Abstract
Optimizing the initial live feed is critical to success in fish larviculture in terms of both larval health and productivity. It is also vitally important due to the significant cost of provisioning live feeds. Glaxiids are high-value diadromous fish species found in parts of the Southern Hemisphere, which are wild-harvested and sold for human consumption in their larval form. In the emerging aquaculture of galaxiids, the live feed makes up a great proportion of the production cycle and is therefore a significant factor in achieving the economic viability of their production. In New Zealand, the endemic galaxiid species giant kōkopu (Galaxias argenteus) is considered suitable for aquaculture; however, little is known of their larval-feeding requirements. This study compares the growth performance and survival of giant kōkopu larvae over a four-week period when fed different proportions of first-molt Artemia nauplii (instar-I) and enriched, second-molt-onward Artemia (instar-II+) for different durations. The larvae in the treatment group which received the greatest proportion of Instar-I Artemia for the longest duration had the lowest mean wet weight and survival, leading to the production of the lowest total biomass of fish larvae when compared to the other feed treatments. The feed treatment that received only instar-II+ Artemia achieved an equally best total productivity. While the treatment fed a partial initial duration of mixed instar-I and instar-II+ Artemia achieved the same total productivity, the individual larvae were heavier and their body length was longer. The latter feed treatment appears to offer the best balance between the availability of feed particles in the optimum size range and a higher level of digestibility given that instar-I Artemia had higher levels of highly unsaturated fatty acids and polyunsaturated fatty acids.
Key Contribution:
This study demonstrates the benefits to the growth and survival of giant kōkopu larvae through the earlier provision of an increased proportion of instar-II+ Artemia, which have the potential to translate to significant cost benefit gains in commercial production.
1. Introduction
The feed and feed preparation often make up the largest single cost for fin-fish aquaculture businesses, typically accounting for 30–70% of the total cost of production [1]. Furthermore, the relative lack of understanding of the specific nutritional requirements of larval fish [2,3] means that the research focusing on optimizing the use of feeds often provides the greatest improvements in cost efficiencies, especially for start-up aquaculture businesses [4].
Live feeds are one of the most expensive components of rearing larval fish due to the cost of supplying raw materials, extensive requirements for labor and capital equipment for live-feed preparation, and the need to supply high-quality water and production facilities [5]. However, it is the use of live feeds, in particular, Artemia spp. nauplii, which has unlocked the commercial aquaculture potential of many fin-fish species [6,7]. The high level of reliance on live feeds in fin-fish larviculture is frequently due to the biological requirements for the initiation of exogenous feeding, which in the larvae of many fin-fish species is triggered by prey movement [8]. These first days of feeding are vital for achieving the optimum growth and survival of the larvae under culture conditions, and so, the identification of the ideal first-prey items and subsequent weaning to artificial inert diets is critical to commercial success in aquaculture production [9]. Therefore, the reliance on costly live feeds in commercial fin-fish larviculture means that the research to optimize production and minimize costs should be a priority.
Whitebait, a generic name for the larval form of the galaxiid species, have been identified as a potential aquaculture species for their high market price, which is currently US$85 kg−1 [10]. However, as is the case with many fin-fish aquaculture species, the whitebait species have largely remained in the pilot stage of development due to the challenges in larval production [11].
New Zealand Premium Whitebait Ltd. (NZPWL) is a start-up company which has developed the fledgling technology to farm whitebait, specifically giant kōkopu, Galaxias argenteus, on a commercial basis. As is the case for several other diadromous whitebait species, egg incubation was the former most significant step preventing the scaling of whitebait production, attributed to the asynchronous spawning of broodstock and natural incubation of eggs in terrestrial habitats [11,12,13,14,15]. Using proprietary technologies and methods, NZPWL has developed a scalable, cost- and labor-efficient incubation protocol which enables the reliable mass production of high-quality giant kōkopu larvae. Now, the critical challenge in the larviculture of whitebait remains identifying the nutritional requirements and suitable diets [11,14].
In the early efforts to raise larvae in the closely related īnanga (Galaxias maculatus), also known as “puye” in South America, the larvae were fed only Artemia, reaching 40 mm length in 160 days [14]. More recent work on this species describes the feeding regime of the same species requiring 20 days of feeding on rotifers, then 20 days on a mix of rotifers and Artemia, followed by 140 days of artificial feed, reaching 40–60 mm [13].
The current NZPWL larval-rearing protocol used for the commercial-scale production of giant kōkopu larvae has been developed incrementally, largely through trial and error rather than any systematic experimental research. Under the NZPWL protocol, Artemia nauplii are used as live feed for approximately 29 days, commencing two days after hatching (DAH), and being fed out three times a day at 0830, 1230, and 1630 h. For the first 14 days of feeding, only instar-I Artemia are provided, followed by a week, in which the proportion of enriched instar-II Artemia and later stages (i.e., referred to as Artemia-II+) fed to the larvae increases from 0 to 100%. Therefore, in the final week of live-food provision, only instar-II+ Artemia are provided. For the remaining approximately 47 days, only artificial feed is provided with the larvae reaching a length of 32 mm and wet weight (WW) of 177.05 mg [16]. This feeding protocol has been implemented by NZPWL because it has been shown to provide a slow and conservative transition in feed-particle size and is based on NZPWL’s experience with a closely related species (īnanga); however, to date, no directed experimentation on larval feeding has taken place for giant kōkopu, which have a larger mouth gape [16]. There are marked morphometric differences between the larvae of īnanga and giant kōkopu at comparable ages, most notably mouth-gape width and total length [16], which would suggest their feeding abilities are also likely to differ. The high cost of providing the feed for the production of giant kōkopu provides a strong incentive for research that may lead to a reduction in feed costs.
Therefore, the purpose of this study is to better understand the growth and survival of larval giant kōkopu fed Artemia diets, differing by the proportions and timings of instar-I and instar-II+ Artemia. This information, together with the knowledge of the relative costs of preparation of instar-II+ versus instar-I Artemia, will enable the opportunity to maximize fish production and minimize feed costs through optimizing the use of Artemia cysts. These data are useful for a comparison with the larval-rearing protocols for many other fin-fish species for which provisioning instar-I and instar-II+ Artemia is a significant cost of larval production.
2. Methods
2.1. Experimental Animals
Approximately 2.4 million giant kōkopu fertilized eggs from 160 female and 20 male captive broodstock were hatched on 9 September 2016 into commercial larval-rearing tanks consisting of conical 2500 L tanks. Three days later, 18,000 larval fish were randomly sampled from the commercial larval-rearing tank and divided evenly into nine 20 L experimental tanks, i.e., around 2000 larvae per tank. This was achieved by estimating the total number of fish per liter in the transfer vessel by careful mixing and taking random 200 mL samples and then counting the number of fish in each sample to produce a mean estimate of the total number of fish.
“Instar-I” Artemia refer to the first naupliar stage, as emerged from the cyst. The term “instar-II+” is used out of convenience due to the continual development and molting of Artemia-I to Artemia-II and subsequent advancing to later metanaupliar stages throughout the enrichment process.
2.2. Tank Design and Recirculation System
Nine identical experimental tanks were manufactured from round 20 L plastic (HDPE) pails, blue in color, 270 mm in diameter, and 380 mm high. Tanks were designed to hold 18 L of water by situating an outflow pipe 80 mm below the rim of the pail. The outflow pipe was fitted with a banjo filter using 600 µm filter mesh to prevent the escape of larvae while allowing the passage of suspended particles. Black PVC adhesive tape was used to line the wall of the tank from 25 mm below to 25 mm above water level to inhibit the climbing ability of the fish larvae.
All nine experimental tanks were operating on the same recirculation system. Outflow from each tank was directed to a filter basket through which seawater passed through a 5 µm filter mat into the sump, with the mat being changed daily. The sump was filled to 300 L and operated as the biological filter containing 40 L of plastic Kaldnes-K3 media (Krüger Kaldnes AS, Sandefjord, Norway). Each day, 100 L of seawater was removed from the sump and replaced with natural seawater of 35 ppt filtered to 5 µm and UV sterilized. Manual skimming of protein from the sump was undertaken daily or as necessary.
