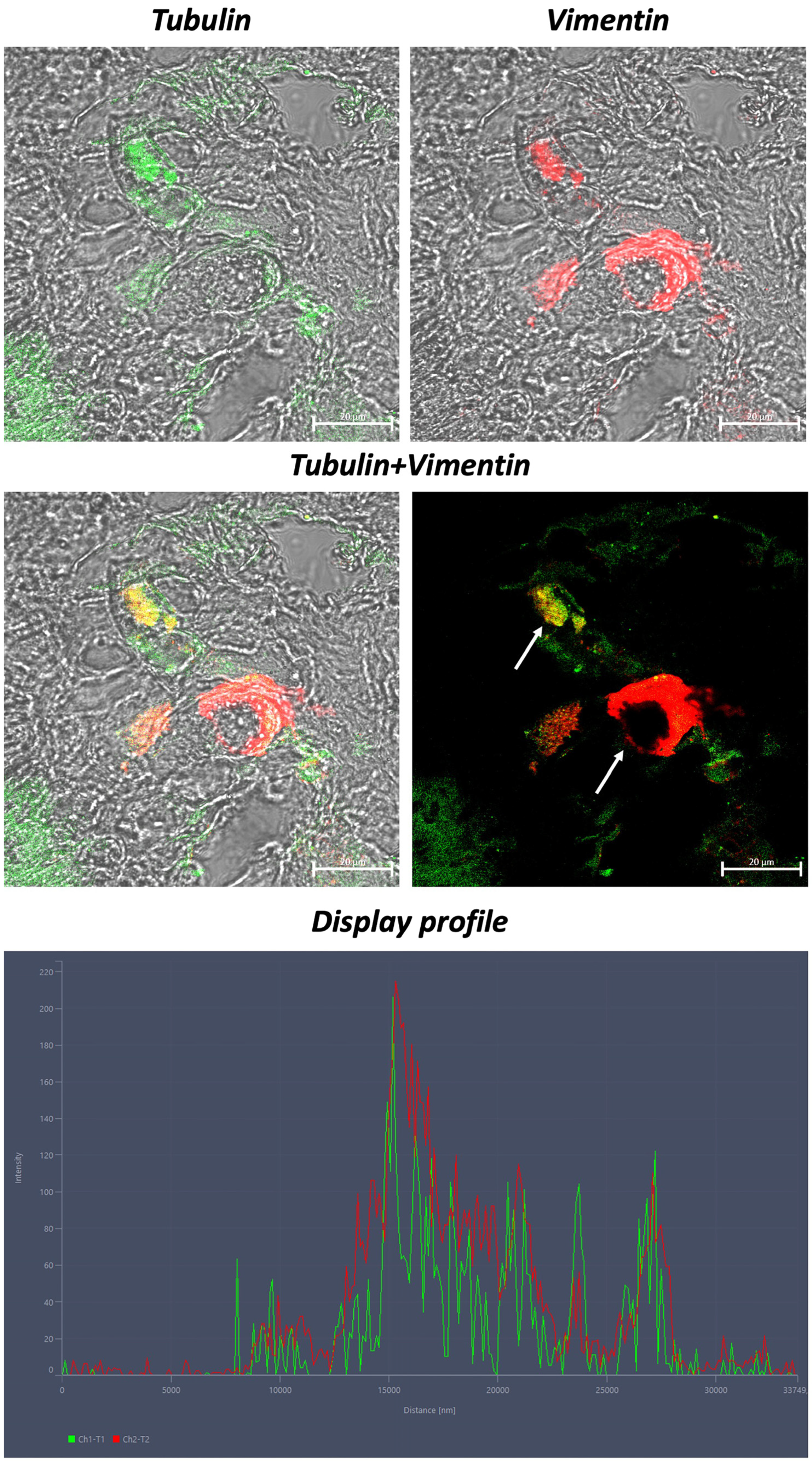
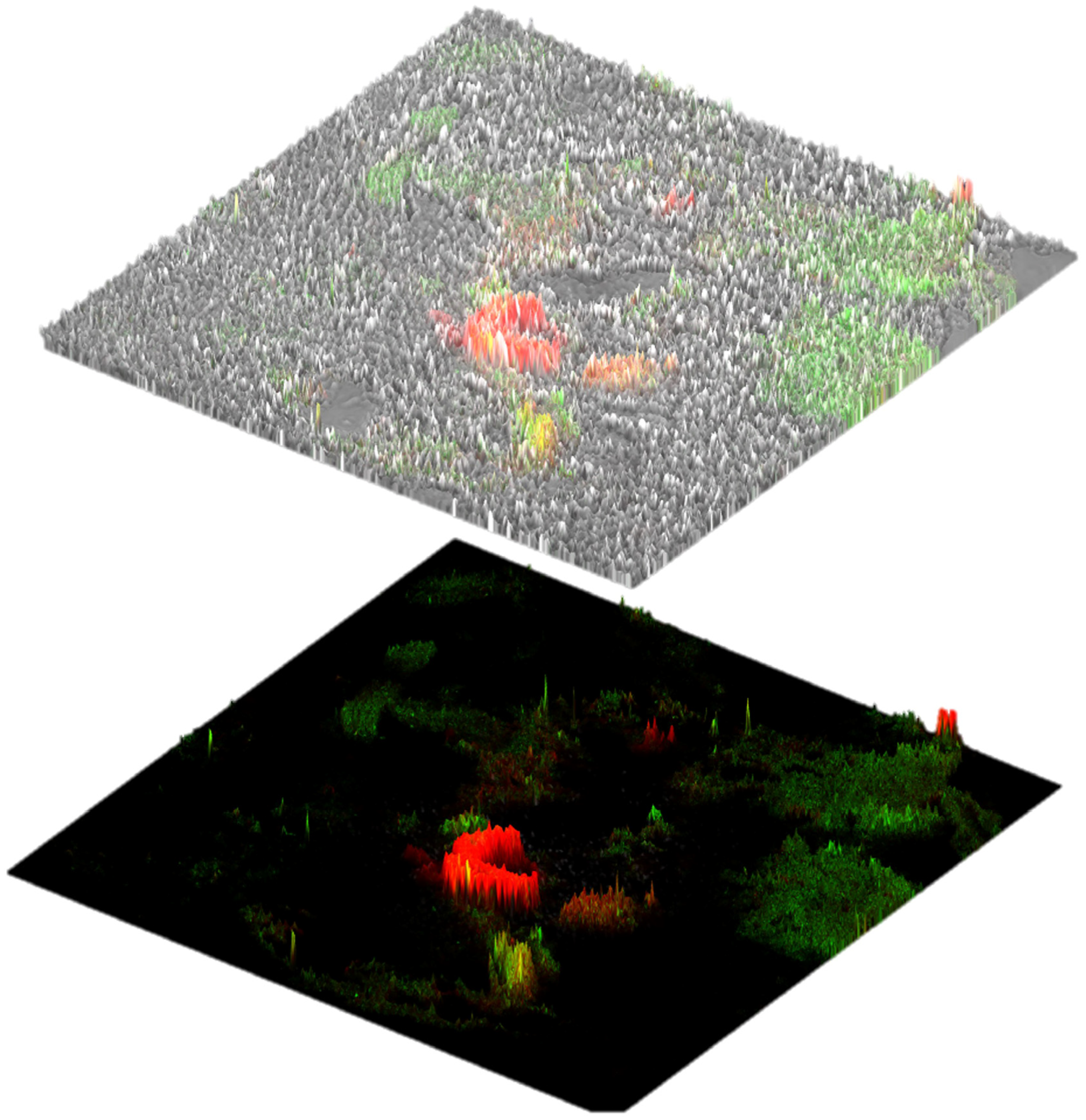
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Fumia, A.; Lauriano, E.R. Neuronal Regeneration: Vertebrates Comparative Overview and New Perspectives for Neurodegenerative Diseases. Acta Zool. 2022, 103, 129–140. [Google Scholar] [CrossRef]
- Brockes, J.P.; Kumar, A. Comparative Aspects of Animal Regeneration. Annu. Rev. Cell Dev. Biol. 2008, 24, 525–549. [Google Scholar] [CrossRef] [PubMed]
- Sinigaglia, C.; Averof, M. The Multifaceted Role of Nerves in Animal Regeneration. Curr. Opin. Genet. Dev. 2019, 57, 98–105. [Google Scholar] [CrossRef]
- Blundon, J.A.; Sheller, R.A.; Moehlenbruck, J.W.; Bittner, G.D. Effect of Temperature on Long-Term Survival of Anucleate Giant Axons in Crayfish and Goldfish. J. Comp. Neurol. 1990, 297, 377–391. [Google Scholar] [CrossRef]
- Krause, T.L.; Bittner, G.D. Rapid Morphological Fusion of Severed Myelinated Axons by Polyethylene Glycol. Proc. Natl. Acad. Sci. USA 1990, 87, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, D.M.; Zaccone, G.; Alesci, A.; Kuciel, M.; Hussein, M.T.; Sayed, R.K.A. Main Components of Fish Immunity: An Overview of the Fish Immune System. Fishes 2023, 8, 93. [Google Scholar] [CrossRef]
- Beschin, A.; Bilej, M.; Torreele, E.; De Baetselier, P. On the Existence of Cytokines in Invertebrates. Cell. Mol. Life Sci. 2001, 58, 801–814. [Google Scholar] [CrossRef]
- Chiaramonte, M.; Russo, R. The Echinoderm Innate Humoral Immune Response. Ital. J. Zool. 2015, 82, 300–308. [Google Scholar] [CrossRef]
- Schmidt, M. The Olfactory Pathway of Decapod Crustaceans—An Invertebrate Model for Life-Long Neurogenesis. Chem. Senses 2007, 32, 365–384. [Google Scholar] [CrossRef]
- da Silva, P.C.; de Abreu, I.S.; Cavalcante, L.A.; De Barros, C.M.; Allodi, S. Role of Hemocytes in Invertebrate Adult Neurogenesis and Brain Repair. Invertebr. Surviv. J. 2015, 12, 142–154. [Google Scholar]
- De Abreu, I.S.; Wajsenzon, I.J.R.; Dias, J.C.; Allodi, S.; Monteiro-de-Barros, C. Central Nervous System Regeneration in Ascidians: Cell Migration and Differentiation. Cell Tissue Res. 2022, 390, 335–354. [Google Scholar] [CrossRef] [PubMed]
- Sardar, S.; Saha, N.; Ghosh, A. Microglia, the Sentinel of Brain in the Evolution of Nervous System from Invertebrate to Vertebrate: A Short Review. J. Sci. Res. 2022, 14, 685. [Google Scholar] [CrossRef]
- Benton, J.L.; Kery, R.; Li, J.; Noonin, C.; Söderhäll, I.; Beltz, B.S. Cells from the Immune System Generate Adult-Born Neurons in Crayfish. Dev. Cell 2014, 30, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Baylor, D.A.; Nicholls, J.G. Patterns of Regeneration between Individual Nerve Cells in the Central Nervous System of the Leech. Nature 1971, 232, 268–270. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.K.S.; Nicholls, J.G. Regeneration and Changes in Synaptic Connections between Individual Nerve Cells in the Central Nervous System of the Leech. Proc. Natl. Acad. Sci. USA 1972, 69, 636–639. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.J.; Muller, K.J. Sprouting and Regeneration of Sensory Axons after Destruction of Ensheathing Glial Cells in the Leech Central Nervous System. J. Neurosci. 1983, 3, 1994–2006. [Google Scholar] [CrossRef]
