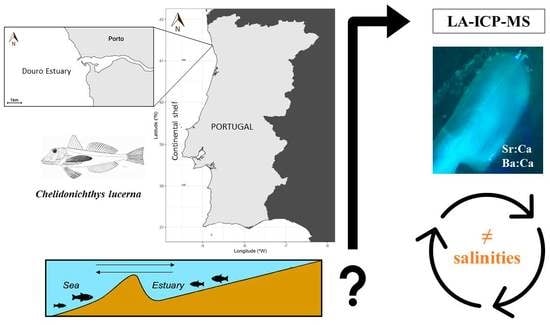
Is Chelidonichthys lucerna (Linnaeus, 1758) a Marine Estuarine-Dependent Fish? Insights from Saccular Otolith Microchemistry
Abstract
1. Introduction
2. Materials and Methods
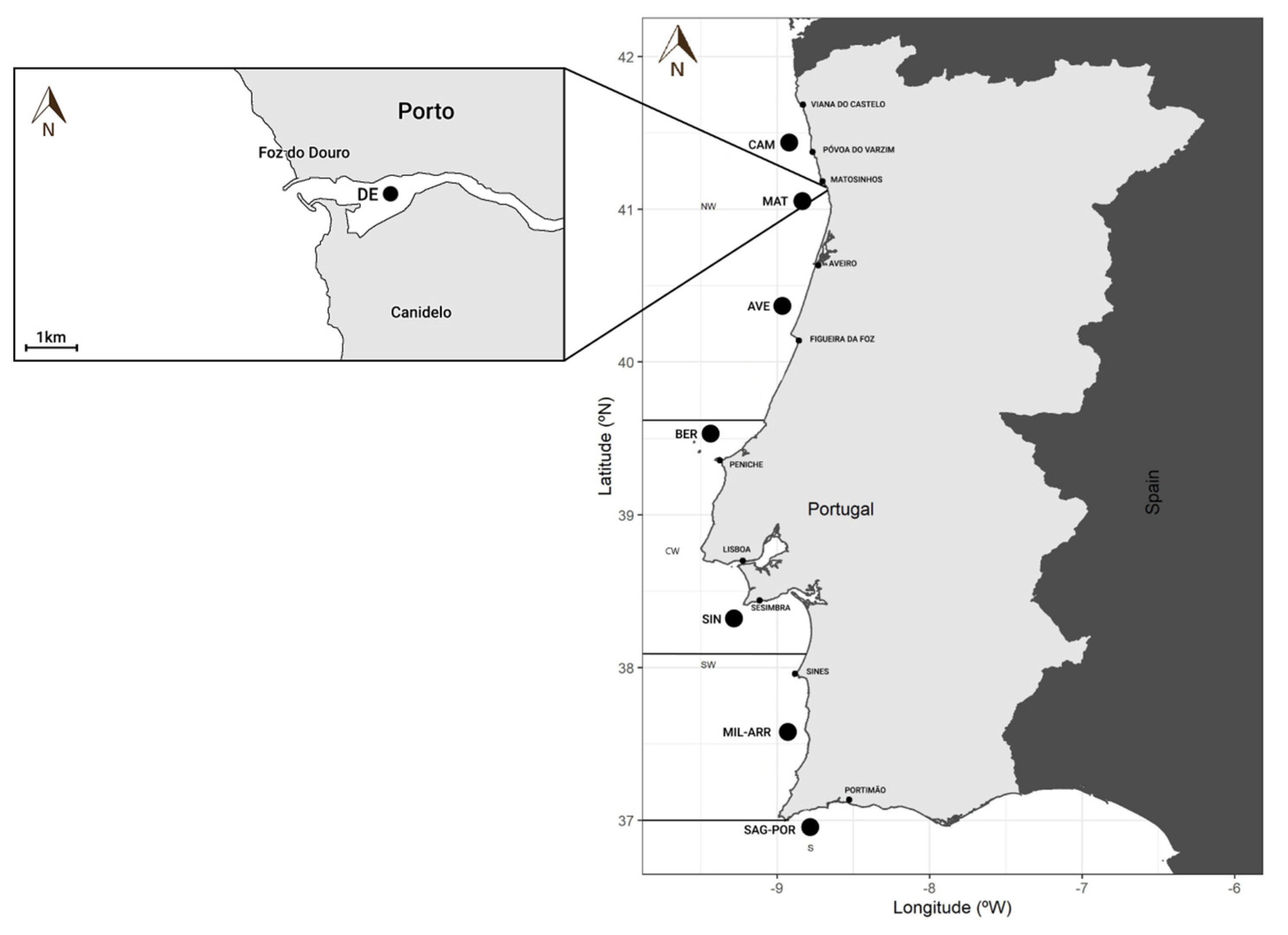
2.1. Fish Sampling
2.2. Otolith Preparation
2.3. Data Analysis
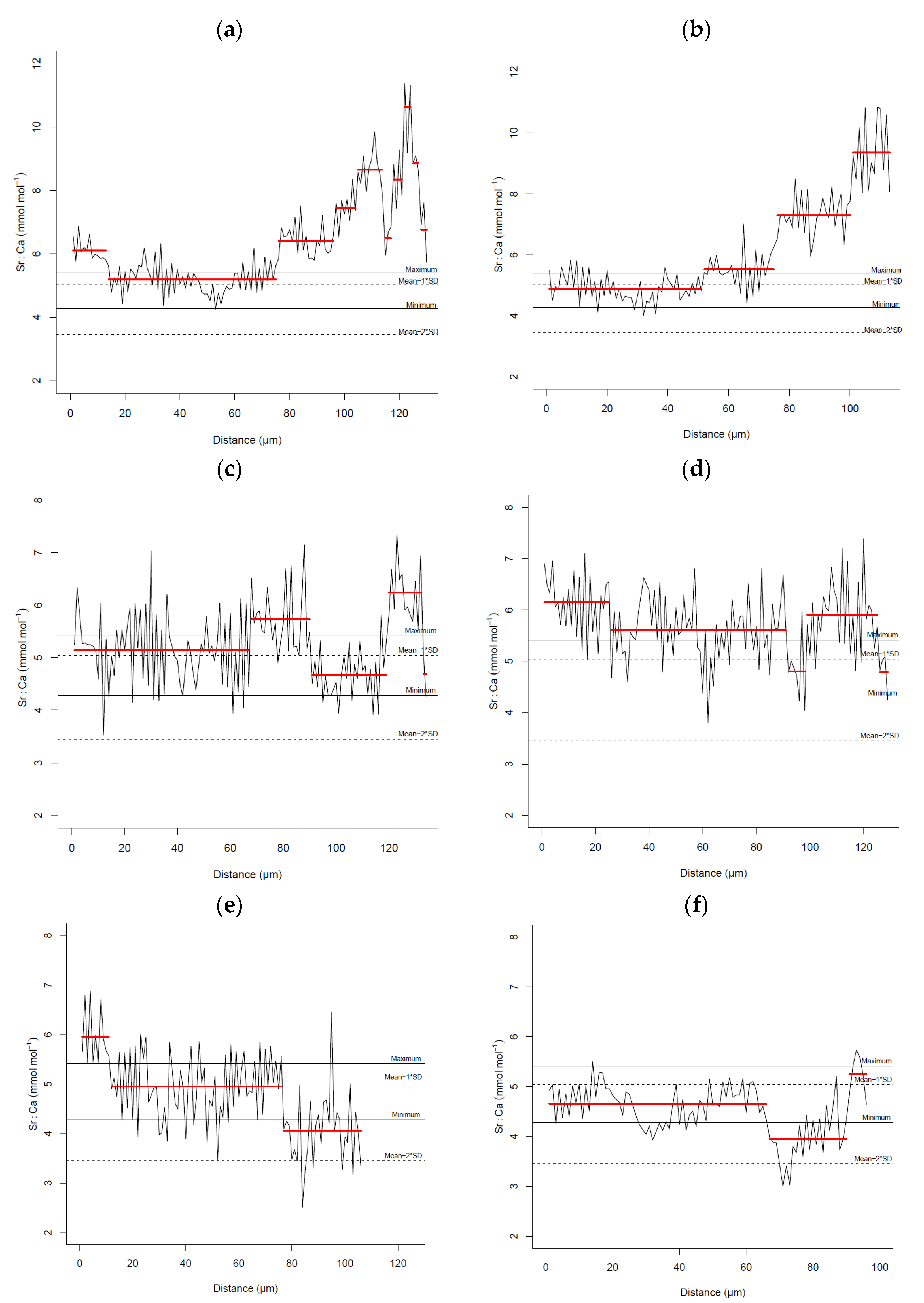
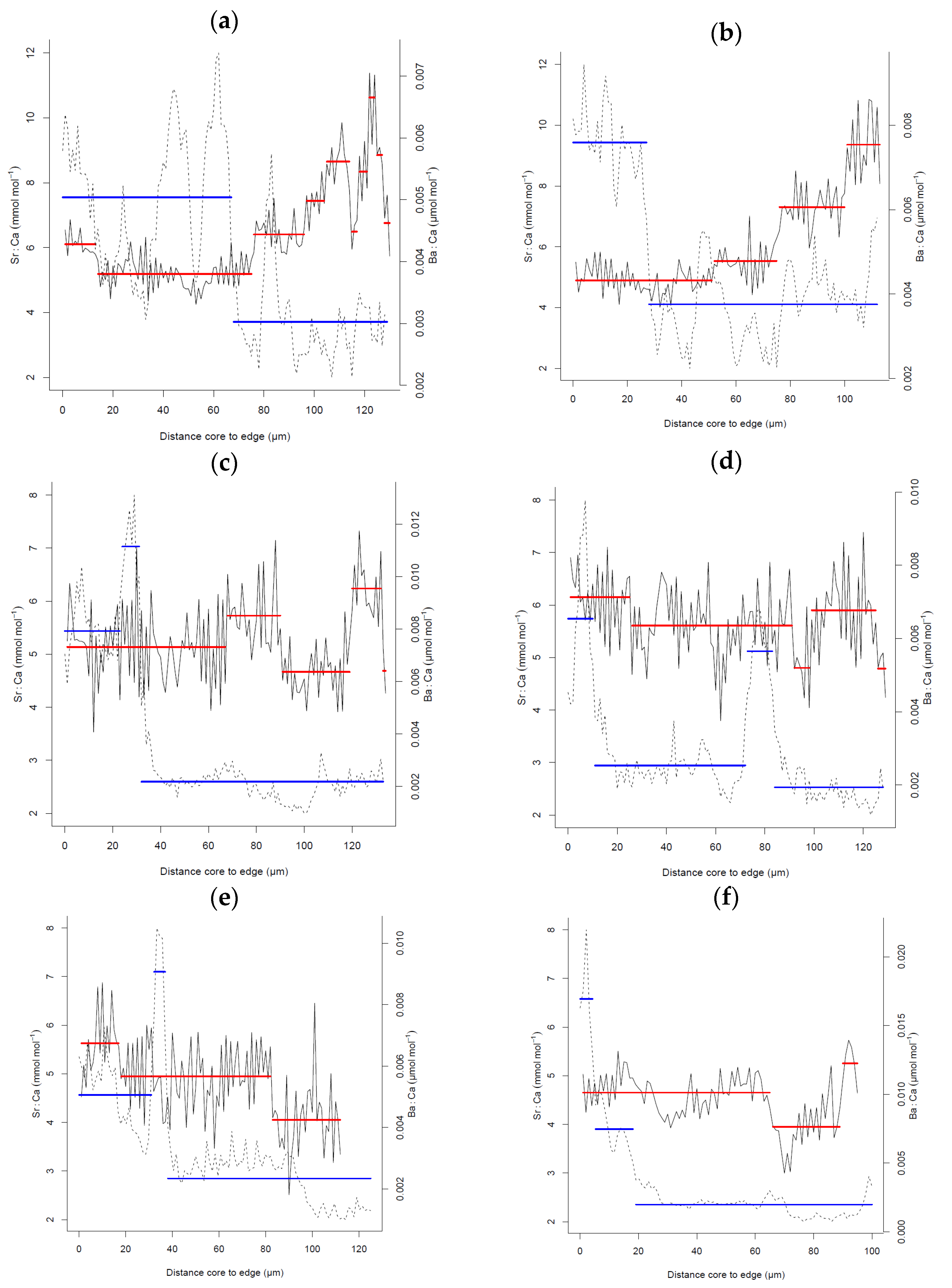
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dahlgren, C.; Kellison, G.; Adams, A.; Gillanders, B.; Kendall; Layman, C.; Ley, J.; Nagelkerken, I.; Serafy, J. Marine nurseries and effective juvenile habitats: Concepts and applications. Mar. Ecol. Prog. Ser. 2006, 312, 291–295. [Google Scholar] [CrossRef]
- Sharpe, C.; Carr-Harris, C.; Arbeider, M.; Wilson, S.M.; Moore, J.W. Estuary habitat associations for juvenile Pacific salmon and pelagic fish: Implications for coastal planning processes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1636–1656. [Google Scholar] [CrossRef]
- Arevalo, E.; Cabral, H.N.; Villeneuve, B.; Possémé, C.; Lepage, M. Fish larvae dynamics in temperate estuaries: A review on processes, patterns and factors that determine recruitment. Fish Fish. 2023, 24, 466–487. [Google Scholar] [CrossRef]
- Wilson, J. Productivity, Fisheries and Aquaculture in Temperate Estuaries. Estuarine, Coast. Shelf Sci. 2002, 55, 953–967. [Google Scholar] [CrossRef]
- Potter, I.C.; Warwick, R.M.; Hall, N.G.; Tweedley, J.R. The physico-chemical characteristics, biota and fisheries of estuaries. In Freshwater Fisheries Ecology; Wiley Blackwell: Chichester, UK, 2015; pp. 48–79. [Google Scholar] [CrossRef]
- Potter, I.C.; Tweedley, J.R.; Elliott, M.; Whitfield, A.K. The ways in which fish use estuaries: A refinement and expansion of the guild approach. Fish Fish. 2013, 16, 230–239. [Google Scholar] [CrossRef]
- Dando, P.R. Reproduction in Estuarine Fish. In Fish Reproduction: Strategies and Tactics; Academic Press: London, UK, 1984; pp. 155–170. [Google Scholar]
