Growth and Oxidative Stress of Clownfish Amphiprion ocellaris Reared at Different Salinities
Abstract
1. Introduction
2. Materials and Methods
2.1. Larval Stage and Ethics
2.2. General Methodology
2.2.1. Water Quality Analysis
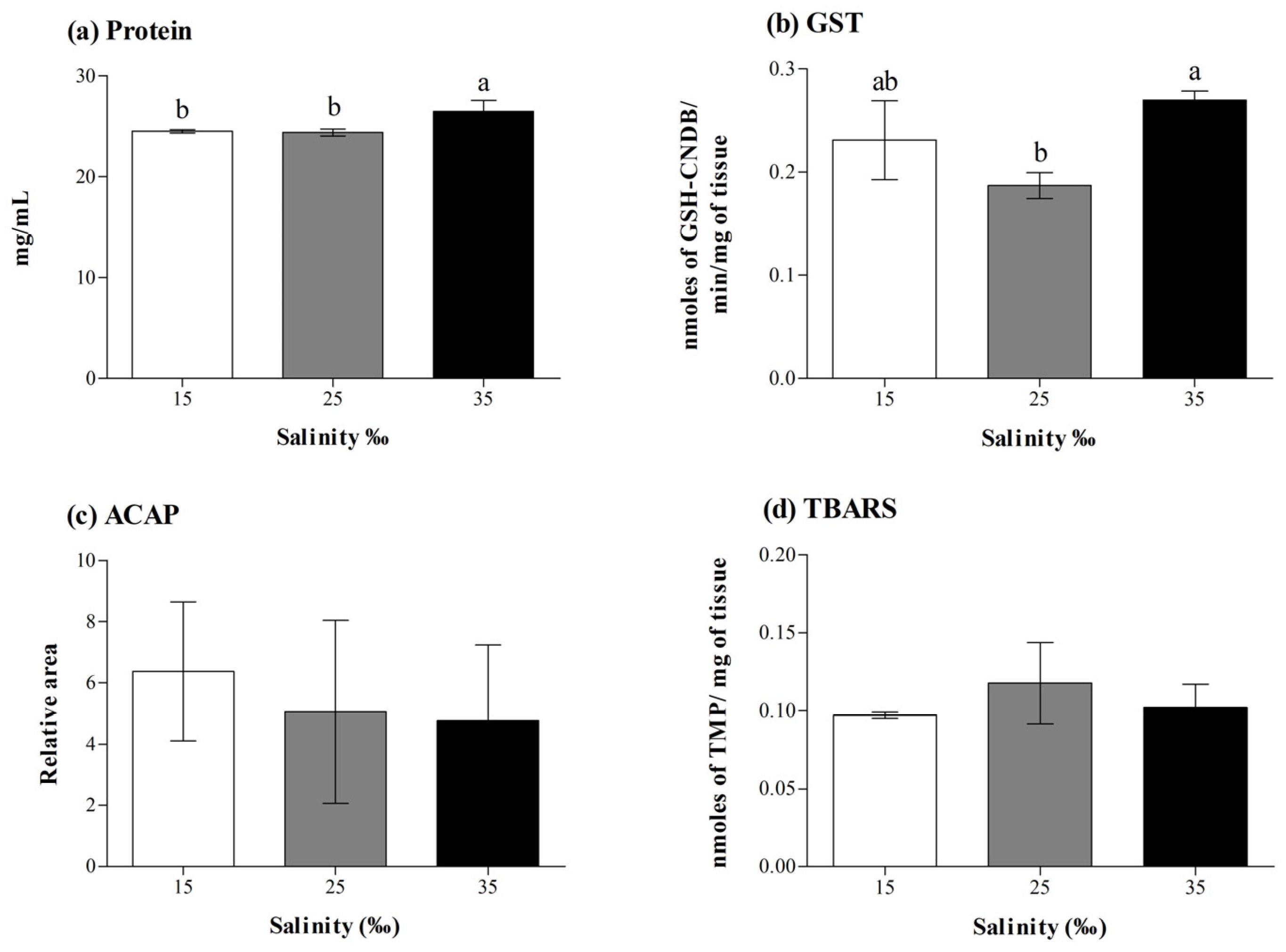
2.2.2. Whole-Body Oxidative Status
2.3. Experiments
2.3.1. Trial 1: Effect of Salinity on Survival, Growth, and Oxidative Status of A. ocellaris
2.3.2. Trial 2: Response of A. ocellaris to Acute Transference from Brackish to Seawater
2.4. Formulas and Statistics
2.4.1. Survival and Growth
2.4.2. Statiscal Analysis
3. Results
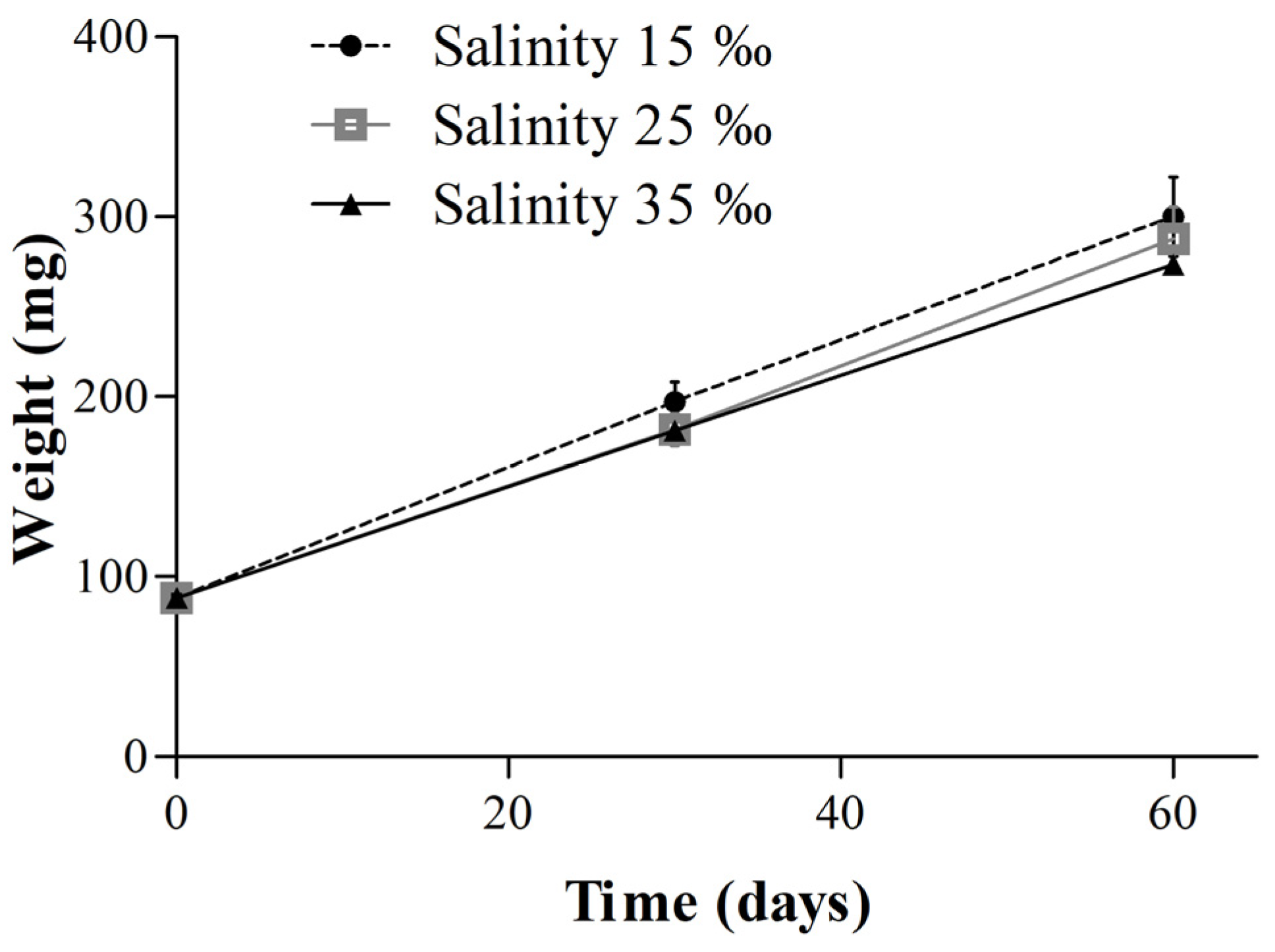
3.1. Effect of Salinity on Survival, Growth, and Oxidative Status of Juvenile Clownfish
3.2. Response of A. ocellaris to Acute Transference from Brackish Water to Seawater
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pouil, S.; Tlusty, M.F.; Rhyne, A.L. Aquaculture of Marine Ornamental Fish: Overview of the Production Trends and the Role of Academia in Research Progress. Rev. Aquac. 2019, 12, 1217–1230. [Google Scholar] [CrossRef]
- Rhyne, A.L.; Tlusty, M.F.; Schofield, P.J.; Kaufman, L.; Morris, J.A.; Bruckner, A.W. Revealing the Appetite of the Marine Aquarium Fish Trade: The Volume and Biodiversity of Fish Imported into the United States. PLoS ONE 2012, 7, e35808. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.F.; Behrens, M.D.; Max, L.M.; Daszak, P. US Drowning in Unidentified Fishes: Scope, Implications, and Regulation of Live Fish Import. Conserv. Lett. 2008, 1, 103–109. [Google Scholar] [CrossRef]
- Calado, R. The Need for Cultured Specimens. In Marine Ornamental Species Aquaculture; Wiley: Hoboken, NJ, USA, 2017; pp. 15–22. [Google Scholar] [CrossRef]
- Palmtag, M.R. The Marine Ornamental Species Trade. In Marine Ornamental Species Aquaculture; Wiley: Hoboken, NJ, USA, 2017; pp. 3–14. [Google Scholar] [CrossRef]
- Fautin, D.; Allen, G.R. Anemonefishes and Their Host Sea Anemones; Western Australian Museum: Perth, Australia, 1992.