- Boidin-Wichlacz, C.; Vergote, D.; Slomianny, C.; Jouy, N.; Salzet, M.; Tasiemski, A. Morphological and Functional Characterization of Leech Circulating Blood Cells: Role in Immunity and Neural Repair. Cell. Mol. Life Sci. 2012, 69, 1717–1731. [Google Scholar] [CrossRef]
- Chaves da Silva, P.G.; Corrêa, C.L.; de Carvalho, S.L.; Allodi, S. The Crustacean Central Nervous System in Focus: Subacute Neurodegeneration Induces a Specific Innate Immune Response. PLoS ONE 2013, 8, e80896. [Google Scholar] [CrossRef][Green Version]
- Chaves-da-Silva, P.G.; De Barros, C.M.; Lima, F.R.S.; Biancalana, A.; Martinez, A.M.B.; Allodi, S. Identity of the Cells Recruited to a Lesion in the Central Nervous System of a Decapod Crustacean. Cell Tissue Res. 2010, 342, 179–189. [Google Scholar] [CrossRef]
- Shapiro, H.M.; Mandy, F. Cytometry in Malaria: Moving beyond Giemsa. Cytometry 2007, 71 Pt A, 643–645. [Google Scholar] [CrossRef]
- Woods, G.L.; Walker, D.H. Detection of Infection or Infectious Agents by Use of Cytologic and Histologic Stains. Clin. Microbiol. Rev. 1996, 9, 382–404. [Google Scholar] [CrossRef]
- Damsgaard, T.E.; Olesen, A.B.; Sørensen, F.B.; Thestrup-Pedersen, K.; Schiøtz, P.O. Mast Cells and Atopic Dermatitis. Stereological Quantification of Mast Cells in Atopic Dermatitis and Normal Human Skin. Arch. Dermatol. Res. 1997, 289, 256–260. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lombardo, G.P.; Messina, E.; D’Angelo, R.; Cascio, P.L.; Sayed, R.K. Morpho-Structural Adaptations of the Integument in Different Aquatic Organisms. Acta Histochem. 2023, 125, 152031. [Google Scholar] [CrossRef] [PubMed]
- LaDouceur, E.E.B.; Wynne, J.; Garner, M.M.; Nyaoke, A.; Keel, M.K. Lesions of Copper Toxicosis in Captive Marine Invertebrates with Comparisons to Normal Histology. Vet. Pathol. 2016, 53, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.T.; Davis, A.S.; Law, J.M.; Lewbart, G.A.; Christian, L.S.; Harms, C.A. Gross and Histologic Evaluation of 5 Suture Materials in the Skin and Subcutaneous Tissue of the California Sea Hare (Aplysia Californica). J. Am. Assoc. Lab. Anim. Sci. 2010, 49, 64–68. [Google Scholar]
- Ponder, W.F.; Lindberg, D.R.; Ponder, J.M. Biology and Evolution of the Mollusca; CRC Press: Boca Raton, FL, USA, 2019; Volume 1. [Google Scholar]
- Cunha, C.M.; Simone, L.R.L. Morphological Re-Description of Aplysia Depilans (Gastropoda: Anaspidea): New Insights into the Anatomy of the Anaspideans. J. Mar. Biol. Assoc. UK 2019, 99, 595–610. [Google Scholar] [CrossRef]
