Metazoan Parasites of Mozambique tilapia (Oreochromis mossambicus) Native to Lake Urema, Mozambique
Abstract
1. Introduction
2. Methods
2.1. Site Description
2.2. Sampling of Fish and Parasite Collection
2.3. Water Parameters
2.4. Ethical Considerations
3. Results
3.1. Parasites Retrieved
3.2. Water Parameters
4. Discussion
4.1. Lake Condition and Parasite Burden
4.2. Known Parasite Records and Distribution
4.3. Zoonotic Importance
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gilbert, B.M.; Avenant-Oldewage, A. Parasites and pollution: The effectiveness of tiny organisms in assessing the quality of aquatic ecosystems, with a focus on Africa. Environ. Sci. Pollut. Res. 2017, 24, 18742–18769. [Google Scholar] [CrossRef] [PubMed]
- Gagne, R.B.; Crooks, K.R.; Craft, M.E.; Chiu, E.S.; Fountain-Jones, N.M.; Malmberg, J.L.; Carver, S.; Funk, W.C.; VandeWoude, S. Parasites as conservation tools. Conserv. Biol. 2022, 36, e13719. [Google Scholar] [CrossRef]
- Marick, J.; Patra, B.K.; Ash, A. Loss of Biodiversity and Ecosystem Services: Told and Untold Stories from Parasite World. Proc. Zool. Soc. 2023. [Google Scholar] [CrossRef]
- Wilson, J.R.; Saunders, R.J.; Hutson, K.S. Parasites of the invasive tilapia Oreochromis mossambicus: Evidence for co-introduction. Aquat. Invasions 2019, 14, 332–349. [Google Scholar] [CrossRef]
- Šimková, A.; Řehulkova, E.; Rasoloariniaina, J.R.; Jorissen, M.W.P.; Scholz, T.; Faltýnková, A.; Mašová, Š.; Vanhove, M.P. Transmission of parasites from introduced tilapias: A new threat to endemic Malagasy ichthyofauna. Biol. Invasions 2019, 21, 803–819. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. Towards Blue Transformation; FAO: Rome, Italy, 2022. [Google Scholar]
- Böhme, B. Geo-ecology of the Lake Urema/Central Moçambique. Freib. Online Geol. 2005, 14, 1–114. [Google Scholar]
- Tinley, K.L. Framework of the Gorongosa Ecosystem. Ph.D. Thesis, University of Pretoria, Pretoria, South Africa, 1977. [Google Scholar]
- Arvidsson, K.; Stenberg, L.; Chirindja, F.; Dahlin, T.; Owen, R.; Steinbruch, F. A hydrogeological study of the Nhandugue River, Moçambique—A major groundwater recharge zone. Phys. Chem. Earth 2011, 36, 789–797. [Google Scholar] [CrossRef]
- SWECO and Associates I. Development of the Pungwe River Basin. Joint Integrated Water Resources Management Strategy. Monograph Report. Annex I. Sector Study on: Surface Water Resources of the Pungwe River Basin. Client: Government of the Republic of Mozambique, Government of the Republic of Zimbabwe; Swedish International Development Cooperation Agency (Sida). 2004. Available online: https://biblioteca.biofund.org.mz/biblioteca_virtual/development-of-the-pungwe-river-basin-joint-integrated-water-resources-management-strategy-annex-x-sector-study-on-conservation-areas-wildlife-and-tourism/ (accessed on 10 May 2023).
- Madanire-Moyo, G.N.; Luus-Powell, W.J.; Olivier, P.A. Diversity of metazoan parasites of the Mozambique tilapia; Oreochromis mossambicus (Peters, 1852), as indicators of pollution in the Limpopo and Olifants River Systems. Onderstepoort J. Vet. Res. 2012, 79, 146–152. [Google Scholar] [CrossRef]
- Douëllou, L. Monogeneans of the genus Cichlidogyrus Paperna, 1960 (Dactylogyridae: Ancyrocephalinae) from cichlid fishes of Lake Kariba (Zimbabwe) with descriptions of five new species. Syst. Parasitol. 1993, 25, 159–186. [Google Scholar] [CrossRef]
- Pariselle, A.; Euzet, L. Systematic revision of dactylogyridean parasites (Monogenea) from cichlid fishes in Africa; the Levant and Madagascar. Zoosystema 2009, 31, 849–898. [Google Scholar] [CrossRef]
- Gibson, D.I.; Bray, R.A.; Jones, A. Keys to the Trematoda; Oxford University Press: Oxford, UK, 2008; Volume 3. [Google Scholar]
- Chibwana, F.D.; Nkwengulila, G. Variation in the morphometrics of diplostomid metacercariae (Digenea: Trematoda) infecting the catfish, Clarias gariepinus in Tanzania. J. Helminthol. 2010, 84, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.C. Nematode Parasites of Vertebrates: Their Development and Transmission; CAB International: Wallingford, UK, 1992. [Google Scholar]
- Oldewage, W.H.; van As, J.G. A key for the identification of African piscine parasitic Ergasilidae (Copepoda: Poecilostomatoida). Suid-Afrik. Tydskr. Vir Dierkd. 1988, 23, 42–46. [Google Scholar]
- Robinson, J.; Avenant-Oldewage, A. Aspects of the morphology of the parasitic copepod Lernaea cyprinacea Linnaeus, 1758 and notes on its distribution in Africa. Crustaceana 1996, 69, 610–626. [Google Scholar]
- Reiczigel, J.; Rózsa, L. Quantitative Parasitology 3.0. Budapest, Distributed by the Authors. 2005. Available online: https://www.zoologia.hu/qp/ (accessed on 25 October 2022).