Seawater was pumped from the sump via a UV filter and entered each tank via 4 mm tubing delivering water to both the surface and the bottom of the tank. For the first seven days of the experiment, water flow rate was 0.28 L min−1 to each tank, with water entering perpendicular to the tank wall. From day seven to day 14, the flow was angled to be parallel to the tank wall to produce circular flow in the tank. From day 14 onwards, flow rate was increased to 0.37 L min−1 with flow remaining parallel to tank wall.
Tanks were aerated by an air-stone at the bottom of the tank producing two medium-sized (0.5 mm diameter) bubbles per second.
The tanks were illuminated by three 58 W fluorescent tubes, suspended 100 cm above the top edge of the tanks. Lights were operated between the 0745 and 1800 h with shade cloth being used to dim the light intensity for 30 min after switching on and before turning off.
Seawater temperature was not controlled but was measured every 6 h during the experimental period and found to vary between 14 and 18 °C over the course of the experiment but was consistent among the nine experimental tanks.
Nitrate, nitrite, ammonia, carbonate hardness, and pH were measured every second day using API© test kits (Aquarium Pharmaceuticals Incorporated, Chalfont, PA, USA) and acceptable levels were maintained throughout the experiment, while temperature was measured with a glass thermometer (Aqua One, Sydney, Australia).
2.3. Experimental Design
Three experimental live-food treatments for feeding larval giant kōkopu were tested over a period of 28 days starting on the first day of feeding (3 DAH). Each experimental tank received 3 g of feed three times a day at 0830, 1230, and 1630 h throughout the experimental period (i.e., 3–31 DAH). Each feed treatment was conducted in three randomly selected replicate tanks.
The first treatment, “NZPWL”, conformed to the existing commercial feeding regime in terms of the timing and transition of instar-I to enriched instar-II+ Artemia as used for the commercial production of larval giant kōkopu by NZPWL. For the initial 14 days of feeding, fish were fed only instar-I Artemia followed by a transitional phase with two days of a 2:1 ratio by wet weight of instar-I to enriched instar-II+ Artemia at each feed, followed by two days of 1:1 ratio, and finally two days of 1:2 ratio. For the remainder of the experimental period (8 days), the larvae were fed only enriched instar-II+ Artemia (Table 1).

Table 1.
Feed-particle density in each experimental tank by treatment throughout the experimental period given as the number of live-fed particles per liter (L−1). Three grams of live feeds were added to each tank containing 2000 larvae for each feeding event in the respective ratios of instar-I and instar-II+ Artemia. The range presented for the NZPWL treatment at 17–23 DAH reflects the change in feed-particle density from the start to the finish of the week as the ratio of instar-I to instar-II+ Artemia changed during this period.
The two other experimental feeding treatments were “Instar-II+” and “5050”. For the Instar-II+ treatment, only enriched instar-II+ Artemia were provided to the fish in this treatment for the 28-day duration of the experiment. This treatment was selected as earlier work [16] identified the potential for larger feed items to be consumed as first feeds. These later-stage Artemia molts enable the testing of this theory as they are larger than instar-I and importantly are readily produced in commercial-scale culture. For the 5050 treatment, the larvae were fed with a 1:1 ratio of Instar-I to enriched instar-II+ Artemia for the first 7 days by wet weight, and, for the remaining 21 days, the fish were provided with only enriched instar-II+ Artemia (Table 1). The 50:50 treatment acts as a middle ground between the other two treatments where the average size of feed particle is concerned.
Artemia cysts used throughout this experiment were A. franciscana from GSL Sep-Art (INVE Aquaculture Inc, Salt Lake City, UT, USA) from the same batch. Feeds were administered by total wet weight, by pouring harvested Artemia through a 100 µm sieve and placing the sieve on a towel to drip dry for 1 min before measuring with tared electronic scales to the nearest 0.1 g. This method was used to standardise feed volumes and had no impact on the performance or quality of Artemia. The number of Instar-I Artemia per gram were in the order of 110,000 g−1, while for Instar-II+, there were 85,000 g−1. At the initiation of live feeding, in NZPWL’s commercial larviculture tanks, feed-particle density is 8800 L−1. By the end of live feeding (30 DAH), in commercial-scale tanks, particle density nears 15,300 L−1.
Upon hatching of giant kōkopu eggs, the commercial tanks at NZPWL are stocked with fish larvae at densities in the region of 500 L−1, and the number of feed particles per larva is estimated to begin at 18 per feeding event. At the conclusion of live feeding, this increases to at least 32 food particles per larva per feeding event. In contrast to commercial conditions, the experimental tanks were stocked at 111 larvae L−1 as a result of the smaller diameter of the experimental tanks. Due to the five-fold difference in larval fish stocking densities between commercial rearing and experimental tanks, feed particles per larva are greater for the duration of this experiment than would be used under typical commercial production conditions. This is a function of the practical limitations of conducting small-scale experiments on fish larvae, as a result of an inability to run replicated commercial-scale experiments owing to the logistic and infrastructure constraints of running such large-scale experimental studies.
Instar-I Artemia production involved a 17 h incubation of cysts in 250 L Artemia cones at 29 °C at 35 ppt with constant, vigorous bubbling while exposed to light. Live Artemia were then separated from cysts via magnet and rinsed in a 100 µm sieve with clean 35 ppt salt water. Instar-I Artemia that were fed to larvae at 0830 h had been cold stored at a density of 1.67 g mL−1 at 4 °C for 16 h after their harvest late the previous day. Instar-I Artemia fed to giant kōkopu larvae at 1230 h were cold stored for 2 h, while those used for the 1630 h feed had undergone 6 h of cold storage.
To produce enriched instar-II+ Artemia for all experimental treatments, the cysts were incubated for 27 h at 29 °C at 35 ppt with constant, vigorous aeration while exposed to a 58 W tube light 100 cm above the water surface. Live animals were then separated from cysts via magnet and rinsed in a 100 µm sieve with 35 ppt clean seawater prior to the 21–29 h enrichment period. The enrichment was specially formulated by NZPWL using a proprietary combination of commercially available concentrated instant algae products (Rotigrow Plus, Nanno 3600, Tetraselmis 3600, Reed Mariculture Inc., Campbell, CA, USA). An aliquot of 60 mL of enrichment formula was provided upon the initial transfer of instar-II+ Artemia to the enrichment tank, with another 60 mL added 18 h later. Instar-II+ Artemia fed to larvae at 0830 h had been enriched for 21 h, while those fed out at 1230 h were enriched for 25 h, and those fed at 1630 h for a period of 29 h.
2.4. Sampling of Larvae
Experimental giant kōkopu larvae were sampled twice: on the first day (Day 0, age 3 DAH) and the last day (Day 28, 31 DAH) of the experiment. The first sampling event was taken from the temporary transfer vessel used to move fish from the commercial larval-rearing tank to the experimental tanks. Three samples containing 50 fish were taken at random for subsequent determination of mean wet weight and mean dry weight (DW), which was measured by freeze drying, re-weighing, and dividing by the total number of fish. The total length (i.e., snout to tip of tail) and body depth (i.e., centre of body at the anus across to the lateral surface) were measured in a further 50 randomly sampled fish. The length:depth ratio (LDR) was calculated by dividing the total length of each fish by its depth. Total length and depth of larvae were measured by taking a digital image of each fish placed on a 460 µm grid using an Olympus TG-4 camera and processing images with the computer software ImageJ64 to derive the measures.
At the final sampling event, three samples of 20 fish each were taken at random from each of the replicate experimental tanks. Moreover, for each of the nine tanks, the total length and depth of 20 fish were measured in the same manner as described previously.
Mortality of larvae was estimated at 2- and 3-day intervals by carefully siphoning the floor of each tank, and the number of dead fish that were removed were counted. Mortality in the first seven days was too great to count and appeared to be consistent among tanks and was most likely related to the stress of handling the larvae for the transfer to the small tanks. As a consequence, the first count of mortality occurred at 9 DAH. Mortality in the first seven days of the experiment was estimated by back calculation using the knowledge of how many fish were alive at the end of the experiment, how many were in each tank at the start, and how many died at each siphoning event throughout the experiment. Cumulative mortality, calculated as a percentage of the initial tank population, was determined for each tank by adding the number of dead fish at each siphoning event. Mean cumulative mortality for each treatment was calculated from the three replicate tanks within each treatment.