- Yu, Y.; Cao, Y.; Xia, Y.; Liu, F. Wright–Giemsa Staining to Observe Phagocytes in Locusta Migratoria Infected with Metarhizium Acridum. J. Invertebr. Pathol. 2016, 139, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Shao, M.; Hokang, K. Classification of Haematopoietic Cells and Haemocytes in Chinese Prawn Fenneropenaeus Chinensis. Fish Shellfish Immunol. 2006, 21, 159–169. [Google Scholar] [CrossRef]
- Battison, A.; Cawthorn, R.; Horney, B. Classification of Homarus Americanus Hemocytes and the Use of Differential Hemocyte Counts in Lobsters Infected with Aerococcus Viridans Var. Homari (Gaffkemia). J. Invertebr. Pathol. 2003, 84, 177–197. [Google Scholar] [CrossRef]
- Cima, F.; Matozzo, V.; Marin, M.G.; Ballarin, L. Haemocytes of the Clam Tapes Philippinarum (Adams & Reeve, 1850): Morphofunctional Characterisation. Fish Shellfish Immunol. 2000, 10, 677–693. [Google Scholar] [CrossRef]
- Jeyachandran, S.; Park, K.; Kwak, I.; Baskaralingam, V. Morphological and Functional Characterization of Circulating Hemocytes Using Microscopy Techniques. Microsc. Res. Tech. 2020, 83, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Vommaro, M.L.; Kurtz, J.; Giglio, A. Morphological Characterisation of Haemocytes in the Mealworm Beetle Tenebrio Molitor (Coleoptera, Tenebrionidae). Insects 2021, 12, 423. [Google Scholar] [CrossRef] [PubMed]
- Impellitteri, F.; Curpăn, A.-S.; Plăvan, G.; Ciobica, A.; Faggio, C. Hemocytes: A Useful Tool for Assessing the Toxicity of Microplastics, Heavy Metals, and Pesticides on Aquatic Invertebrates. Int. J. Environ. Res. Public Health 2022, 19, 16830. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Fumia, A.; Albano, M.; Messina, E.; D’Angelo, R.; Mangano, A.; Miller, A.; Spanò, N.; Savoca, S.; Capillo, G. Investigating the Internal System of Defense of Gastropoda Aplysia Depilans (Gmelin, 1791): Focus on Hemocytes. Fish Shellfish Immunol. 2023, 137, 108791. [Google Scholar] [CrossRef]
- Frazier, W.T.; Kandel, E.R.; Kupfermann, I.; Waziri, R.; Coggeshall, R.E. Morphological and Functional Properties of Identified Neurons in the Abdominal Ganglion of Aplysia Californica. J. Neurophysiol. 1967, 30, 1288–1351. [Google Scholar] [CrossRef]
- Kriegstein, A.R. Development of the Nervous System of Aplysia Californica. Proc. Natl. Acad. Sci. USA 1977, 74, 375–378. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Fumia, A.; Albano, M.; Messina, E.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Coelomocytes of the Oligochaeta Earthworm Lumbricus Terrestris (Linnaeus, 1758) as Evolutionary Key of Defense: A Morphological Study. Zool. Lett. 2023, 9, 5. [Google Scholar] [CrossRef]
- Minc-Golomb, D.; Tsarfaty, I.; Schwartz, J.P. Expression of Inducible Nitric Oxide Synthase by Neurones Following Exposure to Endotoxin and Cytokine. Br. J. Pharmacol. 1994, 112, 720–722. [Google Scholar] [CrossRef]