- James, N.C.; Leslie, T.D.; Potts, W.M.; Whitfield, A.K.; Rajkaran, A. The importance of different juvenile habitats as nursery areas for a ubiquitous estuarine-dependent marine fish species. Estuar. Coast. Shelf Sci. 2019, 226, 106270. [Google Scholar] [CrossRef]
- Soeth, M.; Spach, H.L.; Daros, F.A.; Castro, J.P.; Correia, A.T. Use of otolith elemental signatures to unravel lifetime movement patterns of Atlantic spadefish, Chaetodipterus faber, in the Southwest Atlantic Ocean. J. Sea Res. 2020, 158, 101873. [Google Scholar] [CrossRef]
- Bartes, S.; Simpfendorfer, C.; Walker, T.I.; King, C.; Loneragan, N.; Braccini, M. Conventional tagging of sharks in Western Australia: The main commercial species exhibit contrasting movement patterns. Mar. Freshw. Res. 2021, 72, 1643–1656. [Google Scholar] [CrossRef]
- Braun, C.D.; Gaube, P.; Afonso, P.; Fontes, J.; Skomal, G.B.; Thorrold, S.R. Assimilating electronic tagging, oceanographic modelling, and fisheries data to estimate movements and connectivity of swordfish in the North Atlantic. ICES J. Mar. Sci. 2019, 76, 2305–2317. [Google Scholar] [CrossRef]
- Popper, A.N.; Ramcharitar, J.; Campana, S.E. Why otoliths? Insights from inner ear physiology and fisheries biology. Mar. Freshw. Res. 2005, 56, 497–504. [Google Scholar] [CrossRef]
- Rodriguez-Mendoza, R. Otoliths and their Applications in Fishery Science. Croat. J. Fish. 2006, 64, 89–102. [Google Scholar]
- Volpedo, A.V.; Vaz-dos-Santos, A.M. Métodos de Estudios con Otolitos: Principios y Aplicaciones/Métodos de Estudos com Otólitos: Princípios e Aplicações, 1st ed.; CAFP-BA-PIESCI: Ciudad Autónoma de Buenos Aires, Argentina, 2015. [Google Scholar]
- Ibáñez, C.M.; Riera, R.; Leite, T.; Díaz-Santana-Iturrios, M.; Rosa, R.; Pardo-Gandarillas, M.C. Stomach content analysis in cephalopods: Past research, current challenges, and future directions. Rev. Fish Biol. Fish. 2021, 31, 505–522. [Google Scholar] [CrossRef]