- Drew, J.; Allen, G.R.; Kaufman, L.; Barber, P.H. Endemism and Regional Color and Genetic Differences in Five Putatively Cosmopolitan Reef Fishes. Conserv. Biol. 2008, 22, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Tlusty, M. The Benefits and Risks of Aquacultural Production for the Aquarium Trade. Aquaculture 2002, 205, 203–219. [Google Scholar] [CrossRef]
- Calado, R. Location. In Marine Ornamental Species Aquaculture; Wiley: Hoboken, NJ, USA, 2017; pp. 75–79. [Google Scholar] [CrossRef]
- Timmons, M.B.; Ebeling, J.M. Recirculating Aquaculture, 2nd ed.; Northeastern Regional Aquaculture Center: Ithaca, NY, USA, 2010; ISBN 9780971264625. [Google Scholar]
- Kültz, D. Physiological Mechanisms Used by Fish to Cope with Salinity Stress. J. Exp. Biol. 2015, 218, 1907–1914. [Google Scholar] [CrossRef] [PubMed]
- Bœuf, G.; Payan, P. How Should Salinity Influence Fish Growth? Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Mozanzadeh, M.T.; Safari, O.; Oosooli, R.; Mehrjooyan, S.; Najafabadi, M.Z.; Hoseini, S.J.; Saghavi, H.; Monem, J. The Effect of Salinity on Growth Performance, Digestive and Antioxidant Enzymes, Humoral Immunity and Stress Indices in Two Euryhaline Fish Species: Yellowfin Seabream (Acanthopagrus latus) and Asian Seabass (Lates calcarifer). Aquaculture 2021, 534, 736329. [Google Scholar] [CrossRef]
- Resley, M.J.; Webb, K.A., Jr.; Holt, G.J. Growth and Survival of Juvenile Cobia, Rachycentron canadum, at Different Salinities in a Recirculating Aquaculture System. Aquaculture 2006, 253, 398–407. [Google Scholar] [CrossRef]
- Mylonas, C.C.; Pavlidis, M.; Papandroulakis, N.; Zaiss, M.M.; Tsafarakis, D.; Papadakis, I.E.; Varsamos, S. Growth Performance and Osmoregulation in the Shi Drum (Umbrina cirrosa) Adapted to Different Environmental Salinities. Aquaculture 2009, 287, 203–210. [Google Scholar] [CrossRef]
- Dhaneesh, K.V.; Nanthini Devi, K.; Ajith Kumar, T.T.; Balasubramanian, T.; Tissera, K. Breeding, Embryonic Development and Salinity Tolerance of Skunk Clownfish Amphiprion akallopisos. J. King Saud Univ. Sci. 2012, 24, 201–209. [Google Scholar] [CrossRef]
- Dhaneesh, K.V.; Ajith Kumar, T.T.; Swagat, G.; Balasubramanian, T. Breeding and Mass Scale Rearing of Clownfish Amphiprion percula: Feeding and Rearing in Brackishwater. Chin. J. Oceanol. Limnol. 2012, 30, 528–534. [Google Scholar] [CrossRef]