- Franchini, A.; Conte, A.; Ottaviani, E. Nitric Oxide: An Ancestral Immunocyte Effector Molecule. Adv. Neuroimmunol. 1995, 5, 463–478. [Google Scholar] [CrossRef]
- Zahoor, Z.; Davies, A.J.; Kirk, R.S.; Rollinson, D.; Walker, A.J. Nitric Oxide Production by Biomphalaria Glabrata Haemocytes: Effects of Schistosoma Mansoni ESPs and Regulation through the Extracellular Signal-Regulated Kinase Pathway. Parasites Vectors 2009, 2, 18. [Google Scholar] [CrossRef]
- Wright, B.; Lacchini, A.H.; Davies, A.J.; Walker, A.J. Regulation of Nitric Oxide Production in Snail (Lymnaea Stagnalis) Defence Cells: A Role for PKC and ERK Signalling Pathways. Biol. Cell 2006, 98, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Kämpfer, H.; Wetzler, C.; Pfeilschifter, J. Nitric Oxide Drives Skin Repair: Novel Functions of an Established Mediator. Kidney Int. 2002, 61, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Stallmeyer, B.; Kämpfer, H.; Kolb, N.; Pfeilschifter, J.; Frank, S. The Function of Nitric Oxide in Wound Repair: Inhibition of Inducible Nitric Oxide-Synthase Severely Impairs Wound Reepithelialization. J. Investig. Dermatol. 1999, 113, 1090–1098. [Google Scholar] [CrossRef] [PubMed]
- Cals-Grierson, M.-M.; Ormerod, A.D. Nitric Oxide Function in the Skin. Nitric Oxide 2004, 10, 179–193. [Google Scholar] [CrossRef]
- Mansfield, K.; Naik, S. Unraveling Immune-Epithelial Interactions in Skin Homeostasis and Injury. Yale J. Biol. Med. 2020, 93, 133–143. [Google Scholar] [PubMed]
- Alesci, A.; Lauriano, E.R.; Fumia, A.; Irrera, N.; Mastrantonio, E.; Vaccaro, M.; Gangemi, S.; Santini, A.; Cicero, N.; Pergolizzi, S. Relationship between Immune Cells, Depression, Stress, and Psoriasis: Could the Use of Natural Products Be Helpful? Molecules 2022, 27, 1953. [Google Scholar] [CrossRef]
- Zheng, L.; Zhang, L.; Lin, H.; McIntosh, M.T.; Malacrida, A.R. Toll-like Receptors in Invertebrate Innate Immunity. Invertebr. Surviv. J. 2005, 2, 105–113. [Google Scholar]
- Chou, S.-T.; Lin, T.-M.; Yang, H.-Y.; Fugmann, S.D. Functional Characterization of the MyD88 Homologs in Strongylocentrotus Purpuratus. Dev. Comp. Immunol. 2023, 139, 104580. [Google Scholar] [CrossRef]
- Zhang, L.; Li, L.; Zhang, G. A Crassostrea Gigas Toll-like Receptor and Comparative Analysis of TLR Pathway in Invertebrates. Fish Shellfish Immunol. 2011, 30, 653–660. [Google Scholar] [CrossRef]
- Alesci, A.; Albano, M.; Fumia, A.; Messina, E.; Miller, A.; Di Fresco, D.; de Oliveira Fernandes, J.M.; Spanò, N.; Savoca, S.; Capillo, G. Shell Formation in Two Species of Bivalves: The Role of Mantle Cells and Haemocytes. Zool. J. Linn. Soc. 2023, zlad099. [Google Scholar] [CrossRef]