- D’iglio, C.; Natale, S.; Albano, M.; Savoca, S.; Famulari, S.; Gervasi, C.; Lanteri, G.; Panarello, G.; Spanò, N.; Capillo, G. Otolith Analyses Highlight Morpho-Functional Differences of Three Species of Mullet (Mugilidae) from Transitional Water. Sustainability 2021, 14, 398. [Google Scholar] [CrossRef]
- Korostelev, N.; Frey, P.; Orlov, A. Using different hard structures to estimate the age of deep-sea fishes: A case study of the Pacific flatnose, Antimora microlepis (Moridae, Gadiformes, Teleostei). Fish. Res. 2020, 232, 105731. [Google Scholar] [CrossRef]
- Soeth, M.; Spach, H.L.; Daros, F.A.; Adelir-Alves, J.; de Almeida, A.C.O.; Correia, A.T. Stock structure of Atlantic spadefish Chaetodipterus faber from Southwest Atlantic Ocean inferred from otolith elemental and shape signatures. Fish. Res. 2018, 211, 81–90. [Google Scholar] [CrossRef]
- Schroeder, R.; Schwingel, P.R.; Correia, A.T. Population structure of the Brazilian sardine (Sardinella brasiliensis) in the Southwest Atlantic inferred from body morphology and otolith shape signatures. Hydrobiologia 2021, 849, 1367–1381. [Google Scholar] [CrossRef]
- Franco, A.; Franzoi, P.; Malavasi, S.; Riccato, F.; Torricelli, P.; Mainardi, D. Use of shallow water habitats by fish assemblages in a Mediterranean coastal lagoon. Estuar. Coast. Shelf Sci. 2006, 66, 67–83. [Google Scholar] [CrossRef]
- Daros, F.A.; Spach, H.L.; Correia, A.T. Habitat residency and movement patterns of Centropomus parallelus juveniles in a subtropical estuarine complex. J. Fish Biol. 2016, 88, 1796–1810. [Google Scholar] [CrossRef]
- Avigliano, E.; Leisen, M.; Romero, R.; Carvalho, B.; Velasco, G.; Vianna, M.; Barra, F.; Volpedo, A.V. Fluvio-marine travelers from South America: Cyclic amphidromy and freshwater residency, typical behaviors in Genidens barbus inferred by otolith chemistry. Fish. Res. 2017, 193, 184–194. [Google Scholar] [CrossRef]
- Ferreira, I.; Santos, D.; Moreira, C.; Feijó, D.; Rocha, A.; Correia, A.T. Population structure of Chelidonichthys lucerna in Portugal mainland using otolith shape and elemental signatures. Mar. Biol. Res. 2019, 15, 500–512. [Google Scholar] [CrossRef]