- Rózsa, L.; Reiczigel, J.; Majoros, G. Quantifying parasites in samples of hosts. J. Parasitol. 2000, 86, 228–232. [Google Scholar] [CrossRef]
- South African National Standard 10386; The Care and Use of Animals for Scientific Purposes. SABS Standards Division: Groenkloof, South Africa, 2008.
- Koskivaara, M.; Valtonen, E.T.; Vuori, K.M. Microhabitat distribution and coexistence of Dactylogyrus species (Monogenea) on the gills of roach. Parasitology 1992, 104, 273–281. [Google Scholar] [CrossRef]
- Villar-Torres, M.; Montero, F.E.; Raga, J.A.; Repullés-Albelda, A. Come rain or come shine: Environmental effects on the infective stages of Sparicotyle chrysophrii, a key pathogen in Mediterranean aquaculture. Parasites Vectors 2018, 11, 558. [Google Scholar] [CrossRef]
- Cavaleiro, F.I.; Santos, M.J. Seasonality of metazoan ectoparasites in marine European flounder Platichthys flesus (Teleostei: Pleuronectidae). Parasitology 2009, 136, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Firmat, C.; Alibert, P.; Mutin, G.; Losseau, M.; Pariselle, A.; Sasal, P. A case of complete loss of gill parasites in the invasive cichlid Oreochromis mossambicus. Parasitol. Res. 2016, 115, 3657–3661. [Google Scholar] [CrossRef]
- García-Vásquez, A.; Hansen, H.; Christison, K.W.; RubioGodoy, M.; Bron, J.E.; Shinn, A.P. Gyrodactylids (Gyrodactylidae; Monogenea) infecting Oreochromis niloticus niloticus (L.) and O. mossambicus (Peters) (Cichlidae): A pan-global survey. Acta Parasitol. 2010, 55, 215–229. [Google Scholar] [CrossRef]
- Zahradníčková, P.; Barson, M.; Luus-Powell, W.J.; Přikrylová, I. Species of Gyrodactylus von Nordmann; 1832 (Platyhelminthes: Monogenea) from cichlids from Zambezi and Limpopo river basins in Zimbabwe and South Africa: Evidence for unexplored species richness. Syst. Parasitol. 2016, 93, 679–680. [Google Scholar] [CrossRef]