- Alesci, A.; Di Paola, D.; Fumia, A.; Marino, S.; D’Iglio, C.; Famulari, S.; Albano, M.; Spanò, N.; Lauriano, E.R. Internal Defense System of Mytilus Galloprovincialis (Lamarck, 1819): Ecological Role of Hemocytes as Biomarkers for Thiacloprid and Benzo[a]Pyrene Pollution. Toxics 2023, 11, 731. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Pergolizzi, S.; Lo Cascio, P.; Capillo, G.; Lauriano, E.R. Localization of Vasoactive Intestinal Peptide and Toll-like Receptor 2 Immunoreactive Cells in Endostyle of Urochordate Styela Plicata (Lesueur, 1823). Microsc. Res. Tech. 2022, 85, 2651–2658. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Savoca, S.; Fumia, A.; Mangano, A.; Albano, M.; Messina, E.; Aragona, M.; Lo Cascio, P.; Capillo, G. Detecting Intestinal Goblet Cells of the Broadgilled Hagfish Eptatretus Cirrhatus (Forster, 1801): A Confocal Microscopy Evaluation. Biology 2022, 11, 1366. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.R.; Pergolizzi, S.; Aragona, M.; Montalbano, G.; Guerrera, M.C.; Crupi, R.; Faggio, C.; Capillo, G. Intestinal Immunity of Dogfish Scyliorhinus Canicula Spiral Valve: A Histochemical, Immunohistochemical and Confocal Study. Fish Shellfish Immunol. 2019, 87, 490–498. [Google Scholar] [CrossRef]
- Alesci, A.; Pergolizzi, S.; Fumia, A.; Calabrò, C.; Lo Cascio, P.; Lauriano, E.R. Mast Cells in Goldfish (Carassius Auratus) Gut: Immunohistochemical Characterization. Acta Zool. 2023, 104, 366–379. [Google Scholar] [CrossRef]
- Alesci, A.; Capillo, G.; Fumia, A.; Messina, E.; Albano, M.; Aragona, M.; Lo Cascio, P.; Spanò, N.; Pergolizzi, S.; Lauriano, E.R. Confocal Characterization of Intestinal Dendritic Cells from Myxines to Teleosts. Biology 2022, 11, 1045. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Capillo, G.; Mokhtar, D.M.; Fumia, A.; D’Angelo, R.; Lo Cascio, P.; Albano, M.; Guerrera, M.C.; Sayed, R.K.; Spanò, N. Expression of Antimicrobic Peptide Piscidin1 in Gills Mast Cells of Giant Mudskipper Periophthalmodon Schlosseri (Pallas, 1770). Int. J. Mol. Sci. 2022, 23, 13707. [Google Scholar] [CrossRef] [PubMed]
- Alesci, A.; Albano, M.; Savoca, S.; Mokhtar, D.M.; Fumia, A.; Aragona, M.; Lo Cascio, P.; Hussein, M.M.; Capillo, G.; Pergolizzi, S. Confocal Identification of Immune Molecules in Skin Club Cells of Zebrafish (Danio Rerio, Hamilton 1882) and Their Possible Role in Immunity. Biology 2022, 11, 1653. [Google Scholar] [CrossRef]
- Lauriano, E.R.; Silvestri, G.; Kuciel, M.; Żuwala, K.; Zaccone, D.; Palombieri, D.; Alesci, A.; Pergolizzi, S. Immunohistochemical Localization of Toll-like Receptor 2 in Skin Langerhans’ Cells of Striped Dolphin (Stenella Coeruleoalba). Tissue Cell 2014, 46, 113–121. [Google Scholar] [CrossRef]
- Gerdol, M.; Venier, P.; Pallavicini, A. The Genome of the Pacific Oyster Crassostrea Gigas Brings New Insights on the Massive Expansion of the C1q Gene Family in Bivalvia. Dev. Comp. Immunol. 2015, 49, 59–71. [Google Scholar] [CrossRef]