Total production was calculated by multiplying the total number of fish alive by the mean wet weight of fish for each tank at the end of the experiment. Fish mean wet weight was determined for each tank by taking the average of three samples of randomly selected 10 fish that were weighed to the nearest 0.001 g.
2.5. Sampling of Artemia
Triplicate samples of 10 g wet weight Artemia were taken at time points 0 h (i.e., at harvest after the 17 h incubation), then after 2, 6, and 16 h cold storage for instar-I, and sampling was repeated for a total of three batches of instar-I production/feed. The same sampling program was also used for instar-II+ Artemia; however, 0 h sample was taken after 27 h incubation, then 21, 25, and 29 h enrichment.
The lipid proportion of the Artemia samples was determined with a modified Bligh and Dyer method [17] solvent extraction [18]. In brief, after lyophilizing the samples of larvae, the lipids were extracted from the tissues into a mix of chloroform and methanol solvents and the lipid recovered by fractionating with the addition of deionized water. The lipid fraction was recovered and solvent removed through evaporation, and the remaining lipid was weighed and lipid mass was then divided by the total dry weight of the Artemia sample and multiplied by 100 and presented as (%DW).
A modified bicinchoninic acid (BCA) assay (Micro BCA™ Protein Assay Kit, ThermoFisher Scientific, Auckland, New Zealand) was used to determine the protein proportion of the Artemia sample [19]. In brief, the Artemia samples were hydrolysed with heated sodium hydroxide and then cuprous cations generated from the reduction of Cu2+ to Cu1+ by protein were measured via a colorimetric reaction with BCA against a set of bovine serum albumin standards. Protein content was then calculated as a percentage of dry weight (%DW).
Fatty acid (FA) analyses were undertaken on the previously extracted lipid using a derivatization and preparation process [20,21]. In brief, the derivatized samples were analysed using an Agilent 7890B gas chromatograph coupled to a 5977C mass spectrometer (GC-MS) with a split/splitless inlet [22,23]. The measured FA was calculated as a proportion of Artemia dry weight.
2.6. Statistical Analyses
The final mean weight, total length and depth of larvae, as well as total tank production were compared among treatments using one-way ANOVA. Normality and equality of variances were confirmed using the Shapiro–Wilk’s and Levene’s tests before analyses took place. For each of these response variables, a linear mixed model was fitted to control for the random effects of the tanks. When overall experimental treatment effects were identified by ANOVA, pairwise Tukey’s comparisons of treatment means, with adjustment for false discovery, were made to identify differences between the individual treatments. The same methods were used to compare differences in lipid and protein proportion as % DW; however, these response variables were first arc-sine-transformed to correct for any data-distribution bias associated with percentage data [24]. The difference between instar-I and instar-II+ Artemia fatty acid profiles was found using the non-parametric Kruskal–Wallis test due to the difference in number of samples in each group and non-normality. Estimated mean difference and 95% confidence intervals were calculated and are presented.
The response variable, mortality, is a binary measure so a generalized mixed effects model with binomial distribution (logistic regression) was used to compare mortality among treatments over the course of the experiment, whilst also controlling for the random effects of the tanks. Where ANOVA identified effects of experimental differences at age–time points, a Z-score test was implemented to establish whether the difference was significant.
All statistical analyses were performed using R (RStudio, ver. 1.2.1335, Boston, MA, USA). All measures of variability of means are reported as standard error of the mean.
3. Results
3.1. Weight
At the start of the experiment, the mean WW for the larvae that were distributed at random among all the treatment tanks was 2.30 ± 0.02 mg.
The mean WW of the larvae was significantly different among the treatment groups at the conclusion of the experiment (F(2,6) = 26.49, p < 0.01). The mean WW of the fish from the 5050 treatment was 10.48 ± 0.35 mg, which was between 1.32 and 1.62 times greater than the mean WW for the NZPWL treatment which was 7.19 ± 0.26 mg (p < 0.01) (Figure 1). The mean WW of the larvae fed the 5050 treatment was also between 1.16 and 1.42 times greater than for the Instar-II+ treatment which had a mean WW of 8.18 ± 0.17 mg (p < 0.01) (Figure 1). The mean WW of the larvae for the Instar-II+ treatment was also between 1.03 and 1.27 times greater than the mean WW for the NZPWL treatment (p < 0.05).
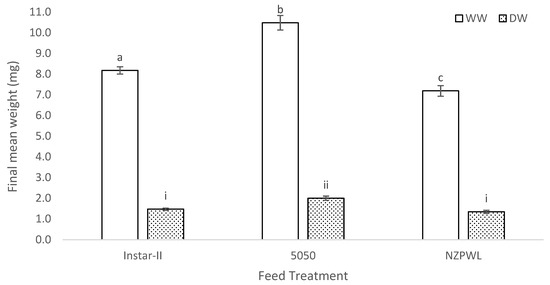
Figure 1.
Final mean wet weight (WW) and mean dry weight (DW) for larval giant kōkopu from three different feed treatments, Instar-II+, 5050, and NZPWL, at the end of a 28-day experimental period (mean ± SE). Means with different superscripts are significantly different for WW or DW (p < 0.05).
The mean DW of the larval giant kōkopu at the start of the experiment was 0.380 ± 0.001 mg. However, the mean DW of the larvae by the end of the experiment was different among the three feed treatments (F(2,6) = 10.67, p = 0.01). The mean DW of the larvae in the 5050 treatment was 2.00 ± 0.15 mg, which was between 1.24 and 1.77 times larger than for the NZPWL treatment which had a mean DW of 1.35 ± 0.09 mg (p = 0.01) (Figure 1). The mean DW of the larvae in the 5050 treatment at the end of the experiment was also 1.13 to 1.61 times larger than for the Instar-II+ treatment which had a mean DW of 1.47 ± 0.04 mg (p = 0.02) (Figure 1). The mean DW of the larvae in the Instar-II+ and NZPWL treatments were not significantly different (p = 0.31).
3.2. Length and Depth
The mean total length of the fish at the start of the experiment was 10.20 ± 0.10 mm. However, the mean total length of the giant kōkopu larvae at the end of the experiment differed significantly among the treatment groups (F(2,6) = 21.44, p < 0.01). The mean total length of the fish in the 5050 treatment was 15.55 ± 0.25 mm, which was between 0.16 and 0.30 mm longer than those in the NZPWL treatment with a mean total length of 13.28 ± 0.26 mm (p < 0.01) (Figure 2). The mean final total length of the larvae in the 5050 treatment was between 0.08 and 0.22 mm longer than the Instar-II+ treatment with a mean of 14.03 ± 0.25 mm (p < 0.01) (Figure 2). There was no difference between the mean total length of the larvae in the Instar-II+ and NZPWL treatments (p = 0.08).
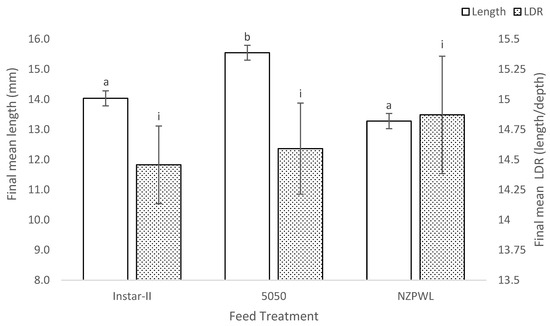
Figure 2.
Final mean total length (primary axis) and mean length:depth ratio (secondary axis) of larval giant kōkopu for three different feed treatments, Instar-II+, 5050, and NZPWL, at the end of the 28-day experimental period (mean ± SE). Means with different superscripts are significantly different among either final mean total length or mean length:depth ratio (p < 0.05).
There was no significant difference in the mean LDR (F(2,6) = 0.277, p = 0.77): Instar-II+—14.46 ± 0.32, 5050—14.59 ± 0.38, and NZPWL—14.87 ± 0.49 (Figure 2).