- Park, M.S.; Shin, H.S.; Choi, C.Y.; Na Kim, N.; Park, D.-W.; Kil, G.-S.; Lee, J. Effect of Hypoosmotic and Thermal Stress on Gene Expression and the Activity of Antioxidant Enzymes in the Cinnamon Clownfish, Amphiprion Melanopus. Anim. Cells Syst. 2011, 15, 219–225. [Google Scholar] [CrossRef]
- Sies, H. Oxidative Stress: Concept and Some Practical Aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Lushchak, V.I. Contaminant-Induced Oxidative Stress in Fish: A Mechanistic Approach. Fish Physiol. Biochem. 2016, 42, 711–747. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.D.D.; García-Mesa, S.; Sampaio, L.A.; Planas, M. Primary, Secondary, and Tertiary Stress Responses of Juvenile Seahorse Hippocampus reidi Exposed to Acute Acid Stress in Brackish and Seawater. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2021, 255, 110592. [Google Scholar] [CrossRef]
- Martínez-Álvarez, R.M.; Hidalgo, M.C.; Domezain, A.; Morales, A.E.; García-Gallego, M.; Sanz, A. Physiological Changes of Sturgeon Acipenser naccarii Caused by Increasing Environmental Salinity. J. Exp. Biol. 2002, 205, 3699–3706. [Google Scholar] [CrossRef] [PubMed]
- Carneiro, M.D.D.; Garcí, S.; Sampaio, L.A.; Planas, M. Implications of Salinity and Acidic Environments on Fitness and Oxidative Stress Parameters in Early Developing Seahorses Hippocampus Reidi. Animals 2022, 12, 3227. [Google Scholar] [CrossRef]
- Zanette, J.; de Almeida, E.A.; da Silva, A.Z.; Guzenski, J.; Ferreira, J.F.; Di Mascio, P.; Marques, M.R.F.; Bainy, A.C.D. Salinity Influences Glutathione S-Transferase Activity and Lipid Peroxidation Responses in the Crassostrea gigas Oyster Exposed to Diesel Oil. Sci. Total Environ. 2011, 409, 1976–1983. [Google Scholar] [CrossRef]
- Blanchette, B.; Feng, X.; Singh, B.R. Marine Glutathione S-Transferases. Mar. Biotechnol. 2007, 9, 513–542. [Google Scholar] [CrossRef]
- Amado, L.L.; Garcia, M.L.; Ramos, P.B.; Freitas, R.F.; Zafalon, B.; Ferreira, J.L.R.; Yunes, J.S.; Monserrat, J.M. A Method to Measure Total Antioxidant Capacity against Peroxyl Radicals in Aquatic Organisms: Application to Evaluate Microcystins Toxicity. Sci. Total Environ. 2009, 407, 2115–2123. [Google Scholar] [CrossRef]