- Ren, Q.; Lan, J.-F.; Zhong, X.; Song, X.-J.; Ma, F.; Hui, K.-M.; Wang, W.; Yu, X.-Q.; Wang, J.-X. A Novel Toll like Receptor with Two TIR Domains (HcToll-2) Is Involved in Regulation of Antimicrobial Peptide Gene Expression of Hyriopsis Cumingii. Dev. Comp. Immunol. 2014, 45, 198–208. [Google Scholar] [CrossRef] [PubMed]
- Elvitigala, D.A.S.; Premachandra, H.K.A.; Whang, I.; Nam, B.-H.; Lee, J. Molecular Insights of the First Gastropod TLR Counterpart from Disk Abalone (Haliotis Discus Discus), Revealing Its Transcriptional Modulation under Pathogenic Stress. Fish Shellfish Immunol. 2013, 35, 334–342. [Google Scholar] [CrossRef]
- Pila, E.A.; Tarrabain, M.; Kabore, A.L.; Hanington, P.C. A Novel Toll-like Receptor (TLR) Influences Compatibility between the Gastropod Biomphalaria Glabrata, and the Digenean Trematode Schistosoma Mansoni. PLoS Pathog. 2016, 12, e1005513. [Google Scholar] [CrossRef] [PubMed]
- Breviario, D.; Gianì, S.; Morello, L. Multiple Tubulins: Evolutionary Aspects and Biological Implications. Plant J. 2013, 75, 202–218. [Google Scholar] [CrossRef]
- Breuss, M.W.; Leca, I.; Gstrein, T.; Hansen, A.H.; Keays, D.A. Tubulins and Brain Development—The Origins of Functional Specification. Mol. Cell. Neurosci. 2017, 84, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, B.B.; Gioio, A.E.; Capano, C.P.; Crispino, M.; Giuditta, A. β-Actin and β-Tubulin Are Components of a Heterogeneous mRNA Population Present in the Squid Giant Axon. Mol. Cell. Neurosci. 1992, 3, 133–144. [Google Scholar] [CrossRef]
- Nejatbakhsh, N.; Guo, C.-H.; Lu, T.Z.; Pei, L.; Smit, A.B.; Sun, H.-S.; Van Kesteren, R.E.; Feng, Z.-P. Caltubin, a Novel Molluscan Tubulin-Interacting Protein, Promotes Axonal Growth and Attenuates Axonal Degeneration of Rodent Neurons. J. Neurosci. 2011, 31, 15231–15244. [Google Scholar] [CrossRef]
- Ludueña, R.F. Possible Roles of Specific Amino Acids in β-Tubulin Isotypes in the Growth and Maintenance of Neurons: Novel Insights From Cephalopod Mollusks. Front. Mol. Neurosci. 2022, 15, 838393. [Google Scholar] [CrossRef]
- Moccia, R.; Chen, D.; Lyles, V.; Kapuya, E.; Yaping, E.; Kalachikov, S.; Spahn, C.M.T.; Frank, J.; Kandel, E.R.; Barad, M.; et al. An Unbiased cDNA Library Prepared from Isolated Aplysia Sensory Neuron Processes Is Enriched for Cytoskeletal and Translational mRNAs. J. Neurosci. 2003, 23, 9409–9417. [Google Scholar] [CrossRef]
- Kidd, M.E.; Shumaker, D.K.; Ridge, K.M. The Role of Vimentin Intermediate Filaments in the Progression of Lung Cancer. Am. J. Respir. Cell Mol. Biol. 2014, 50, 1–6. [Google Scholar] [CrossRef]
- Chen, Z.; Fang, Z.; Ma, J. Regulatory Mechanisms and Clinical Significance of Vimentin in Breast Cancer. Biomed. Pharmacother. 2021, 133, 111068. [Google Scholar] [CrossRef] [PubMed]