- Olivier, P.A.; Luus-Powell, W.J.; Saayman, J.E. Report on some monogenean and clinostomid infestations of freshwater fish and waterbird hosts in Middle Letaba Dam, Limpopo Province, South Africa. Onderstepoort J. Vet. Res. 2009, 76, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Pariselle, A.; Morand, S.; Deveney, M.; Pouyaud, L. Parasite species richness of closely related hosts: Historical scenario and “genetic” hypothesis. In Hommage à Louis Euzet—Taxonomie; Écologie et Évolution des Métazoaires Parasites. Taxonomy; Ecology and Evolution of Metazoan Parasites; Combes, C., Jourdane, J., Eds.; Presses Universitaires de Perpignan: Perpignan, France, 2003; pp. 147–166. [Google Scholar]
- Ilgová, J.; Salát, J.; Kašný, M. Molecular communication between the monogenea and fish immune system. Fish Shellfish. Immunol. 2021, 112, 179–190. [Google Scholar] [CrossRef]
- Madanire-Moyo, G.N.; Matla, M.M.; Olivier, P.A.; Luus-Powell, W.J. Population dynamics and spatial distribution of Monogeneans on the gills of Oreochromis mossambicus (Peters, 1852) from two lakes of the Limpopo River System, South Africa. J. Helminthol. 2011, 85, 146–152. [Google Scholar] [CrossRef]
- Sara, J.R.; Smit, W.J.; Erasmus, L.J.; Ramalepe, T.P.; Mogashoa, M.E.; Raphahlelo, M.E.; Theron, J.; Luus-Powell, W.J. Ecological status of Hout River Dam; Limpopo province, South Africa, using fish condition and health assessment index protocols: A preliminary investigation. Afr. J. Aquat. Sci. 2014, 39, 35–43. [Google Scholar] [CrossRef]
- Maneepitaksanti, W.; Nagasawa, K. Monogeneans of Cichlidogyrus Paperna, 1960 (Dactylogyridae); gill parasites of tilapias; from Okinawa Prefecture, Japan. Biogeography 2012, 14, 111–119. [Google Scholar]
- Khalil, L.F.; Polling, L. Checklist of the Helminth Parasites of African Freshwater Fishes; University of the North: Pietersburg, South Africa, 1997. [Google Scholar]