- Moura, A.; Muniz, A.; Mullis, E.; Wilson, J.; Vieira, R.; Almeida, A.; Pinto, E.; Brummer, G.; Gaever, P.; Gonçalves, J.; et al. Population structure and dynamics of the Atlantic mackerel (Scomber scombrus) in the North Atlantic inferred from otolith chemical and shape signatures. Fish. Res. 2020, 230, 105621. [Google Scholar] [CrossRef]
- Daros, F.A.; Spach, H.L.; Sial, A.N.; Correia, A.T. Otolith fingerprints of the coral reef fish Stegastes fuscus in southeast Brazil: A useful tool for population and connectivity studies. Reg. Stud. Mar. Sci. 2016, 3, 262–272. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Sial, A.; Caeiro, A.; Vaz-Pires, P.; Correia, A. Population structure of the blue jack mackerel (Trachurus picturatus) in the NE Atlantic inferred from otolith microchemistry. Fish. Res. 2018, 197, 113–122. [Google Scholar] [CrossRef]
- Correia, A.; Moura, A.; Triay-Portella, R.; Santos, P.; Pinto, E.; Almeida, A.; Sial, A.; Muniz, A. Population structure of the chub mackerel (Scomber colias) in the NE Atlantic inferred from otolith elemental and isotopic signatures. Fish. Res. 2020, 234, 105785. [Google Scholar] [CrossRef]
- Correia, A.; Pipa, T.; Gonçalves, J.; Erzini, K.; Hamer, P. Insights into population structure of Diplodus vulgaris along the SW Portuguese coast from otolith elemental signatures. Fish. Res. 2011, 111, 82–91. [Google Scholar] [CrossRef]
- Moreira, C.; Froufe, E.; Vaz-Pires, P.; Triay-Portella, R.; Méndez, A.; Castro, J.P.; Correia, A.T. Unravelling the spatial-temporal population structure of Trachurus picturatus across the North-East Atlantic using otolith fingerprinting. Estuar. Coast. Shelf Sci. 2022, 272, 107860. [Google Scholar] [CrossRef]
- Tabouret, H.; Bareille, G.; Claverie, F.; Pécheyran, C.; Prouzet, P.; Donard, O. Simultaneous use of strontium:calcium and barium:calcium ratios in otoliths as markers of habitat: Application to the European eel (Anguilla anguilla) in the Adour basin, South West France. Mar. Environ. Res. 2010, 70, 35–45. [Google Scholar] [CrossRef]