- Kuna, A.T. Mutated Citrullinated Vimentin Antibodies in Rheumatoid Arthritis. Clin. Chim. Acta 2012, 413, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Musaelyan, A.; Lapin, S.; Nazarov, V.; Tkachenko, O.; Gilburd, B.; Mazing, A.; Mikhailova, L.; Shoenfeld, Y. Vimentin as Antigenic Target in Autoimmunity: A Comprehensive Review. Autoimmun. Rev. 2018, 17, 926–934. [Google Scholar] [CrossRef]
- Ivaska, J.; Pallari, H.-M.; Nevo, J.; Eriksson, J.E. Novel Functions of Vimentin in Cell Adhesion, Migration, and Signaling. Exp. Cell Res. 2007, 313, 2050–2062. [Google Scholar] [CrossRef]
- Patteson, A.E.; Vahabikashi, A.; Goldman, R.D.; Janmey, P.A. Mechanical and Non-Mechanical Functions of Filamentous and Non-Filamentous Vimentin. Bioessays 2020, 42, 2000078. [Google Scholar] [CrossRef] [PubMed]
- Ridge, K.M.; Eriksson, J.E.; Pekny, M.; Goldman, R.D. Roles of Vimentin in Health and Disease. Genes Dev. 2022, 36, 391–407. [Google Scholar] [CrossRef]
- Menet, V.; Prieto, M.; Privat, A.; Ribotta, M.G. y Axonal Plasticity and Functional Recovery after Spinal Cord Injury in Mice Deficient in Both Glial Fibrillary Acidic Protein and Vimentin Genes. Proc. Natl. Acad. Sci. USA 2003, 100, 8999–9004. [Google Scholar] [CrossRef] [PubMed]
- Toyooka, T.; Nawashiro, H.; Shinomiya, N.; Shima, K. Down-Regulation of Glial Fibrillary Acidic Protein and Vimentin by RNA Interference Improves Acute Urinary Dysfunction Associated with Spinal Cord Injury in Rats. J. Neurotrauma 2011, 28, 607–618. [Google Scholar] [CrossRef]
- Desclaux, M.; Perrin, F.E.; Do-Thi, A.; Prieto-Cappellini, M.; Gimenez, Y.; Ribotta, M.; Mallet, J.; Privat, A. Lentiviral-Mediated Silencing of Glial Fibrillary Acidic Protein and Vimentin Promotes Anatomical Plasticity and Functional Recovery after Spinal Cord Injury: Glial Scar Suppression in Mouse SCI. J. Neurosci. Res. 2015, 93, 43–55. [Google Scholar] [CrossRef]
- Ludin, B.; Matus, A. The Neuronal Cytoskeleton and Its Role in Axonal and Dendritic Plasticity. Hippocampus 1993, 3, 61–71. [Google Scholar] [CrossRef]
- Shigyo, M.; Tohda, C. Extracellular Vimentin Is a Novel Axonal Growth Facilitator for Functional Recovery in Spinal Cord-Injured Mice. Sci. Rep. 2016, 6, 28293. [Google Scholar] [CrossRef] [PubMed]
- Morrow, C.S.; Porter, T.J.; Xu, N.; Arndt, Z.P.; Ako-Asare, K.; Heo, H.J.; Thompson, E.A.; Moore, D.L. Vimentin Coordinates Protein Turnover at the Aggresome during Neural Stem Cell Quiescence Exit. Cell Stem Cell 2020, 26, 558–568. [Google Scholar] [CrossRef] [PubMed]
- Kron, N.S. In Search of the Aplysia Immunome: An In Silico Study. BMC Genom. 2022, 23, 543. [Google Scholar] [CrossRef] [PubMed]
- Greer, J.B.; Schmale, M.C.; Fieber, L.A. Whole-Transcriptome Changes in Gene Expression Accompany Aging of Sensory Neurons in Aplysia Californica. BMC Genom. 2018, 19, 529. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.B.; Pereira, D.M.; Jiménez, C.; Rodríguez, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentão, P. Anti-Inflammatory Effects of 5α,8α-Epidioxycholest-6-En-3β-Ol, a Steroidal Endoperoxide Isolated from Aplysia Depilans, Based on Bioguided Fractionation and NMR Analysis. Mar. Drugs 2019, 17, 330. [Google Scholar] [CrossRef]