- Scholz, T.; Vanhove, M.P.; Smit, N.; Jayasundera, Z.; Gelnar, M. A Guide to the Parasites of African Freshwater Fishes. Abc Taxa 2018, 18, 1–425. [Google Scholar]
- Tavakol, S.; Smit, W.J.; Sara, J.R.; Halajian, A.; Luus-Powell, W.J. Distribution of Contracaecum (Nematoda: Anisakidae) larvae in freshwater fish from the northern regions of South Africa. Afr. Zool. 2015, 50, 133–139. [Google Scholar] [CrossRef]
- Dumbo, J.C. Estudo de Parasitas Metazoários de Clarias gariepinus (Burchell; 1822) que ocorre no Lago Urema, Parque Nacional da Gorongosa. Master’s Thesis, Universidade Eduardo Mondlane, Maputo, Mozambique, 2009. [Google Scholar]
- Boane, C.; Cruze, C.; Saraiva, A. Metazoan Parasites of Cyprinus carpio L. (Cyprinidae) from Mozambique. Aquaculture 2008, 284, 59–61. [Google Scholar] [CrossRef]
- Britz, J.; van As, J.G.; Saayman, J.E. Occurrence and distribution of Clinostomum tilapiae Ukoli, 1966 and Euclinostomum heterostomum (Rudolphi; 1809) metacercarial infections of freshwater fish in Venda and Lebowa, southern Africa. J. Fish Biol. 1985, 26, 21–28. [Google Scholar] [CrossRef]
- Oldewage, W.H.; van As, J.G. A new fish-ectoparasitic ergasilid (Crustacea: Copepoda) from the Pongola River system. S. Afr. J. Zool. 1987, 22, 62–65. [Google Scholar] [CrossRef]
- Oldewage, W.H.; Avenant-Oldewage, A. Checklist of the piscine parasitic Copepoda (Crustacea) of African fishes. Zool. Meded. 1993, 23, 2–28. [Google Scholar]
- Abdel-Hady, O.K.; Bayoumy, E.M.; Osman, H.A.M. New copepodal ergasilid parasitic on Tilapia zilli from Lake Temsah with special reference to its pathological effect. Glob. Vet. 2008, 2, 123–129. [Google Scholar]
- Douëllou, L.; Erlwanger, K.H. Crustacean parasites of fishes in Lake Kariba, Zimbabwe, preliminary results. Hidrobiologica 1994, 287, 233–242. [Google Scholar] [CrossRef]
- Yashuv, V.A. On the biology of Lernaea cyprinacea in fish ponds. Bull. Fish Cult. 1959, 11, 80–89. [Google Scholar]