- Stanley, R.R.E.; Bradbury, I.R.; DiBacco, C.; Snelgrove, P.V.R.; Thorrold, S.R.; Killen, S.S. Environmentally mediated trends in otolith composition of juvenile Atlantic cod (Gadus morhua). ICES J. Mar. Sci. 2015, 72, 2350–2363. [Google Scholar] [CrossRef]
- Macdonald, J.; Crook, D. Variability in Sr:Ca and Ba:Ca ratios in water and fish otoliths across an estuarine salinity gradient. Mar. Ecol. Prog. Ser. 2010, 413, 147–161. [Google Scholar] [CrossRef]
- Menezes, R.; Moura, P.E.; Santos, A.C.; Moraes, L.E.; Condini, M.V.; Rosa, R.S.; Albuquerque, C.Q. Habitat use plasticity by the dog snapper (Lutjanus jocu) across the Abrolhos Bank shelf, eastern Brazil, inferred from otolith chemistry. Estuar. Coast. Shelf Sci. 2021, 263, 107637. [Google Scholar] [CrossRef]
- Gillanders, B.M. Otolith chemistry to determine movements of diadromous and freshwater fish. Aquat. Living Resour. 2005, 18, 291–300. [Google Scholar] [CrossRef]
- Walther, B.D.; Limburg, K.E. The use of otolith chemistry to characterize diadromous migrations. J. Fish Biol. 2012, 81, 796–825. [Google Scholar] [CrossRef]
- Brown, R.J.; Severin, K.P.; Martin, J.; Rougemont, Q.; Drouineau, H.; Launey, S.; Jatteau, P.; Bareille, G.; Berail, S.; Pécheyran, C.; et al. Otolith chemistry analyses indicate that water Sr:Ca is the primary factor influencing otolith Sr:Ca for freshwater and diadromous fish but not for marine fish. Can. J. Fish. Aquat. Sci. 2009, 66, 1790–1808. [Google Scholar] [CrossRef]
- Işmen, A.; Işmen, P.; Başusta, N. Age, Growth and Reproduction of Tub Gurnard (Chelidonichthys lucerna L. 1758) in the Bay of İskenderun in the Eastern Mediterranean. Turk. J. Vet. Anim. Sci. 2004, 28, 289–295. [Google Scholar]
- Quigley, D. Gurnards (Triglidae) in Irish and European Atlantic Seas. Sherkin Comment. 2005, p. 21. Available online: https://www.researchgate.net/publication/277305043 (accessed on 4 May 2023).