- Evangelista, I.R.; dos Santos, L.N.; dos Santos, A.F.G.N. Influence of Salinity, Temperature and Photoperiod on Eye Asymmetry of Amphirion ocellaris Larvae. Aquaculture 2020, 521, 734976. [Google Scholar] [CrossRef]
- Carneiro, M.D.D.; Maltez, L.C.; Rodrigues, R.V.; Planas, M.; Sampaio, L.A. Does Acidification Lead to Impairments on Oxidative Status and Survival of Orange Clownfish Amphiprion percula Juveniles? Fish Physiol. Biochem. 2021, 47, 841–848. [Google Scholar] [CrossRef]
- Eaton, A.; Clesceri, L.; Greenberg, A. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- UNESCO. Chemical Methods for Use in Marine Environmental Monitoring. Manual and Guides 12; Intergovernamental Oceanographic Commissiony: Paris, France, 1983. [Google Scholar]
- Bendschneider, K.; Robinson, R. A New Spectrophotometric Method for the Determination of nitrite in Sea Water. J. Mar. Res. 1952, 11, 87–96. [Google Scholar]
- Aminot, A.; Chaussepied, M. Manuel Des Analyses Chimiques En Milieu Marin; Editions Jouve, CNEXO: Paris, France, 1983. [Google Scholar]
- Castro, C.; Pérez-Jiménez, A.; Guerreiro, I.; Peres, H.; Castro-Cunha, M.; Oliva-Teles, A. Effects of Temperature and Dietary Protein Level on Hepatic Oxidative Status of Senegalese Sole Juveniles (Solea senegalensis). Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W. Glutathione S-Transferases: The First Enzymatic Step in Mercapturic Acid Formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Oakes, K.D.; Van Der Kraak, G.J. Utility of the TBARS Assay in Detecting Oxidative Stress in White Sucker (Catostomus Commersoni) Populations Exposed to Pulp Mill Effluent. Aquat. Toxicol. 2003, 63, 447–463. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. The Use of Ranks to Avoid the Assumption of Normality Implicit in the Analysis of Variance. J. Am. Stat. Assoc. 1937, 32, 675–701. [Google Scholar] [CrossRef]
- Sneddon, L.U. Evolution of Nociception and Pain: Evidence from Fish Models. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 20190290. [Google Scholar] [CrossRef]