- Paperna, I. Parasites; Infections and Diseases of Fishes in Africa: An Update; FAO/CIFA Technical Paper No. 31; FAO Publications: Rome, Italy, 1996. [Google Scholar]
- Piasecki, W.; Goodwin, A.E.; Eiras, J.C.; Nowak, B.F. Importance of Copepoda in freshwater aquaculture. Zool. Stud. 2004, 43, 193–205. [Google Scholar]
- Hoffman, G.L. Intercontinental and transcontinental dissemination and transfaunation of fish parasites with emphasis on whirling disease (Myxosoma cerebralis). In A Symposium on Diseases of Fish and Shellfish; Snieszko, S.F., Ed.; American Fisheries Society: Bethesda, MD, USA, 1970; Volume 5, pp. 69–81. [Google Scholar]
- Marcogliese, D.J. Seasonal occurrence of Lernaea cyprinacea on fishes in Belews Lake, North Carolina. J. Parasitol. 1991, 77, 326–327. [Google Scholar] [CrossRef]
- Medeiros, E.S.; Maltchik, L. The effects of hydrological disturbance on the intensity of infestation of Lernaea cyprinacea in an intermittent stream fish community. J. Arid. Environ. 1999, 43, 351–356. [Google Scholar] [CrossRef]
- Barson, M.; Mulonga, A.; Nhiwatiwa, T. Investigation of a parasitic outbreak of Lernaea cyprinacea Linnaeus (Crustacea: Copepoda) in fish from Zimbabwe. J. Afr. Zool. 2008, 43, 175–183. [Google Scholar] [CrossRef]
- Dalu, T.; Nhiwatiwa, T.; Clegg, B.; Barson, M. Impact of Lernaea cyprinacea Linnaeus 1758 (Crustacea: Copepoda) almost a decade after an initial parasitic outbreak in fish of Malilangwe Reservoir, Zimbabwe. Knowl. Manag. Aquat. Ecosyst. 2012, 406, 1–9. [Google Scholar]
- Van As, J.G.; Basson, L. Checklist of freshwater parasites from southern Africa. S. Afr. J. Wildl. 1984, 14, 49–61. [Google Scholar]
- Viljoen, B.C.S. A seasonal investigation of the genus Lernaea (Crustacea: Copepoda) on cyprinid fish in Boskop Dam; Transvaal; South Africa. S. Afr. J. Wildl. 1986, 16, 27–31. [Google Scholar]