- FAO. Fisheries and Aquaculture Department, Species Fact Sheets, Chelidonichthys lucerna (Linnaeus, 1758). Rome. 2023. Available online: https://www.fao.org/figis/pdf/fishery/species/2530/en?title=FAO%20Fisheries%20%26%20Aquaculture%20-%20Species%20Fact%20Sheets%20-%20Chelidonichthys%20lucerna%20(Linnaeus%2C%201758) (accessed on 20 May 2023).
- Feijó, D.; Rocha, A.; Santos, P.; Saborido-Rey, F. Statistical Species characterization of Gurnard Landings in North of Portugal. Conference handbook (ICES CM 2008/K:15). In Proceedings of the ICES Annual Science Conference, Halifax, NS, Canada, 22–26 September 2008. [Google Scholar]
- Instituto Nacional de Estatística. Estatísticas da Pesca: 2021. Lisboa. 2022. Available online: https://www.ine.pt/xurl/pub/36828280 (accessed on 1 July 2023).
- Instituto Nacional de Estatística. Estatísticas da Pesca: 2022. Lisboa. 2023. Available online: https://www.ine.pt/xurl/pub/66322600 (accessed on 1 July 2023).
- Colloca, F.; Ardizzone, G.D.; Gravina, M.F. Trophic ecology of gurnards (Pisces: Triglidae) in the Central Mediterranean Sea. Mar. Life 1994, 4, 45–57. Available online: https://www.researchgate.net/publication/284081952 (accessed on 4 May 2023).
- Montanini, S.; Stagioni, M.; Benni, E.; Vallisneri, M. Feeding strategy and ontogenetic changes in diet of gurnards (Teleostea: Scorpaeniformes: Triglidae) from the Adriatic Sea. Eur. Zool. J. 2017, 84, 356–367. [Google Scholar] [CrossRef]
- El-Serafy, S.S.; El-Gammal, F.I.; Mehanna, S.F.; Abdel-Hamid, N.-A.H.; Farrag, E.-S.F. Age, Growth and Reproduction of the Tub Gurnard, Chelidonichthys lucerna (Linnaeus, 1758) from the Egyptian Mediterranean waters off, Alexandria. Int. J. Fish. Aquat. Sci. 2015, 4, 13–20. [Google Scholar] [CrossRef]
- McCarthy, I.D.; Marriott, A.L. Age, growth and maturity of tub gurnard (Chelidonichthys lucerna Linnaeus 1758; Triglidae) in the inshore coastal waters of Northwest Wales, UK. J. Appl. Ichthyol. 2018, 34, 581–589. [Google Scholar] [CrossRef]
- Campos, J.; Dias, S.C.; Bio, A.; Santos, P.T.; Jorge, I. Age and Growth of Tub Gurnard Chelidonichthys lucerna (Linnaeus, 1758) during Estuarine Occupation of a Temperate Atlantic Nursery. Int. J. Environ. Sci. Nat. Resour. 2022, 31, 1–14. [Google Scholar] [CrossRef]
- Azevedo, I.C.; Bordalo, A.A.; Duarte, P. Influence of freshwater inflow variability on the Douro estuary primary productivity: A modelling study. Ecol. Model. 2014, 272, 1–15. [Google Scholar] [CrossRef]
- Rodrigues, S.M.; Silva, D.; Cunha, J.; Pereira, R.; Freitas, V.; Ramos, S. Environmental influences, particularly river flow alteration, on larval fish assemblages in the Douro Estuary, Portugal. Reg. Stud. Mar. Sci. 2022, 56, 102617. [Google Scholar] [CrossRef]
- Ambar, I.; Serra, N.; Brogueira, M.; Cabeçadas, G.; Abrantes, F.; Freitas, P.; Gonçalves, C.; Gonzalez, N. Physical, chemical and sedimentological aspects of the Mediterranean outflow off Iberia. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2002, 49, 4163–4177. [Google Scholar] [CrossRef]
- Correia, A.T.; Ramos, A.A.; Barros, F.; Silva, G.; Hamer, P.; Morais, P.; Cunha, R.L.; Castilho, R. Population structure and connectivity of the European conger eel (Conger conger) across the north-eastern Atlantic and western Mediterranean: Integrating molecular and otolith elemental approaches. Mar. Biol. 2012, 159, 1509–1525. [Google Scholar] [CrossRef]
- Correia, A.; Hamer, P.; Carocinho, B.; Silva, A. Evidence for meta-population structure of Sardina pilchardus in the Atlantic Iberian waters from otolith elemental signatures of a strong cohort. Fish. Res. 2013, 149, 76–85. [Google Scholar] [CrossRef]
- Campana, S.E. Chemistry and composition of fish otoliths:pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef]
- Sirot, C.; Ferraton, F.; Panfili, J.; Childs, A.; Guilhaumon, F.; Darnaude, A. elementr: An R package for reducing elemental data from LA-ICPMS analysis of biological calcified structures. Methods Ecol. Evol. 2017, 8, 1659–1667. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria. 2023. Available online: https://www.r-project.org/ (accessed on 6 June 2023).