3.3. Mortality
The initial mortality was very high for all treatment groups in the first four days of the experiment. Mortality rates then slowed in all groups for the next seven-day period and plateaued with little additional mortality from age 14 DAH until the completion of the experiment at age 31 DAH. Significant differences in the cumulative mortality were found among the treatments at several different times during this experiment (χ2 = 426.0, p < 0.001). The mortality at 7 DAH did not differ significantly among treatment groups, i.e., Instar-II+—63.3 ± 2.13%, 5050—66.65 ± 2.72%, and NZPWL—67.72 ± 4.30% (p > 0.05).
At 14 DAH, the mean cumulative mortality of thet larvae in the NZPWL treatment (i.e., 90.3 ± 2.2% SE) was greater than for the Instar-II+ treatment (i.e., 81.9 ± 1.3%) (p < 0.01) and the 5050 treatment, which had a mean mortality of 83.2 ± 3.0% (p < 0.01) (Figure 3).
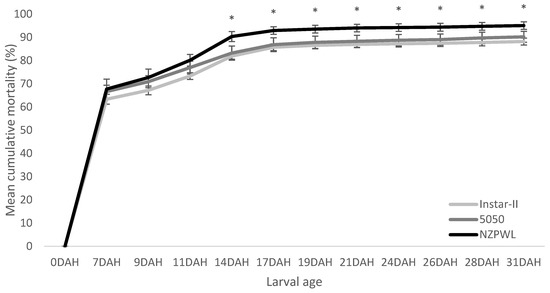
Figure 3.
Mean cumulative mortality of larval giant kōkopu in three different feed treatments, Instar-II+, 5050, and NZPWL, over the 28-day experimental period (mean ± SE). Sets of three treatment means marked with asterisk are significantly different for that DAH sampling event (p < 0.05).
The mean cumulative mortality of the NZPWL treatment remained significantly higher than both of the other treatment groups for the remainder of the experiment (Figure 3). At no point in the experiment did the cumulative mean mortalities of the 5050 and Instar-II+ treatments differ.
At the conclusion of this experiment, the mean cumulative mortality of the larvae in NZPWL (i.e., 95.1 ± 1.6%) was greater than that of Instar-II+ by between 1.7 and 4.1% (p < 0.01) with a cumulative mean mortality of 88.2 ± 1.5% (Figure 3). The NZPWL cumulative mortality was also greater than that of the 5050 treatment which had a mean mortality of 90.2 ± 2.4%, which was between 1.4 and 3.3% lower than for the NZPWL treatment (p < 0.01).
3.4. Artemia Lipid and Protein Composition
The lipid proportion was very consistent among instar-I samples, showing no significant difference even after a 16 h (in-1:16h-f) cold storage when compared to immediately post-harvest (in-1:0 h) (t = −0.24, p = 0.91) (Figure 4). It is also notable that there was no difference in the total lipid proportion between instar-I and instar-II+ immediately after harvest (in-1:0 h and in-2:0 h) (t = −0.02, p = 0.99) (Figure 4). However, all of the enriched instar-II+ Artemia treatments (in-2:21 h-e, in-2:25 h-e, and in-2:29 h-e) had significantly lower lipid relative to their dry weight compared with those immediately after harvest (in-2:0 h) (t = 5.72, p < 0.01; t = 6.90, p < 0.01; and t = 6.91, p < 0.01, respectively) (Figure 4).
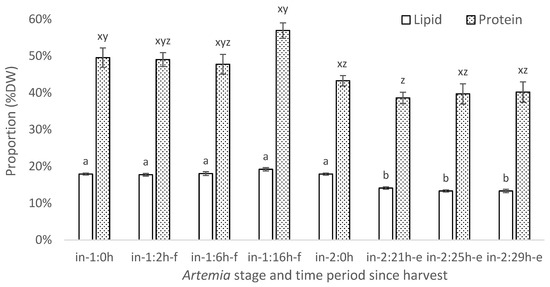
Figure 4.
Mean lipid (no fill) and protein (shaded) proportions of Artemia as % dry weight. Annotations: in-1 refers to instar-I Artemia and 0 h denotes immediately after harvest; 2, 6, and 16 h-f denote 2, 6, and 16 h in cold store, respectively. In-2 refers to instar-II+ Artemia and 0 h immediate sampling on harvest; 21, 25, and 29h-e signify 21, 25, and 29 h enrichment, respectively. Means with different superscripts are significantly different among means for lipid and protein (p < 0.05).
A similar trend was present in the protein proportion in Artemia where there was no difference among any of the instar-I samples from the time of their harvest and subsequent cold storage (i.e., in-1:0 h and in-1:2 h t = 0.21, p = 0.87; in-1:0 h and in-1:6 h t = 0.53, p = 0.73; or in-1:0 h and in-1:16 h t =−2.10, p = 0.11) (Figure 4). All instar-II+ Artemia all had a lower protein proportion than instar-I after a 16 h cold storage (in-2:0 h t = 3.39, p = 0.02; in-2:21 h-e t = 4.47, p = 0.01; in-2:25 h-e t = 4.19, p = 0.01; and in-2:29 h-e t = 4.08, p = 0.01) while a 21 h enriched instar-II+ had a lower protein proportion than freshly hatched instar-I (t = 1.39, p = 0.03). There were no other differences in the protein composition among instar-I and instar-II+ Artemia.
3.5. Artemia Fatty Acid Profile
The fatty acid profiles of instar-I and instar-II+ Artemia were markedly different with instar II+ Artemia, containing higher proportions of some saturated fatty acids on a dry-weight basis (Table 2). For example, instar-II+ were higher than instar-I in C18:0 2.162 ± 0.043% vs. 1.853 ± 0.026% (χχ2 = 29.82, df = 1, p < 0.01), C20:0 0.049 ± 0.001% vs. 0.049 ± 0.001% (χ2 = 30.21, df = 1, p < 0.01), and C22:0 0.095 ± 0.003% vs. 0.051 ± 0.001% (χ2 = 49.60, df = 1, p < 0.01).

Table 2.
Mean percent fatty acid composition (±SE) of total dry weight of instar-I and enriched instar-II+ Artemia. Superscript characters indicate significant differences along the row. ARA: arachidonic acid; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid; PUFA: polyunsaturated FA; HUFA: highly unsaturated FA; ND: not detected; c: -cis isomer; and t: -trans isomer.
No differences were found between instar-I and instar-II+ Artemia for C18:1n-9t (χ2 = 2.34, df = 1, p = 0.13) and C20:4n-6c (ARA) (χ2 = 0.15, df = 1, p = 0.70); however, Instar-II+ had higher C18:1n-7c 0.935 ± 0.017 % vs. 0.671 ± 0.010 % (χ2 = 48.95, df = 1, p < 0.01).
All other fatty acids were in a higher concentration in instar-I than instar-II+ Artemia. For example, C20:5n-3 0.245 ± 0.005% vs. 0.205 ± 0.014% (χ2 = 4.76, df = 1, p < 0.05). The total PUFA and total HUFA were both higher in instar-I Artemia comprising PUFA 6.841 ± 0.079% vs. 4.164 ± 0.206% (χ2 = 48.30, df = 1, p < 0.01), and HUFA 0.518 ± 0.011% vs. 0.401 ± 0.024% (χ2 = 17.10, df = 1, p < 0.01). DHA was not detected at any level in either form of Artemia.
3.6. Total Production
The mean total production of the larvae by weight differed among treatments (F(2,6) = 7.21, p = 0.03). The mean total production of the larvae in the 5050 treatment was 1.82 ± 0.31 g and was between 0.52 and 1.94 g greater than the NZPWL treatment with a mean of 0.59 ± 0.23 g (p = 0.03) (Figure 5). The Instar-II+ treatment mean total production 1.74 ± 0.22 g was between 0.43 and 1.85 g greater than that of the NZWPL treatment (p = 0.03) (Figure 5). There was no difference in the mean total production for the Instar-II+ and 5050 treatments (p = 0.82).
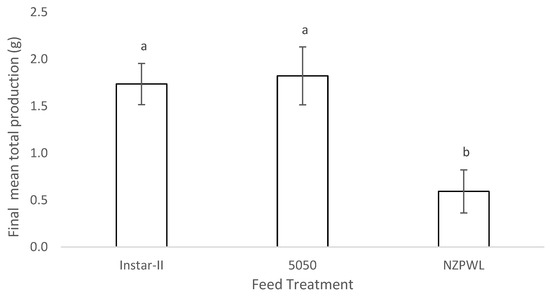
Figure 5.