- Welicky, R.L.; de Swart, J.; Gerber, R.; Netherlands, E.; Smit, N.J. Drought associated absence of alien invasive anchorworm; Lernaea cyprinacea (Copepoda: Lernaeidae); is related to changes in fish health. Int. J. Parasitol. Parasites Wildl. 2017, 6, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Lahav, M.; Sarig, S.; Shilo, M. The eradication of Lernaea in storage ponds of carps through destruction of the copepodidal stage by Dipterex. Bamidgeh 1964, 27, 341–397. [Google Scholar]
- Bulow, F.J.; Winningham, J.R.; Hooper, R.C. Occurrence of the copepod parasite Lernaea cyprinacaea in a stream fish population. Trans. Am. Fish. Soc. 1979, 108, 100–102. [Google Scholar] [CrossRef]
- Hoffman, G.L. Copepod parasites of freshwater fish: Ergasilus, Achtheres and Salmincola. Fish Dis. Leafl. 1977, 48, 1–10. [Google Scholar]
- Dezfuli, B.S.; Glari, L.; Konecny, R.; Jaeger, P.; Manera, M. Immunohistochemistry, ultrastructure and pathology of gills of Abramis brama from Lake Mondsee, Austria, in infected with Ergasilus sieboldi (Copepoda). Dis. Aquat. Org. 2003, 53, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Lester, R.G.; Hayward, C.J. Phylum arthropoda. In Fish Diseases and Disorders: Protozoan and Metazoan Infections; Woo, P.T.K., Ed.; CAB International: Wallingford, UK, 2006; Volume 1, pp. 466–565. [Google Scholar] [CrossRef]
- Vinobaba, P. Histopathological changes induced by ergasilid copepod infections on the gills of food fish from Batticaloa lagoon, Sri Lanka. Sri Lanka J. Aquat. Sci. 2007, 12, 77–87. [Google Scholar] [CrossRef]
- Kabata, Z. Copepoda (Crustacea) parasitic on fishes: Problems and perspectives. Adv. Parasitol. 1982, 19, 1–71. [Google Scholar]
- Khan, M.N.; Aziz, F.; Afzal, M.; Rab, A.; Sahar, L.; Ali, R.; Naqvi, S.M. Parasitic infestation in different fresh water fishes of mini dams of Potohar region, Pakistan. J. Sci. Technol. (Pak.) 2003, 6, 1092–1095. [Google Scholar] [CrossRef]