- Secor, D.H.; Rooker, J.R. Is otolith strontium a useful scalar of life cycles in estuarine fishes? Fish. Res. 2000, 46, 359–371. [Google Scholar] [CrossRef]
- Martinho, F.; Pina, B.; Nunes, M.; Vasconcelos, R.P.; Fonseca, V.; Crespo, D.; Primo, A.; Vaz, A.; Pardal, M.A.; Gillanders, B.M.; et al. Water and Otolith Chemistry: Implications for Discerning Estuarine Nursery Habitat Use of a Juvenile Flatfish. Front. Mar. Sci. 2020, 7, 347. [Google Scholar] [CrossRef]
- Papaconstantinou, C. Age and growth of the yellow gurnard (Trigla lucerna L. 1758) from the Thermaikos Gulf (Greece) with some comments on its biology. Fish. Res. 1984, 2, 243–255. [Google Scholar] [CrossRef]
- Rodrigues, J.; Feijó, D.; Rocha, A.; Erzini, K.; Correia, A.T. Age, growth and reproductive biology of the tub gurnard (Chelidonichthys lucerna) in North-East Portugal. Front. Mar. Sci. 2019, 6, 158. [Google Scholar] [CrossRef]
- Walther, B.D.; Thorrold, S.R. Water, not food, contributes the majority of strontium and barium deposited in the otoliths of a marine fish. Mar. Ecol. Prog. Ser. 2006, 311, 125–130. [Google Scholar] [CrossRef]
- Nelson, T.R.; Powers, S.P. Validation of species specific otolith chemistry and salinity relationships. Environ. Biol. Fishes 2019, 102, 801–815. [Google Scholar] [CrossRef]
- Elsdon, T.S.; Wells, B.K.; Campana, S.E.; Gillanders, B.M.; Jones, C.M.; Limburg, K.E.; Secor, D.H.; Thorrold, S.R.; Walther, B.D. Otolith chemistry to describe movements and life-history parameters of fishes: Hypotheses, assumptions, limitations and inferences. Oceanogr. Mar. Biol. Annu. Rev. 2008, 46, 297–330. Available online: https://www.researchgate.net/publication/231180600 (accessed on 10 May 2023).
- Reis, P.A.; Feijó, D.; Seixas, F.; Pimenta, J.; Rocha, A.; Santos, P. Fishery of Chelidonichthys lucerna (Linnaeus, 1758) in portuguese northwest atlantic coast: Exploratory baseline study. Int. J. Fish. Aquat. Stud. 2020, 8, 5. [Google Scholar] [CrossRef]
- İlhan, D.; Toğulga, M. Age, growth and reproduction of tub gurnard Chelidonichthys lucernus Linnaeus, 1758 (Osteichthtyes: Triglidae) from İzmir Bay, Aegean Sea, Eastern Mediterranean. Acta Adriat. 2007, 48, 173–184. [Google Scholar]
- Vallisneri, M.; Stagioni, M.; Montanini, S.; Tommasini, S. Body size, sexual maturity and diet in Chelidonichthys lucerna (Osteichthyes: Triglidae) from the Adriatic Sea, north eastern Mediterranean. Acta Adriat. 2011, 52, 141–148. [Google Scholar]
- Correia, A.T.; Antunes, C.; Isidro, E.J.; Coimbra, J. Changes in otolith microstructure and microchemistry during larval development of the European conger eel (Conger conger). Mar. Biol. 2003, 142, 777–789. [Google Scholar] [CrossRef]
- Correia, A.T.; Able, K.W.; Antunes, C.; Coimbra, J. Early life history of the American conger eel (Conger oceanicus) as revealed by otolith microstructure and microchemistry of metamorphosing leptocephali. Mar. Biol. 2004, 145, 477–488. [Google Scholar] [CrossRef]
- Ling, Y.; Iizuka, Y.; Tzeng, W. Decreased Sr/Ca ratios in the otoliths of two marine eels, Gymnothorax reticularis and Muraenesox cinereus, during metamorphosis. Mar. Ecol. Prog. Ser. 2005, 304, 201–206. [Google Scholar] [CrossRef]
- Bath, G.E.; Thorrold, S.R.; Jones, C.M.; Campana, S.E.; McLaren, J.W.; Lam, J.W.H. Strontium and barium uptake in aragonitic otoliths of marine fish. Geochim. Cosmochim. Acta 2000, 64, 1705–1714. [Google Scholar] [CrossRef]
- Lin, Y.T.; Wang, C.H.; You, C.F.; Tzeng, W.N. BA/CA ratios in otoliths of southern bluefin tuna (Thunnus maccoyii) as a biological tracer of upwelling in the great Australian bight. J. Mar. Sci. Technol. 2013, 21, 733–741. [Google Scholar] [CrossRef]