- Segner, H.; Sundh, H.; Buchmann, K.; Douxfils, J.; Sundell, K.S.; Mathieu, C.; Ruane, N.; Jutfelt, F.; Toften, H.; Vaughan, L. Health of Farmed Fish: Its Relation to Fish Welfare and Its Utility as Welfare Indicator. Fish Physiol. Biochem. 2012, 38, 85–105. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Cividanes da Hora, M.; Joyeux, J.-C.; Rodrigues, R.V.; de Sousa-Santos, L.P.; Gomes, L.C.; Tsuzuki, M.Y. Tolerance and Growth of the Longsnout Seahorse Hippocampus reidi at Different Salinities. Aquaculture 2016, 463, 1–6. [Google Scholar] [CrossRef]
- Imsland, A.K.; Foss, A.; Gunnarsson, S.; Berntssen, M.H.G.; FitzGerald, R.; Bonga, S.W.; Ham, E.V.; Nævdal, G.; Stefansson, S.O. The Interaction of Temperature and Salinity on Growth and Food Conversion in Juvenile Turbot (Scophthalmus maximus). Aquaculture 2001, 198, 353–367. [Google Scholar] [CrossRef]
- Hoff, F.H. Conditioning, Spawning and Rearing of Fish with Emphasis on Marine Clownfish; Aquaculture Consultants, Incorporated: Florida, FL, USA, 1996; ISBN 978-0966296013. [Google Scholar]
- Turcios, A.E.; Papenbrock, J. Sustainable Treatment of Aquaculture Effluents-What Can We Learn from the Past for the Future? Sustainability 2014, 6, 836–856. [Google Scholar] [CrossRef]
- Lim, L.C.; Dhert, P.; Sorgeloos, P. Recent Developments and Improvements in Ornamental Fish Packaging Systems for Air Transport. Aquac. Res. 2003, 34, 923–935. [Google Scholar] [CrossRef]
- Zeng, L.; Ai, C.X.; Wang, Y.H.; Zhang, J.S.; Wu, C.W. Abrupt Salinity Stress Induces Oxidative Stress via the Nrf2-Keap1 Signaling Pathway in Large Yellow Croaker Pseudosciaena crocea. Fish Physiol. Biochem. 2017, 43, 955–964. [Google Scholar] [CrossRef]
- Evans, T.G.; Kültz, D. The Cellular Stress Response in Fish Exposed to Salinity Fluctuations. J. Exp. Zool. Part A Ecol. Integr. Physiol. 2020, 333, 421–435. [Google Scholar] [CrossRef]

| Parameter | Salinity (‰) | |||
|---|---|---|---|---|
| 5 * | 15 | 25 | 35 | |
| Salinity (‰) | 5.2 ± 0.07 | 15.3 ± 0.03 | 25.4 ± 0.05 | 35.3 ± 0.06 |
| pH | 8.1 ± 0.04 | 8.1 ± 0.01 | 8.1 ± 0.01 | 8.0 ± 0.01 |
| Alkalinity (mg CaCO3/L) | 105.0 ± 5.0 | 152.0 ± 2.0 | 154.0 ± 2.0 | 153.0 ± 2.0 |
| Osmolality (mOsm/kg) | 128.8 ± 2.2 | 431.5 ± 13.5 | 764.5 ± 10.5 | 1060.5 ± 6.5 |
| Na+ (g/L) | 3.5 ± 0.03 | 7.2 ± 0.05 | 12.3 ± 0.1 | 15.5 ± 0.06 |