Mean total production of larval giant kōkopu resulting from three different feed treatments, Instar-II+, 5050, and NZPWL, over the 28-day experimental period (mean ± SE). Means with different superscripts are significantly different (p < 0.05).
4. Discussion
The performance of cultured larval giant kōkopu was influenced by three different Artemia live-feed treatments tested in this research. The 5050 treatment achieved the greatest final mean WW, DW, and total length of the larvae when compared with the NZPWL and the Instar-II+ treatments. The final mean WW of the Instar-II+ treatment was greater than that of the NZPWL treatment; however, there were no other significant differences between these two treatment groups for the remaining performance measures.
Mortality was extensive in the first seven days of this experiment and consistent across all three treatments. This could be attributed to the physical and physiological stress involved in the transfer of the fish from the commercial to experimental tanks. However, the subsequent cumulative mortality was not consistent among the treatments from day 14 when the larval mortality in the NZPWL treatment first became higher than for the other two treatments. This difference increased further over the remainder of the experimental period. In contrast, there was no difference in the cumulative mortality between the Instar-II+ and 5050 treatments at any time during the experiment.
The growth and survival in fish larvae rely to a large extent on the ability to capture and digest food with an appropriate nutritional composition. The nutritional value of the two stages of Artemia nauplii are not equivalent, with instar-I being richer in lipid with a higher proportion of unsaturated fatty acids and marginally higher in protein than instar-II+. Interestingly, the feed treatments containing lower proportions of these two vital energetic and growth nutrients (i.e., Instar-II+ and 5050) achieved a greater growth performance and survival than the NZPWL treatment, which received only instar-I for the first two weeks. The lower EPA, PUFA, and HUFA composition of instar-II+ Artemia compared to instar-I used in these feed treatments did not appear to constrain the growth in these feed treatments despite the higher dietary supply of these essential fatty acids generally being considered to be critical for greater growth performance and survival in larval fish [25,26,27,28,29,30,31,32]; that is not the case in the present study. This suggests that, despite the lower overall EPA, PUFA, and HUFA supplied by the instar-II+ Artemia, it is sufficient to meet the demands for the somatic growth of the larvae. The closely related Galaxias maculatus has shown that, when cultured under high-salinity conditions, higher levels of EPA and PUFA are not critical, while DHA is important in the larval development of the eye and neural networks, which can lead to detrimental impacts on the feeding ability, growth performance, and survival [28,31,33,34,35,36,37]. In the present study, the DHA fatty acid level was absent in both Artemia feeds. This is unsurprising for instar-I as they are naturally deficient in DHA [30,38]; however, the key role of enrichment of instar-II+ Artemia is to provide these essential fatty acids [39,40]. This is a direct result of using enrichment products that are very low in DHA, and the delay between the enrichment and feeding out. This low DHA content is likely to have had a detrimental impact on the early larval performance; however, given the lack of DHA in all treatments, the nutritional profile of the feed items cannot be the sole determinant of the greater success seen in the treatments with earlier and higher provisions of instar-II+ Artemia.
The provision of live prey of a suitable size and type, and stocked at a sufficient density to make them available to larvae for consumption is paramount to achieving optimum productivity [41]. Despite the importance of the first feeding and subsequent weaning from live feed for larval fin-fish growth and survival [9], few studies have directly compared the impacts of the timing and proportion of instar-I and instar-II+ Artemia on the growth performance and survival of cultured larvae.
A primary limitation on the size of prey that can be consumed by larval fin-fish is the size of the mouth opening or mouth gape [42,43,44,45,46,47]. Consequently, it is vital that feed items meet the species-specific capture capabilities of the larval fish in culture. This is of particular importance at the transitional stage from endogenous to exogenous resource utilization when larval fish are highly vulnerable [48]. Any delay in the availability of suitable food items can have immediate and ongoing effects on the growth performance and survival for larval fish.
Larval giant kōkopu have a mouth gape of 348 ± 15 μm upon hatching and, by 7 DAH, the gape has increased to 538 ± 20 μm [16]. The provision of food for the larval giant kōkopu in this current experiment began at 3 DAH, which is consistent with the commercial rearing protocol of NZPWL. The ratio of prey size:mouth gape has been a key determining factor in food-item choice in aquaculture, with 25–60% considered to be optimum [43,49,50,51]. The prey size:mouth gape ratio for giant kōkopu larvae at the initiation of exogenous feeding (at 3 DAH) if provisioned with instar-I Artemia (minimum width 195 μm) is 48%, and 70 % for instar-II+ (width minimum 270 μm) [9,16]. At this stage of development of the larval giant kokopu, their mouth gape could be expected to limit the capture success of the larger instar-II+ Artemia (520 μm length) in comparison to instar-I Artemia (450 length). However, the Instar-II+ group still outperformed the NZPWL group for several measured variables, most importantly the total biomass production. This would suggest any potential size limitation has been overcome to some degree by other characteristics of the instar-II+ Artemia nauplii.
Feed-particle density plays a vital role in the initiation of exogenous feeding in larviculture with foraging success shown to increase with increasing feed-particle density until an asymptote is reached when feeding in excess [52,53,54,55]. Low feed densities result in a lower growth performance and survival due to the increased energy-partitioning-to-foraging effort [53,56,57]. Unfortunately, as a new species being developed for aquaculture, there is no existing information on the larval feed density requirements for the giant kōkopu.
The NZPWL treatment received greater feed-particle density over the first 14 days of this experiment. However, it was the worst-performing treatment in terms of growth performance and achieved the greatest mortality. This suggests that the feed-particle density in this treatment could have exceeded the requirements of the larval giant kōkopu. Reduced growth performance and survival can occur despite increased encounters between predator and prey when the predator’s assimilation capacity is exceeded [58]. For example, the growth performance of Atlantic cod (Gadus morhua) larvae did not increase with a corresponding increase in prey density from 4000 L−1 to 16,000 L−1 [53], a response that is thought to be due to the larval fish continuing to consume prey despite having a full gut, resulting from a lack of satiety feedback [59]. As a consequence of this continued larval feeding, the gut throughput increases, reducing residence times and decreasing the efficiency of digestion. Ultimately, this results in an overall reduction in the net energy budget for the larvae. Additionally, high densities of live-food items frequently cause reduced growth and survival through degrading water quality [53,60]. This may also help to explain the increased mortality observed in the NZPWL treatment from 14 DAH.
Successful diets are not only dependent on the ability of cultured species to capture the feed item or the nutritional quality but also the digestive capabilities of the larval fish which is restricted by their simple gut with little physical processing capacity combined with the relative lack of the quantity, activity, and diversity of digestive enzymes [61,62,63,64]. This restricts the ability of the larval fish to hydrolyse the digesta to break down the fractions which can then be more effectively absorbed and assimilated. Likewise, an inability to physically break down ingested food, such as the tough exoskeleton of crustaceans, can severely inhibit access to the nutrient-rich tissues contained within [61,65,66,67,68]. Instar-II+ Artemia are more readily digestible than instar-I as their mouth is open, providing a second point of entry for the digestive enzymes of the fish larvae [7,65,69]. Furthermore, instar-II+ have a greater diversity of endogenous digestive enzymes which, once released in the gut of the fish, can also help to break down the digesta [65,70,71,72,73,74]. This increased digestibility of instar-II+ Artemia may have played an important role in the improved growth performance of the giant kōkopu larvae by improving their ability to hydrolyse what was a slightly lesser amount of available protein than the fish which received a higher proportion of instar-I Artemia.