- Timi, J.T.; Buchmann, K. A century of parasitology in fisheries and aquaculture. J. Helminthol. 2023, 97, e4. [Google Scholar] [CrossRef]
- Van De, N.; Waikagul, J.; Dalsgaard, A.; Chai, J.Y.; Sohn, W.M.; Murrell, K.D. Fishborne zoonotic intestinal trematodes, Vietnam. Emerg. Infect. Dis. 2007, 13, 1828–1833. [Google Scholar]
- Vollset, K.W. Parasite induced mortality is context dependent in Atlantic salmon: Insights from an individual based model. Sci. Rep. 2019, 9, 17377. [Google Scholar] [CrossRef] [PubMed]
- Cribb, T.H.; Chisholm, L.A.; Bray, R.A. Diversity in the Monogenea and Digenea: Does lifestyle matter? Int. J. Parasitol. 2002, 32, 321–328.93. [Google Scholar] [CrossRef]
- Kaddumukasa, M.; Kaddu, J.B.; Makanga, B. A Survey of nematode infection in Oreochromis niloticus (L.) (Teleostei: Cichlidae) in Lake Kyoga, Uganda. Afr. J. Trop. Hydrobiol. 2013, 13, 12–13. [Google Scholar]
- Bekele, J.; Hussien, D. Prevalence of internal parasites of Oreochromis niloticus and Clarias gariepinus fish species in Lake Ziway, Ethiopia. J. Aquac. Res. 2015, 6, 308. [Google Scholar]
- Reshid, M.; Adugna, M.; Redda, Y.T.; Awol, N.; Teklu, A. A Study of Clinostomum (Trematode) and Contracaecum (Nematode) parasites affecting Oreochromis niloticus in Small Abaya Lake, Silite Zone, Ethiopia. J. Aquac. Res. Dev. 2015, 6, 316. [Google Scholar]
- Thu, N.D.; Dalsgaard, A.; Loan, L.T.; Murrell, K.D. Survey for zoonotic liver and intestinal trematode metacercariae in cultured and wild fish in an Giang Province; Vietnam. Korean J. Parasitol. 2007, 45, 45–54. [Google Scholar] [CrossRef]
- Urban-Econ Development Economists. Nile & Mozambique Tilapia Feasibility Study; Department of Agriculture, Forestry and Fisheries Chief Directorate: Vlaeberg, South Africa; Aquaculture and Economic Development: Vlaeberg, South Africa, 2018. [Google Scholar]
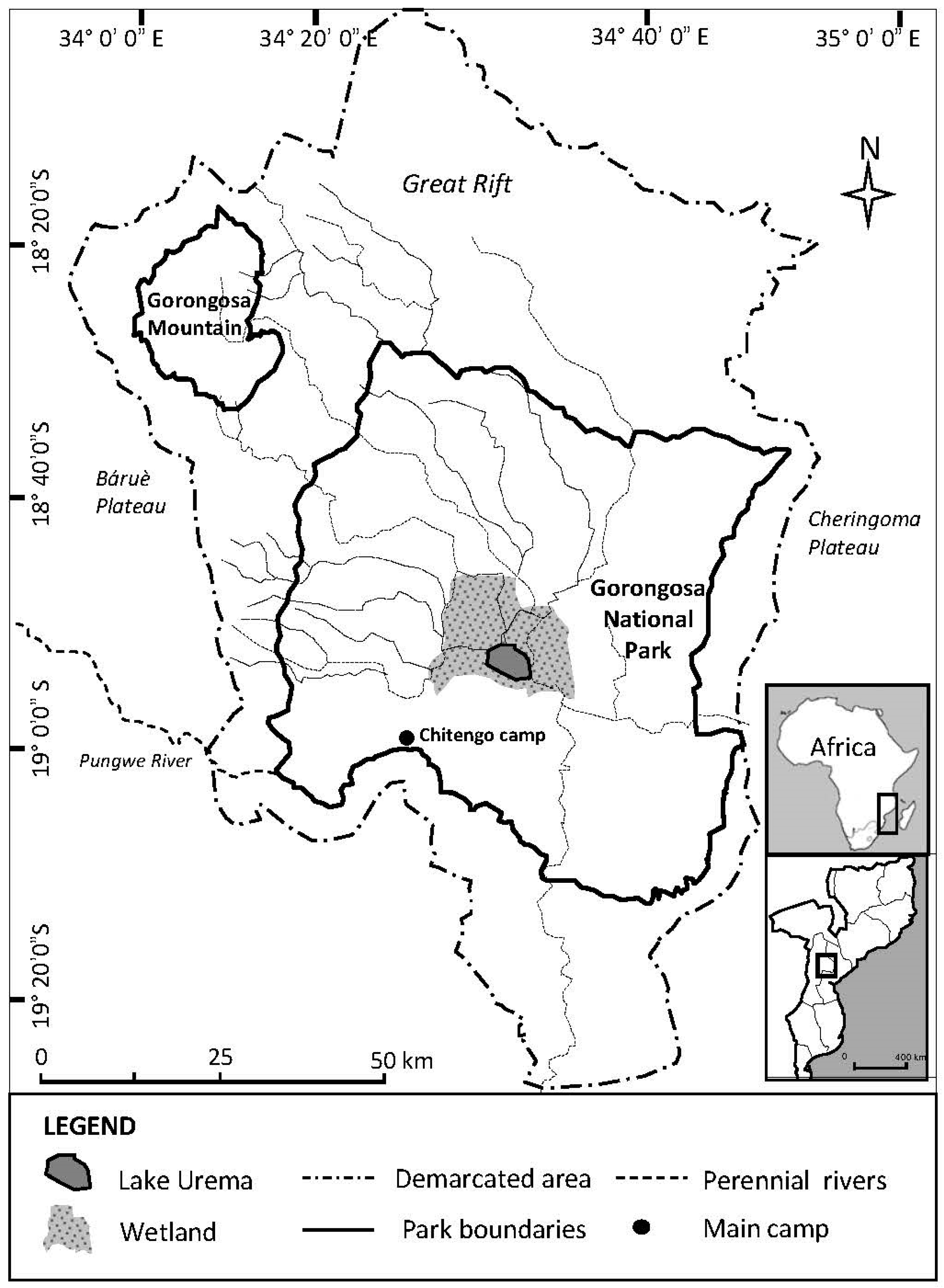