- Bodnárová, M.; Martásek, P.; Moroz, L.L. Calcium/Calmodulin-Dependent Nitric Oxide Synthase Activity in the CNS of Aplysia Californica: Biochemical Characterization and Link to cGMP Pathways. J. Inorg. Biochem. 2005, 99, 922–928. [Google Scholar] [CrossRef]
- Perlson, E.; Hanz, S.; Ben-Yaakov, K.; Segal-Ruder, Y.; Seger, R.; Fainzilber, M. Vimentin-Dependent Spatial Translocation of an Activated MAP Kinase in Injured Nerve. Neuron 2005, 45, 715–726. [Google Scholar] [CrossRef]

| Primary Antibody | Supplier | Catalogue Number | Source | Dilution |
| Anti-iNOS | Santa Cruz Biotechnology, Inc., Dallas, TX, USA | sc-7271 | Mouse | 1:200 |
| Anti-TLR2 | Active Motif, La Hulpe, Belgium | 40981 | Rabbit | 1:100 |
| Anti-α-Tubulin | Sigma-Aldrich, Saint Louis, MO, USA | T6793 | Mouse | 1:200 |
| Anti-Vimentin | Sigma-Aldrich, Saint Louis, MO, USA | SAB1305445 | Rabbit | 1:150 |
| Secondary antibody | Supplier | Catalogue number | Source | Dilution |
| Alexa Fluor 488 Donkey anti-Mouse IgG FITC conjugated | Molecular Probes, Invitrogen, Waltham, Massachusetts, USA | A-21202 | Donkey | 1:300 |
| Alexa Fluor 594 Donkey anti-Rabbit IgG TRITC conjugated | Molecular Probes, Invitrogen, Waltham, Massachusetts, USA | A-21207 | Donkey | 1:300 |
| No. of Hemocytes per Slide | |
|---|---|
| iNOS+ | 347.83 ± 47.92 |
| TLR2+ | 387.32 ± 40.87 |
| iNOS+TLR2+ | 227.36 ± 25.36 |
| Vimentin+ | 325.28 ± 37.47 |
| iNOS+Vimentin+ | 233.47 ± 28.28 |
| α-Tubulin+ | 352.67 ± 38.11 |
| α-Tubulin+Vimentin+ | 216.30 ± 16.58 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alesci, A.; Fumia, A.; Mastrantonio, L.; Marino, S.; Miller, A.; Albano, M. Functional Adaptations of Hemocytes of Aplysia depilans (Gmelin, 1791) and Their Putative Role in Neuronal Regeneration. Fishes 2024, 9, 32. https://doi.org/10.3390/fishes9010032
Alesci A, Fumia A, Mastrantonio L, Marino S, Miller A, Albano M. Functional Adaptations of Hemocytes of Aplysia depilans (Gmelin, 1791) and Their Putative Role in Neuronal Regeneration. Fishes. 2024; 9(1):32. https://doi.org/10.3390/fishes9010032
Chicago/Turabian StyleAlesci, Alessio, Angelo Fumia, Lorenza Mastrantonio, Sebastian Marino, Anthea Miller, and Marco Albano. 2024. "Functional Adaptations of Hemocytes of Aplysia depilans (Gmelin, 1791) and Their Putative Role in Neuronal Regeneration" Fishes 9, no. 1: 32. https://doi.org/10.3390/fishes9010032
APA StyleAlesci, A., Fumia, A., Mastrantonio, L., Marino, S., Miller, A., & Albano, M. (2024). Functional Adaptations of Hemocytes of Aplysia depilans (Gmelin, 1791) and Their Putative Role in Neuronal Regeneration. Fishes, 9(1), 32. https://doi.org/10.3390/fishes9010032