- Woodson, L.; Wells, B.; Grimes, C.; Franks, R.; Santora, J.; Carr, M. Water and otolith chemistry identify exposure of juvenile rockfish to upwelled waters in an open coastal system. Mar. Ecol. Prog. Ser. 2013, 473, 261–273. [Google Scholar] [CrossRef]
- Wheeler, S.; Russell, A.; Fehrenbacher, J.; Morgan, S. Evaluating chemical signatures in a coastal upwelling region to reconstruct water mass associations of settlement-stage rockfishes. Mar. Ecol. Prog. Ser. 2016, 550, 191–206. [Google Scholar] [CrossRef]
- Ferguson, G.J.; Ward, T.M.; Gillanders, B.M. Otolith shape and elemental composition: Complementary tools for stock discrimination of mulloway (Argyrosomus japonicus) in southern Australia. Fish. Res. 2011, 110, 75–83. [Google Scholar] [CrossRef]
- Morat, F.; Letourneur, Y.; Dierking, J.; Pécheyran, C.; Bareille, G.; Blamart, D.; Harmelin-Vivien, M. The Great Melting Pot. Common Sole Population Connectivity Assessed by Otolith and Water Fingerprints. PLoS ONE 2014, 9, e86585. [Google Scholar] [CrossRef] [PubMed]
- De Carvalho, B.M.; Volpedo, A.; Dos Santos, A.M.V.; Spach, H.L. Use of otolith microchemistry as habitat indicator of Anchoa tricolor (Spix and Agassiz, 1829) in a subtropical estuary. Lat. Am. J. Aquat. Res. 2017, 45, 457–465. [Google Scholar] [CrossRef]
- Costa, M.; Cabral, H. Changes in the Tagus nursery function for commercial fish species: Some perspectives for management. Aquat. Ecol. 1999, 33, 287–292. [Google Scholar] [CrossRef]
- Veiga, P.; Machado, D.; Almeida, C.; Bentes, L.; Monteiro, P.; Oliveira, F.; Ruano, M.; Erzini, K.; Gonçalves, J.M.S. Weight-length relationships for 54 species of the Arade estuary, southern Portugal. J. Appl. Ichthyol. 2009, 25, 493–496. [Google Scholar] [CrossRef]

| Location | Date | N | TL (cm) | Sr:Ca (mmol.mol−1) | |
|---|---|---|---|---|---|
| Mean ± SD | Range | ||||
| Sagres-Portimão | 13–14 March 2007 | 5 | 27.16 ± 4.75 | 21.60–32.90 | Mean = 6.63 SD = 1.59 Mean − 1 × SD = 5.04 Mean − 2 × SD = 3.45 |
| Arrábida-Milfontes | 10–12 March 2007 | 5 | 31.68 ± 8.10 | 26.10–45.30 | |
| Sines | 17–18 March 2007 | 5 | 24.54 ± 3.01 | 22.80–29.80 | |
| Berlengas | 30 March 2007 | 5 | 22.56 ± 1.00 | 21.20–23.90 | |
| Aveiro | 29 March 2007 | 5 | 23.04 ± 0.93 | 21.80–23.90 | |
| Matosinhos | 26 March 2007 | 5 | 22.42 ± 0.93 | 21.00–23.30 | |
| Caminha | 25 March 2007 | 5 | 22.50 ± 1.02 | 21.20–23.80 | |
| Douro estuary | 12 December 2016 | 5 | 20.16 ± 8.38 | 18.00–21.60 | Mean = 4.89 |
| Maximum = 5.41 | |||||
| Minimum = 4.28 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferreira, I.; Daros, F.A.; Moreira, C.; Feijó, D.; Rocha, A.; Mendez-Vicente, A.; Pisonero, J.; Correia, A.T. Is Chelidonichthys lucerna (Linnaeus, 1758) a Marine Estuarine-Dependent Fish? Insights from Saccular Otolith Microchemistry. Fishes 2023, 8, 383. https://doi.org/10.3390/fishes8070383
Ferreira I, Daros FA, Moreira C, Feijó D, Rocha A, Mendez-Vicente A, Pisonero J, Correia AT. Is Chelidonichthys lucerna (Linnaeus, 1758) a Marine Estuarine-Dependent Fish? Insights from Saccular Otolith Microchemistry. Fishes. 2023; 8(7):383. https://doi.org/10.3390/fishes8070383
Chicago/Turabian StyleFerreira, Inês, Felippe A. Daros, Cláudia Moreira, Diana Feijó, Alberto Rocha, Ana Mendez-Vicente, Jorge Pisonero, and Alberto Teodorico Correia. 2023. "Is Chelidonichthys lucerna (Linnaeus, 1758) a Marine Estuarine-Dependent Fish? Insights from Saccular Otolith Microchemistry" Fishes 8, no. 7: 383. https://doi.org/10.3390/fishes8070383
APA StyleFerreira, I., Daros, F. A., Moreira, C., Feijó, D., Rocha, A., Mendez-Vicente, A., Pisonero, J., & Correia, A. T. (2023). Is Chelidonichthys lucerna (Linnaeus, 1758) a Marine Estuarine-Dependent Fish? Insights from Saccular Otolith Microchemistry. Fishes, 8(7), 383. https://doi.org/10.3390/fishes8070383