The current study suggests instar-I Artemia could be substituted with instar-II+ for a greater effect in the larviculture of giant kōkopu, a substitution that may be useful for the larviculture of other species. For example, the Senegalese sole, Solea senegalensis, showed no negative consequences when the instar-I Artemia was replaced with instar-II+ in larval rearing [75]. However, the larvae of the mandarin fish, Synchiropus splendidus, achieved greater survival when given instar-II+ Artemia at 25 DAH than 28 DAH; however, at 22 DAH, survival was reduced, suggesting any substitution between the two Artemia stages may also be highly sensitive to the state of larval development [76]. The earlier provision, and the increasing proportion, of instar-II+ Artemia, in the diet of larval giant kōkopu, has the potential to markedly improve the commercial production of this species. A comparison of the total productivity of each of the treatments by their final biomass demonstrates the advantage gained by the Instar-II+ and 5050 feed regimes. The 5050 treatment produced between 88 and 328% more larval giant kōkopu biomass than the NZPWL treatment, while the Instar-II+ treatment produced between 73 and 314% more, which would translate to a significant increase in productivity at the commercial scale. There is potential for further significant gains through the development of a more suitable enrichment formula and protocol for the instar-II Artemia to ensure that the EPA, DHA, and ARA are in suitable proportions.
The most significant savings through switching to one of the proposed feed regimes is the reduced Artemia costs. The Artemia product currently used by NZPWL costs $282NZ per kg of cysts with a total of 11.1 kg required for the live-feeding stage for approximately 2.4 million larval giant kōkopu. Estimating from the 2016 harvests, this number of fish results in approximately 75 kg of whitebait product at harvest. In adopting the timing and proportion of the instar-II+ provision in the 5050 regime, the cost of Artemia cysts required would be reduced by 21%, while the Instar-II+ regime would offer a 25% saving. The additional cost required for enriching the instar-II+ Artemia are in the order of $7NZ per kg wet weight of Artemia, resulting in overall savings of 22% and 18%, respectively, for the Instar-II+ and 5050 feed regimes. Further savings will be made as a result of the lower labor associated with instar-II+ Artemia production.
Furthermore, better conditions (i.e., larger size and better health) have been shown to reduce the age at which the larval fish will accept artificial dry foods [77,78,79]. The increased size of the larvae at a younger age and the improved condition achieved when using the 5050 and Instar-II+ diets have the potential to allow the weaning from live to artificial feeds at an earlier age than currently practiced. This outcome would result in greater productivity and further savings on food-associated costs.
5. Conclusions
The results of this study demonstrate the major potential benefits that the earlier provision, as well as the increased proportion, of enriched instar-II+ Artemia has on the larviculture of giant kōkopu. By improving the growth performance and survival of the larvae, productivity increased substantially, an obvious commercial benefit. These changes to the diet directly reduce the live-feed costs and bolster the commercial production benefits.
To further optimize the live-feeding regime of larval giant kokopu, future research should focus on understanding the growth performance and survival of larvae fed a lower range of feed-particle densities. Additionally, the impacts of the nutrient composition of instar-II+ Artemia with differing enrichment formulae should be assessed on the growth performance and survival of this species. It is evident from this study that attempts to improve the nutritional quality of enriched instar-II+ Artemia may not currently be achieved given the lower levels of EPA, PUFA, and HUFA, and the lack of solid evidence in the form of increased ARA or DHA levels compared to instar-I. As such, the current enrichment protocols used in the current commercial production of giant kōkopu should be assessed with it being likely that the composition, quantity, and timing of enrichment is inadequate to maximise the essential fatty acid composition of the enriched Artemia.
Author Contributions
Conceptualisation, W.M. and A.J.; methodology, W.M.; data analyses, W.M.; writing and editing, W.M. and A.J. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Callaghan Innovation under project NZPWH1501/PROP-47490-FELLOW. Logistic support was provided by New Zealand Premium Whitebait Ltd., Auckland, New Zealand.
Institutional Review Board Statement
Ethical review and approval were not required for this study under New Zealand’s Animal Welfare Act 1999 because it explicitly excludes the larval stages.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author. The data are not publicly available due to stakeholder privacy.
Acknowledgments
We would like to thank New Zealand Premium Whitebait Ltd. for the larvae and, in particular, Paul Decker and Tagried Kurwie for sharing their experiences and expertise on the larval rearing of galaxiids.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Webster, C.D.; Lim, C.E. Nutrient Requirements and Feeding of Finfish for Aquaculture; CABI: Cambridge, UK, 2001; ISBN 978-0-85199-702-5. [Google Scholar]
- Hamre, K.; Yúfera, M.; Rønnestad, I.; Boglione, C.; Conceição, L.E.C.; Izquierdo, M.S. Fish larval nutrition and feed formulation: Knowledge gaps and bottlenecks for advances in larval rearing. Rev. Aquac. 2013, 5, S26–S58. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Koven, W. Larval Fish Nutrition; Holt, G.J., Ed.; Wiley-Blackwell: Oxford, UK, 2011; pp. 1–143. ISBN 978-0-470-95986-2. [Google Scholar]
- Trushenski, J.T.; Kasper, C.S.; Kohler, C.C. Challenges and opportunities in finfish nutrition. N. Am. J. Aquac. 2006, 68, 122–140. [Google Scholar] [CrossRef]
- Southgate, P.C.; Partridge, G.J. Development of artificial diets for marine finfish Larvae. In Tropical Mariculture; Elsevier: Amsterdam, The Netherlands, 1998; pp. 151–169. ISBN 978-0-12-210845-7. [Google Scholar]
- Dhert, P. Rotifers. In Manual on the Production and Use of Live Food for Aquaculture; Sorgeloos, P., Lavens, P., Eds.; Food and Agriculture Organization of the United Nations: Rome, Italy, 1996; pp. 49–78. [Google Scholar]
- Van Stappen, G. Artemia. In Manual on the Production and Use of Live Food for Aquaculture; Lavens, P., Sorgeloos, P., Eds.; FAO Fisheries Technical Paper; Food and Agriculture Organisation: Rome, Italy, 1996; pp. 79–250. [Google Scholar]
- Qin, J.G. Larval fish nutrition and rearing technology: State of the art and future. In Aquaculture Research Trends; Schwartz, S.H., Ed.; Nova Science Publishers: New York, NY, USA, 2008; pp. 113–148. [Google Scholar]
- Hoestenberghe, S.V.; Wille, M.; Swaef, E.D.; Goddeeris, B.M.; Nevejan, N. Effect of weaning age and the use of different sized Artemia nauplii as first feed for jade perch Scortum barcoo. Aquac. Int. 2015, 23, 1539–1552. [Google Scholar] [CrossRef]
- Taunton, E. $140 a Kilogram: Whitebait Back to “Gold-Plated” Prices. Available online: https://www.stuff.co.nz/business/122878457/140-a-kilogram-whitebait-back-to-goldplated-prices (accessed on 25 July 2022).