| Cl– (g/L) | 6.1 ± 0.02 | 8.2 ± 0.01 | 10.8 ± 0.03 | 12.7 ± 0.05 |
| K+ (mg/L) | 109.2 ± 0.8 | 198.8 ± 0.0 | 388.1 ± 4.9 | 430.9 ± 0.0 |
| Ca2+ (mg/L) | 115.1 ± 0.8 | 220.5 ± 1.6 | 353.0 ± 2.1 | 447.8 ± 2.7 |
| Parameter | Salinity (‰) | |
|---|---|---|
| 15 (15–15) | 35 (15–35) | |
| Salinity (‰) | 15.3 ± 0.07 | 35.2 ± 0.1 |
| pH | 8.2 ± 0.05 | 8.1 ± 0.04 |
| Alkalinity (mg CaCO3/L) | 153.0 ± 3.0 | 157.5 ± 2.0 |
| Osmolality (mOsm/kg) | 447.7 ± 8.1 | 1036 ± 12.3 |
| Na+ (g/L) | 7.8 ± 0.06 | 15.7 ± 0.2 |
| Cl− (g/L) | 8.3 ± 0.02 | 12.3 ± 0.02 |
| K+ (mg/L) | 198.5 ± 0.0 | 448.8 ± 5.1 |
| Ca2+ (mg/L) | 214.9 ± 0.9 | 434.9 ± 1.8 |
| Parameter | Salinity (‰) | |||
|---|---|---|---|---|
| 15 | 25 | 35 | p Value | |
| Survival (%) | 95.0 ± 4.4 | 97.0 ± 4.4 | 95.0 ± 4.4 | 0.73 |
| Length (mm) | 23.8 ± 0.6 | 23.4 ± 0.5 | 23.2 ± 0.5 | 0.11 |
| WG (mg) | 211.1 ± 14.4 | 208.9 ± 20.6 | 177.7 ± 9.8 | 0.31 |
| K | 4.4 ± 0.2 | 4.6 ± 0.1 | 4.5 ± 0.1 | 0.55 |
| FCR | 1.9 ± 0.1 | 1.4 ± 0.1 | 1.5 ± 0.01 | 0.06 |
| SGR (%/day) | 2.0 ± 0.1 | 2.1 ± 0.3 | 1.7 ± 0.05 | 0.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carneiro, M.D.D.; Medeiros, R.S.d.; Monserrat, J.M.; Rodrigues, R.V.; Sampaio, L.A. Growth and Oxidative Stress of Clownfish Amphiprion ocellaris Reared at Different Salinities. Fishes 2024, 9, 30. https://doi.org/10.3390/fishes9010030
Carneiro MDD, Medeiros RSd, Monserrat JM, Rodrigues RV, Sampaio LA. Growth and Oxidative Stress of Clownfish Amphiprion ocellaris Reared at Different Salinities. Fishes. 2024; 9(1):30. https://doi.org/10.3390/fishes9010030
Chicago/Turabian StyleCarneiro, Mario Davi Dias, Rafael Soriani de Medeiros, José Maria Monserrat, Ricardo Vieira Rodrigues, and Luís André Sampaio. 2024. "Growth and Oxidative Stress of Clownfish Amphiprion ocellaris Reared at Different Salinities" Fishes 9, no. 1: 30. https://doi.org/10.3390/fishes9010030
APA StyleCarneiro, M. D. D., Medeiros, R. S. d., Monserrat, J. M., Rodrigues, R. V., & Sampaio, L. A. (2024). Growth and Oxidative Stress of Clownfish Amphiprion ocellaris Reared at Different Salinities. Fishes, 9(1), 30. https://doi.org/10.3390/fishes9010030