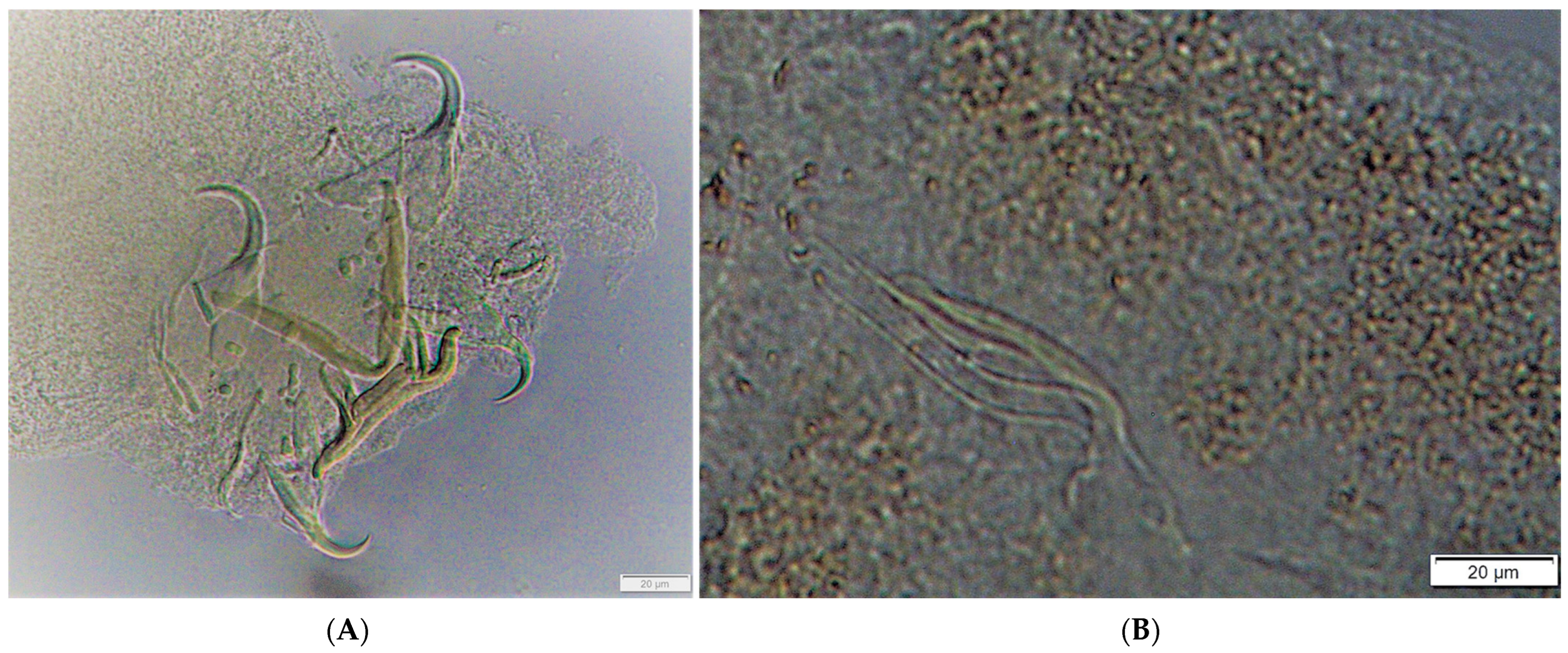
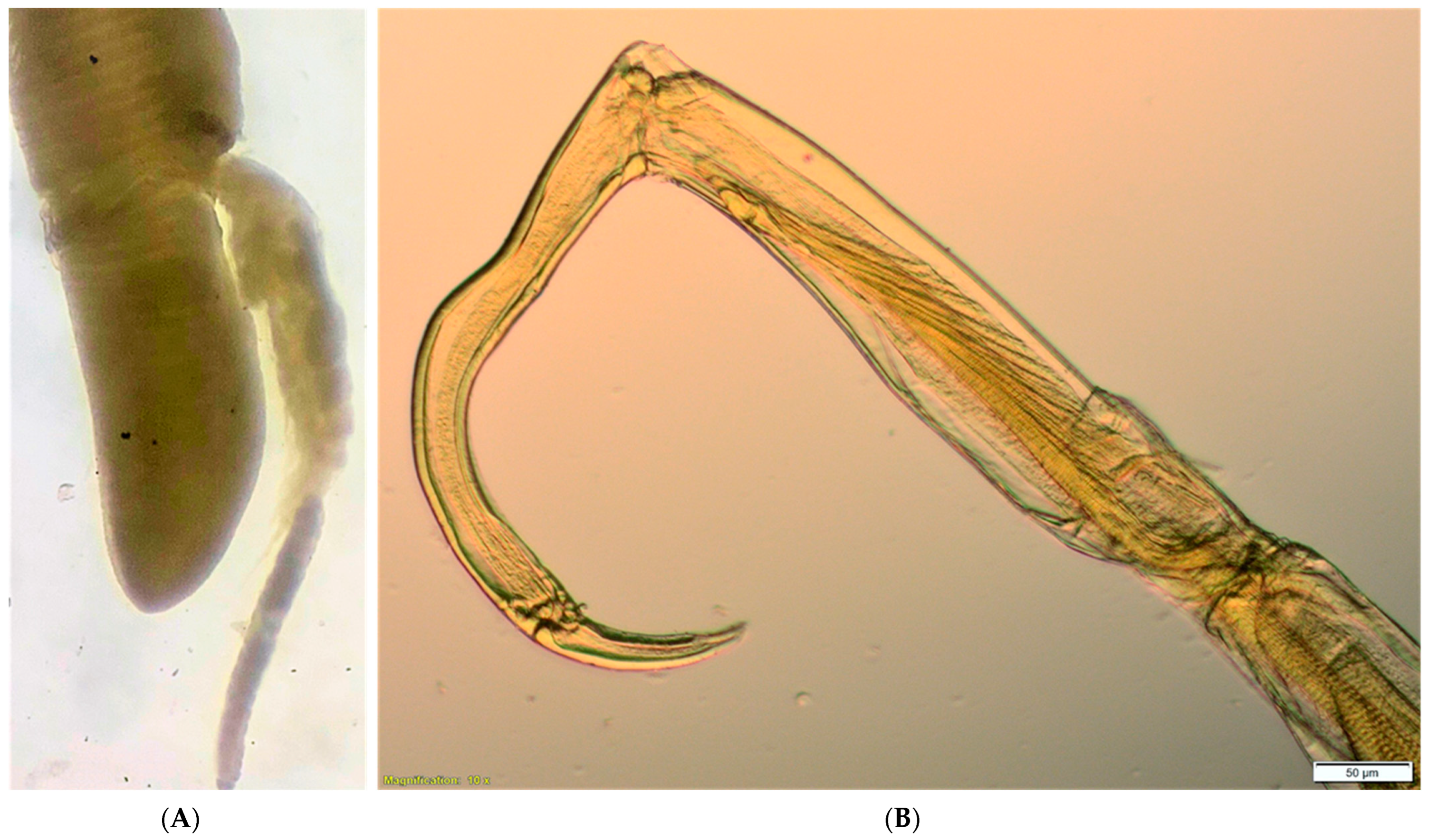
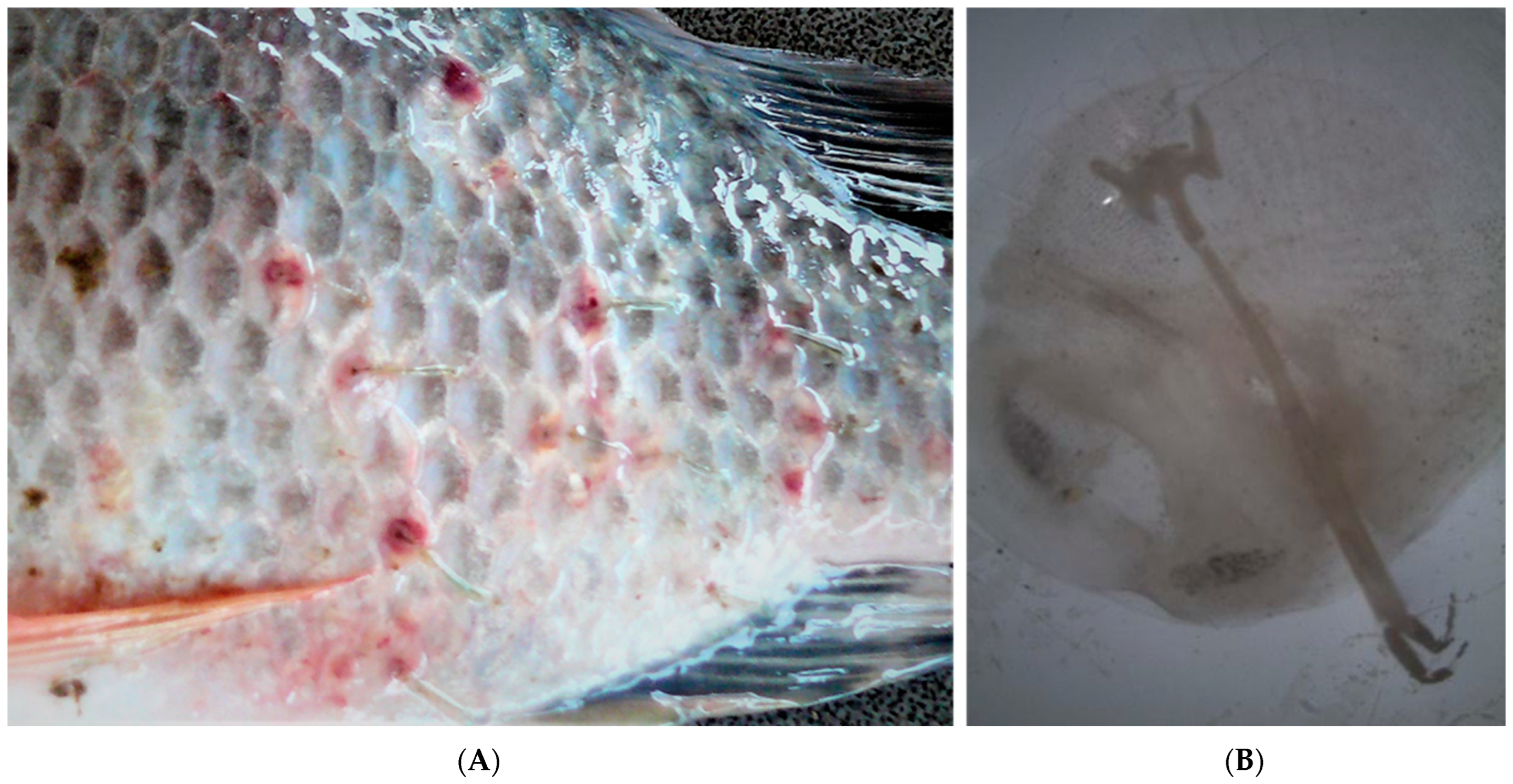
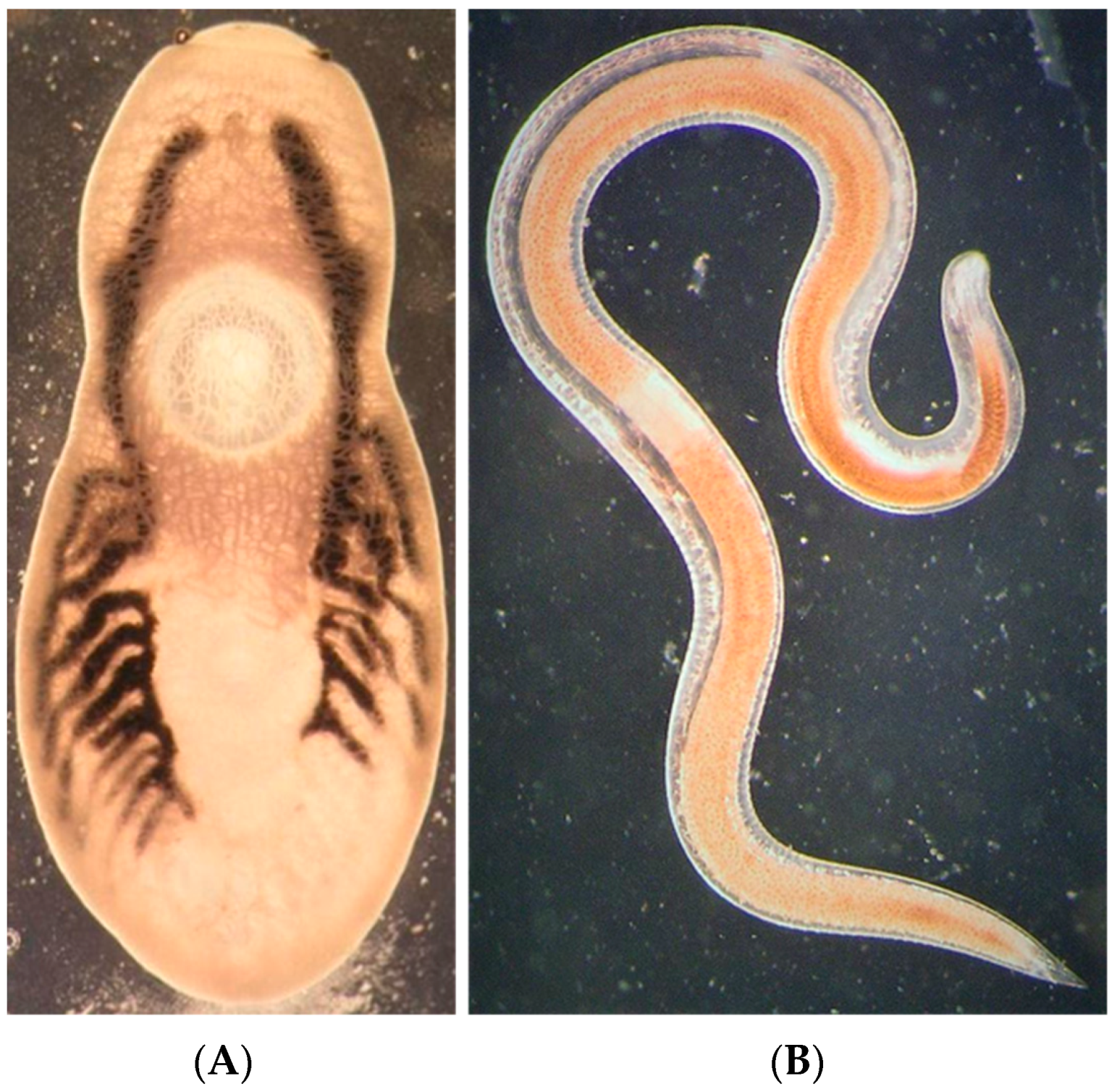
| Parasite Group | Parasite Species | Infected Site | P (%) | 95% CL for P (%) | MA | 95% CL for MA | MI | 95% CL for MI | IR |
|---|---|---|---|---|---|---|---|---|---|
| Monogenea | Cichlidogyrus halli | Gills | 100.0 | (88.4–100.0) | 5.9 | (4.6–7.1) | 5.9 | (4.7–7.1) | 2–15 |
| Trematoda | Euclinostomum sp. * | Muscle | 16.7 | (3.8–30.7) | 0.2 | (0.0–0.3) | 1.0 | (NC) | 1 |
| Nematoda | Contracaecum sp. * | Body cavity | 60.0 | (40.6–77.3) | 0.9 | (0.6–1.2) | 1.5 | (1.2–1.8) | 1–3 |
| Copepoda | Ergasilus mirabilis | Gills | 100.0 | (88.4–100.0) | 8.0 | (6.0–10.7) | 8.0 | (6.0–10.4) | 2–28 |
| Lernaea cyprinacea | Skin | 90.0 | (69.3–96.2) | 10.4 | (7.3–12.5) | 11.6 | (8.7–14.0) | 2–24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Smit, W.J.; Vanhove, M.P.M.; Moyo, N.A.G.; Luus-Powell, W.J. Metazoan Parasites of Mozambique tilapia (Oreochromis mossambicus) Native to Lake Urema, Mozambique. Fishes 2023, 8, 273. https://doi.org/10.3390/fishes8050273
Smit WJ, Vanhove MPM, Moyo NAG, Luus-Powell WJ. Metazoan Parasites of Mozambique tilapia (Oreochromis mossambicus) Native to Lake Urema, Mozambique. Fishes. 2023; 8(5):273. https://doi.org/10.3390/fishes8050273
Chicago/Turabian StyleSmit, Willem J., Maarten P. M. Vanhove, Ngonidzashe A. G. Moyo, and Wilmien J. Luus-Powell. 2023. "Metazoan Parasites of Mozambique tilapia (Oreochromis mossambicus) Native to Lake Urema, Mozambique" Fishes 8, no. 5: 273. https://doi.org/10.3390/fishes8050273
APA StyleSmit, W. J., Vanhove, M. P. M., Moyo, N. A. G., & Luus-Powell, W. J. (2023). Metazoan Parasites of Mozambique tilapia (Oreochromis mossambicus) Native to Lake Urema, Mozambique. Fishes, 8(5), 273. https://doi.org/10.3390/fishes8050273