- Vega, R.; Dantagnan, P.; Mardones, A.; Valdebenito, I.; Zamorano, J.; Encina, F. Bases biológicas para el cultivo del puye Galaxias maculatus (Jenyns, 1842): Una revisión. Lat. Am. J. Aquat. Res. 2013, 41, 369–386. [Google Scholar] [CrossRef]
- Benzie, V. Some ecological aspects of the spawning behaviour and early development of the common whitebait, Galaxias Maculatus Attenuatus (Jenyns). Proc. N. Z. Ecol. Soc. 1968, 15, 31–39. [Google Scholar]
- Mardones, A.; Vega, R.; Encina, F. Cultivation of whitebait (Galaxias maculatus) in Chile. Aquac. Res. 2008, 39, 731–737. [Google Scholar] [CrossRef]
- Mitchell, C.P. Laboratory culture of Galaxias maculatus and potential applications. N. Z. J. Mar. Freshw. Res. 1989, 23, 325–336. [Google Scholar] [CrossRef]
- O’Brien, Q.; Cooper, D. Conservation breeding of shortfin eels (Anguilla australis) and giant kokopu (Galaxias argenteus) at Mahurangi Technical Institute using aquarium and aquaculture techniques. Int. Zoo Yearb. 2013, 47, 120–128. [Google Scholar] [CrossRef]
- McKay, W.J.G.; Jeffs, A.G. Morphometric and energetic development of artificiall reared giant kōkopu (Galaxias argenteus). Aquaculture 2021, 544, 737123. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Wang, W.; Wang, H.; Yu, C.; Jiang, Z. Acute toxicity of ammonia and nitrite to different ages of Pacific cod (Gadus macrocephalus) larvae. Chem. Speciat. Bioavailab. 2015, 27, 147–155. [Google Scholar] [CrossRef]
- Smith, P.K.; Krohn, R.I.; Hermanson, G.T.; Mallia, A.K.; Gartner, F.H.; Provenzano, M.D.; Fujimoto, E.K.; Goeke, N.M.; Olson, B.J.; Klenk, D.C. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985, 150, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Spreitzenbarth, S.; Jeffs, A. effect of hatching time and starvation on morphometrics and biochemical composition of Octopus tetricus paralarvae. Aquac. Res. 2022, 53, 1739–1754. [Google Scholar] [CrossRef]
- Jeffs, A.G.; Phleger, C.F.; Nelson, M.M.; Mooney, B.D.; Nichols, P.D. Marked depletion of polar lipid and non-essential fatty acids following settlement by post-larvae of the spiny lobster Jasus verreauxi. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2002, 131, 305–311. [Google Scholar] [CrossRef]
- Kramer, J.K.G.; Hernandez, M.; Cruz-Hernandez, C.; Kraft, J.; Dugan, M.E.R. Combining results of two GC separations partly achieves determination of all cis and trans 16:1, 18:1, 18:2 and 18:3 except CLA isomers of milk fat as demonstrated using Ag-Ion SPE fractionation. Lipids 2008, 43, 259–273. [Google Scholar] [CrossRef]
- Zar, J.H. Biostatistical Analysis, 4th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 1999; ISBN 978-0-13-081542-2. [Google Scholar]
- Bell, M.V.; Batty, R.S.; Dick, J.R.; Fretwell, K.; Navarro, J.C.; Sargent, J.R. Dietary deficiency of docosahexaenoic acid impairs vision at low light intensities in juvenile herring (Clupea harengus L.). Lipids 1995, 30, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bengtson, D.A.; Léger, P.; Sorgeloos, P. Use of Artemia as a food source for aquaculture. In Artemia Biology; CRC Press: Boca Raton, FL, USA, 1991; Volume 29. [Google Scholar]
- Boglino, A.; Darias, M.J.; Ortiz-Delgado, J.B.; Özcan, F.; Estévez, A.; Andree, K.B.; Hontoria, F.; Sarasquete, C.; Gisbert, E. Commercial products for Artemia enrichment affect growth performance, digestive system maturation, ossification and incidence of skeletal deformities in Senegalese sole (Solea senegalensis) larvae. Aquaculture 2012, 324–325, 290–302. [Google Scholar] [CrossRef]
- Izquierdo, M.S.; Koven, W. Lipids. In Larval Fish Nutrition; Holt, G.J., Ed.; Wiley-Blackwell: Oxford, UK, 2011; pp. 47–81. ISBN 978-0-470-95986-2. [Google Scholar]
- Izquierdo, M.S. Essential fatty acid requirements of cultured marine fish larvae. Aquac. Nutr. 1996, 2, 183–191. [Google Scholar] [CrossRef]
- Koven, W.; Nixon-Shtupler, O.; Lutzky, S.; Ben Atia, S.; Elkayam, A.; Tandler, A. The effect of N-3 HUFA and light intensity on hunting success in gilthead sea bream (Sparus aurata). Isr. J. Aquac.-Bamidgeh 2012, 64, IJA:64.2012.712. [Google Scholar]
- Koven, W.; Nixon, O.; Allon, G.; Gaon, A.; El Sadin, S.; Falcon, J.; Besseau, L.; Escande, M.; Vassallo Agius, R.; Gordin, H.; et al. The effect of dietary DHA and taurine on rotifer capture success, growth, survival and vision in the larvae of Atlantic bluefin tuna (Thunnus thynnus). Aquaculture 2018, 482, 137–145. [Google Scholar] [CrossRef]
- Sargent, J.R.; Tocher, D.R.; Bell, J.G. The Lipids. In Fish Nutrition, 3rd ed.; Halver, J.E., Hardy, R.W., Eds.; Academic Press: San Diego, CA, USA, 2003; pp. 181–257. ISBN 978-0-12-319652-1. [Google Scholar]
- Dantagnan, P.; Bórquez, A.; Hernández, A.; Izquierdo, M.S. Effect of EPA/DHA ratios on the growth and survival of Galaxias maculatus (Jenyns, 1842) larvae reared under different salinity regimes. Aquac. Res. 2010, 41, e239–e244. [Google Scholar] [CrossRef]
- Dantagnan, P.; Bórquez, A.; Pavez, C.; Hernández, A. Feeding ω-3 PUFA enriched rotifers to Galaxias maculatus (Jenyns, 1842) larvae reared at different salinity conditions: Effects on growth parameters, survival and fatty acids profile. Lat. Am. J. Aquat. Res. 2013, 41, 404–411. [Google Scholar] [CrossRef]
- Mourente, G.; Tocher, D.R.; Sargent, J.R. Specific Accumulation of docosahexaenoic acid (22:6n-3) in brain lipids during development of juvenile turbot Scophthalmus maximus L. Lipids 1991, 26, 871–877. [Google Scholar] [CrossRef]
- Mourente, G.; Tocher, D.R. Lipid class and fatty acid composition of brain lipids from Atlantic herring (Clupea harengus) at different stages of development. Mar. Biol. 1992, 112, 553–558. [Google Scholar] [CrossRef]
- Navarro, J.C.; McEvoy, L.A.; Bell, M.V.; Amat, F.; Hontoria, F.; Sargent, J.R. Effect of different dietary levels of docosahexaenoic acid (DHA, 22:6ω-3) on the DHA composition of lipid classes in sea bass larvae eyes. Aquac. Int. 1997, 5, 509–516. [Google Scholar] [CrossRef]
- Peykaran Mana, N.; Vahabzadeh, H.; Seidgar, M.; Hafezieh, M.; Pourali, H. Proximate composition and fatty acids profiles of artemia cysts, and nauplii from different geographical regions of Iran. Iran. J. Fish. Sci. 2014, 13, 761–775. [Google Scholar]
- Navarro, J.C.; Henderson, R.J.; McEvoy, L.A.; Bell, M.V.; Amat, F. Lipid conversions during enrichment of Artemia. Aquaculture 1999, 174, 155–166. [Google Scholar] [CrossRef]
- Viciano, E.; Monroig, Ó.; Salvador, A.; Amat, J.; Fiszman, S.; Navarro, J.C. Enriching Artemia nauplii with a high DHA-containing lipid emulsion: Search for an optimal protocol. Aquac. Res. 2015, 46, 1066–1077. [Google Scholar] [CrossRef]
- van der Meeren, T.; Næss, T. How does cod (Gadus morhua) cope with variability in feeding conditions during early larval stages? Mar. Biol. 1993, 116, 637–647. [Google Scholar] [CrossRef]
- Arts, M.T.; Evans, D.O. Precision micrometer measurement of mouth gape of larval fish. Can. J. Fish. Aquat. Sci. 1987, 44, 1786–1791. [Google Scholar] [CrossRef]
- Bremigan, M.T.; Stein, R.A. Gape-dependent larval foraging and zooplankton size: Implications for fish recruitment across systems. Can. J. Fish. Aquat. Sci. 1994, 51, 913–922. [Google Scholar] [CrossRef]
- Cunha, I.; Planas, M. Optimal Prey Size for early turbot larvae (Scophthalmus maximus L.) based on mouth and ingested Pprey size. Aquaculture 1999, 175, 103–110. [Google Scholar] [CrossRef]
- Krebs, J.M.; Turingan, R.G. Intraspecific variation in gape–prey size relationships and feeding success during early ontogeny in red drum, Sciaenops ocellatus. Environ. Biol. Fishes 2003, 66, 75–84. [Google Scholar] [CrossRef]
- Makrakis, M.C.; Nakatani, K.; Bialetzki, A.; Gomes, L.C.; Sanches, P.V.; Baumgartner, G. Relationship between gape size and feeding selectivity of fish larvae from a Nneotropical reservoir. J. Fish Biol. 2008, 72, 1690–1707. [Google Scholar] [CrossRef]
- Zaret, T.M. Predation and Freshwater Communities; Yale University Press: London, UK, 1980; p. 187. [Google Scholar]
- Black, K.D.; Pickering, A.D. Biology of Farmed Fish; Sheffield Academic Press: Sheffield, UK; CRC Press: Boca Raton, FL, USA, 1998; ISBN 978-0-8493-9731-8. [Google Scholar]
- Fernández-Diaz, C.; Pascual, E.; Yúfera, M. Feeding behaviour and prey size selection of gilthead seabream, Sparus aurata larvae fed on inert and live food. Mar. Biol. 1994, 118, 323–328. [Google Scholar] [CrossRef]
- Østergaard, P.; Munk, P.; Janekarn, V. Contrasting feeding patterns among apecies of fish larvae from the tropical Andaman sea. Mar. Biol. 2005, 146, 595–606. [Google Scholar] [CrossRef]
- Shirota, A. Studies on the mouth size of fish larvae. Bull. Jpn. Soc. Sci. Fish. 1970, 36, 353–368. [Google Scholar] [CrossRef]
- Houde, E.D.; Schekter, R.C. Feeding by marine fish larvae: Developmental and functional responses. Environ. Biol. Fishes 1980, 5, 315–334. [Google Scholar] [CrossRef]
- Puvanendran, V.; Brown, J.A. Foraging, growth and survival of Atlantic cod larvae reared in different prey concentrations. Aquaculture 1999, 175, 77–92. [Google Scholar] [CrossRef]
- Temple, S.; Cerqueira, V.R.; Brown, J.A. The effects of lowering prey density on the growth, survival and foraging behaviour of larval fat snook (Centropomus parallelus, Poey 1860). Aquaculture 2004, 233, 205–217. [Google Scholar] [CrossRef]
- Wyatt, T. Some effects of food density on the growth and behaviour of plaice larvae. Mar. Biol. 1972, 14, 210–216. [Google Scholar] [CrossRef]
- Kiørboe, T.; Munk, P. Feeding and growth of larval herring, Clupea harengus, in relation to Ddensity of copepod nauplii. Environ. Biol. Fishes 1986, 17, 133–139. [Google Scholar] [CrossRef]
- Munk, P.; Kiørboe, T. Feeding behaviour and swimming activity of larval herring (Clupea harengus L.) in relation to density of copepod nauplii. Mar. Ecol.-Prog. Ser. 1985, 24, 15–21. [Google Scholar] [CrossRef]
- Werner, R.G.; Blaxter, J.H.S. Growth and survival of larval herring (Clupea harengus) in relation to prey density. Can. J. Fish. Aquat. Sci. 1980, 37, 1063–1069. [Google Scholar] [CrossRef]
- Rønnestad, I.; Yúfera, M.; Ueberschär, B.; Ribeiro, L.; Sæle, Ø.; Boglione, C. Feeding behaviour and digestive physiology in larval fish: Current knowledge, and gaps and bottlenecks in research. Rev. Aquac. 2013, 5, S59–S98. [Google Scholar] [CrossRef]
- Houde, E.D. Effects of stocking density and food density on survival, growth and yield of laboratory-reared larvae of sea bream Archosargus rhomboidalis (L.) (Sparidae). J. Fish Biol. 1975, 7, 115–127. [Google Scholar] [CrossRef]
- Cara, J.B.; Moyano, F.J.; Cárdenas, S.; Fernández-Díaz, C.; Yúfera, M. Assessment of digestive enzyme activities during larval development of white bream. J. Fish Biol. 2003, 63, 48–58. [Google Scholar] [CrossRef]
- Chen, B.N.; Qin, J.G.; Kumar, M.S.; Hutchinson, W.G.; Clarke, S.M. Ontogenetic development of digestive enzymes in yellowtail kingfish Seriola lalandi larvae. Aquaculture 2006, 260, 264–271. [Google Scholar] [CrossRef]
- Dabrowski, K. The feeding of fish larvae: Present state of the art and perspectives. Reprod. Nutr. Dév. 1984, 24, 807–833. [Google Scholar] [CrossRef]
- Schwartz, S.H. Aquaculture Research Trends; Nova Science Publishers: New York, NY, USA, 2008; ISBN 978-1-60456-217-0. [Google Scholar]
- Léger, P.; Bengtson, D.; Sorgeloos, P.; Simpson, K.; Beck, A. The Nutritional Value of Artemia: A Review. In Artemia Research and Its Applications; Ecology, Culturing, Use in Aquaculture; Sorgeloos, P., Bengtson, D.A., Decleir, W., Jaspers, E., Eds.; Universa Press: Wetteren, Belgium, 1987; Volume 3, 556p. [Google Scholar]
- Luizi, F.S.; Gara, B.; Shields, R.J.; Bromage, N.R. Further description of the development of the digestive organs in Atlantic halibut (Hippoglossus hippoglossus) larvae, with notes on differential absorption of copepod and Artemia prey. Aquaculture 1999, 176, 101–116. [Google Scholar] [CrossRef]
- Schipp, G.R.; Bosmans, J.M.P.; Marshall, A.J. A method for hatchery culture of tropical calanoid copepods, Acartia spp. Aquaculture 1999, 174, 81–88. [Google Scholar] [CrossRef]
- Camus, T. The Improvement of Copepods Intensive Culture Protocols as Live Feeds for Aquaculture Hatcheries. Ph.D. Thesis, James Cook University, Douglas, QLD, Australia, 2012. [Google Scholar]
- Sorgeloos, P.; Dhert, P.; Candreva, P. Use of the brine shrimp, Artemia spp., in marine fish larviculture. Aquaculture 2001, 200, 147–159. [Google Scholar] [CrossRef]
- Abatzopoulos, T.; Clegg, J.; Sorgeloos, P.; Beardmore, J. Artemia: Basic and Applied Biology; Kluwer Academic Publishers: Dordrecht, The Netherlands; Boston, MA, USA, 2002; ISBN 978-1-4020-0746-0. [Google Scholar]
- Dabrowski, K.; Glogowski, J. Studies on the role of exogenous proteolytic enzymes in digestion processes in fish. Hydrobiologia 1977, 54, 129–134. [Google Scholar] [CrossRef]
- Kolkovski, S.; Tandler, A.; Kissil, G.W.; Gertler, A. The Effect of Dietary Exogenous Digestive Enzymes on Ingestion, Assimilation, Growth and Survival of Gilthead Seabream (Sparus aurata, Sparidae, Linnaeus) Larvae. Fish Physiol. Biochem. 1993, 12, 203–209. [Google Scholar] [CrossRef]
- FAO. Manual on the Production and Use of Live Food for Aquaculture; FAO Fisheries Technical Paper; Lavens, P., Sorgeloos, P., Eds.; FAO: Rome, Italy, 1996; ISBN 978-92-5-103934-2. [Google Scholar]
- Naz, M. The changes in the biochemical compositions and enzymatic activities of rotifer (Brachionus plicatilis, Müller) and Artemia during the enrichment and starvation periods. Fish Physiol. Biochem. 2008, 34, 391–404. [Google Scholar] [CrossRef]
- Ferreira de Sá, T.L. Substitution of Instar I by Enriched Instar II Artemia in the First Days of Solea senegalensis Rearing. Master’s Thesis, University of Porto, Porto, Portugal, 2016. [Google Scholar]
- Shao, L. Development of Larval Fish Rrearing Techniques and Nutrient Requirements for the Green Mandarin, Synchiropus splendidus: A Popular Marine Ornamental Fish. Ph.D. Thesis, James Cook University, Douglas, QLD, Australia, 2016. [Google Scholar]
- Kubitza, F.; Lovshin, L.L. Effects of initial weight and genetic strain on feed training largemouth bass Micropterus salmoides using ground fish flesh and freeze dried krill as starter diets. Aquaculture 1997, 148, 179–190. [Google Scholar] [CrossRef]
- Luz, R.K.; Portella, M.C.; Luz, R.K.; Portella, M.C. Effect of prey concentrations and feed training on production of Hoplias lacerdae juvenile. An. Acad. Bras. Ciênc. 2015, 87, 1125–1132. [Google Scholar] [CrossRef]
- Moura, M.a.M.; Kubitza, F.; Cyrino, J.E.P. Feed training of peacock bass (Cichla sp.). Rev. Bras. Biol. 2000, 60, 645–654. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).