Effect of Stocking Density on Growth, Water Quality Changes and Cost Efficiency of Butter Catfish (Ompok bimaculatus) during Seed Rearing in a Biofloc System
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics and Experimental Design
2.2. Microbial Floc Initiation
2.3. Experimental Feed and Feeding
2.4. Water Quality Parameters
2.5. Animal Growth Performance, Feed Utilization and Survival
2.6. Assay of Digestive Enzymes
2.7. Profitability Analysis
2.8. Statistical Data Analysis
3. Results
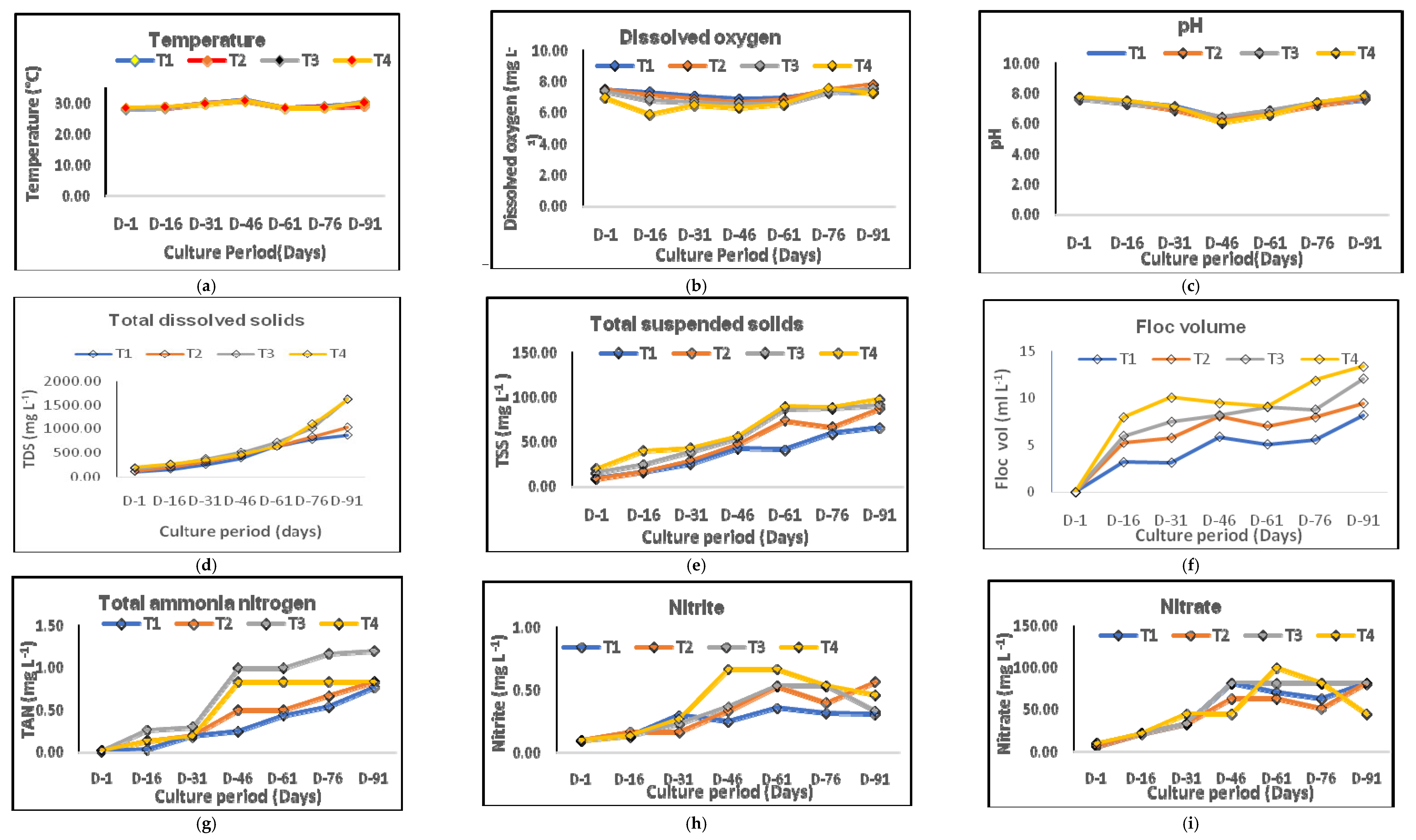
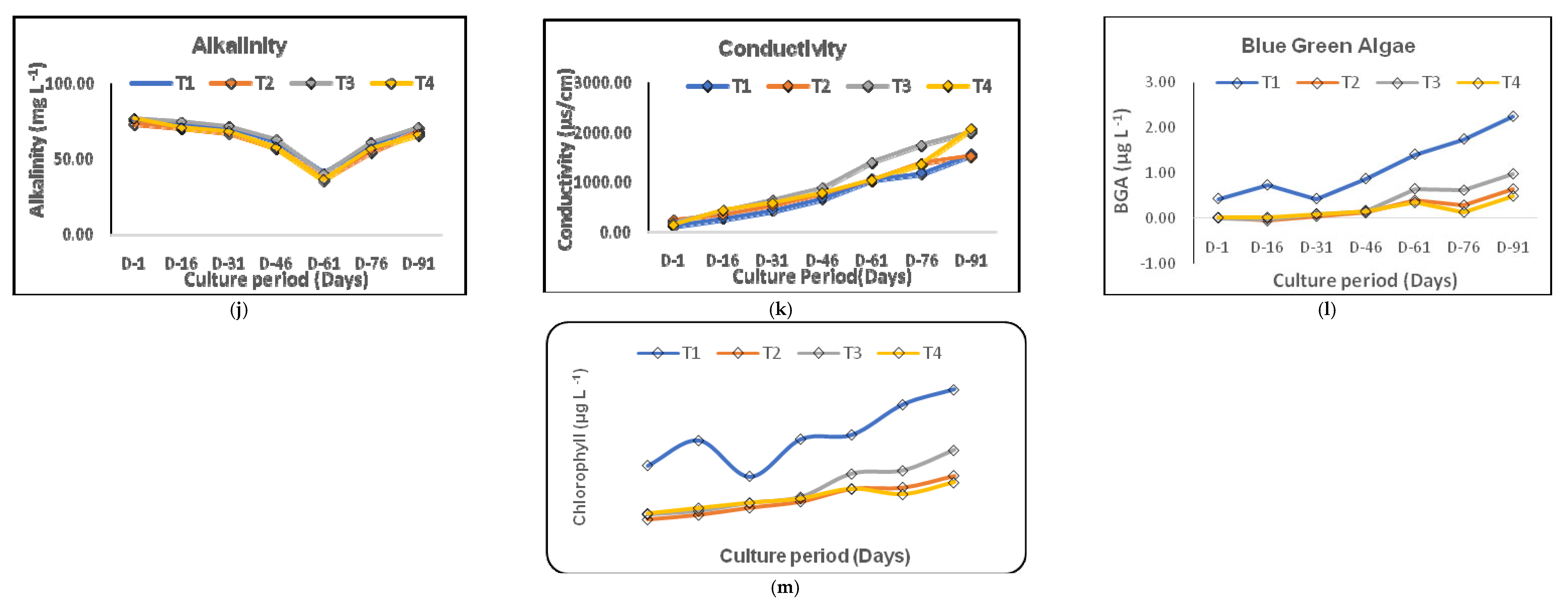
3.1. Variations in Water Quality
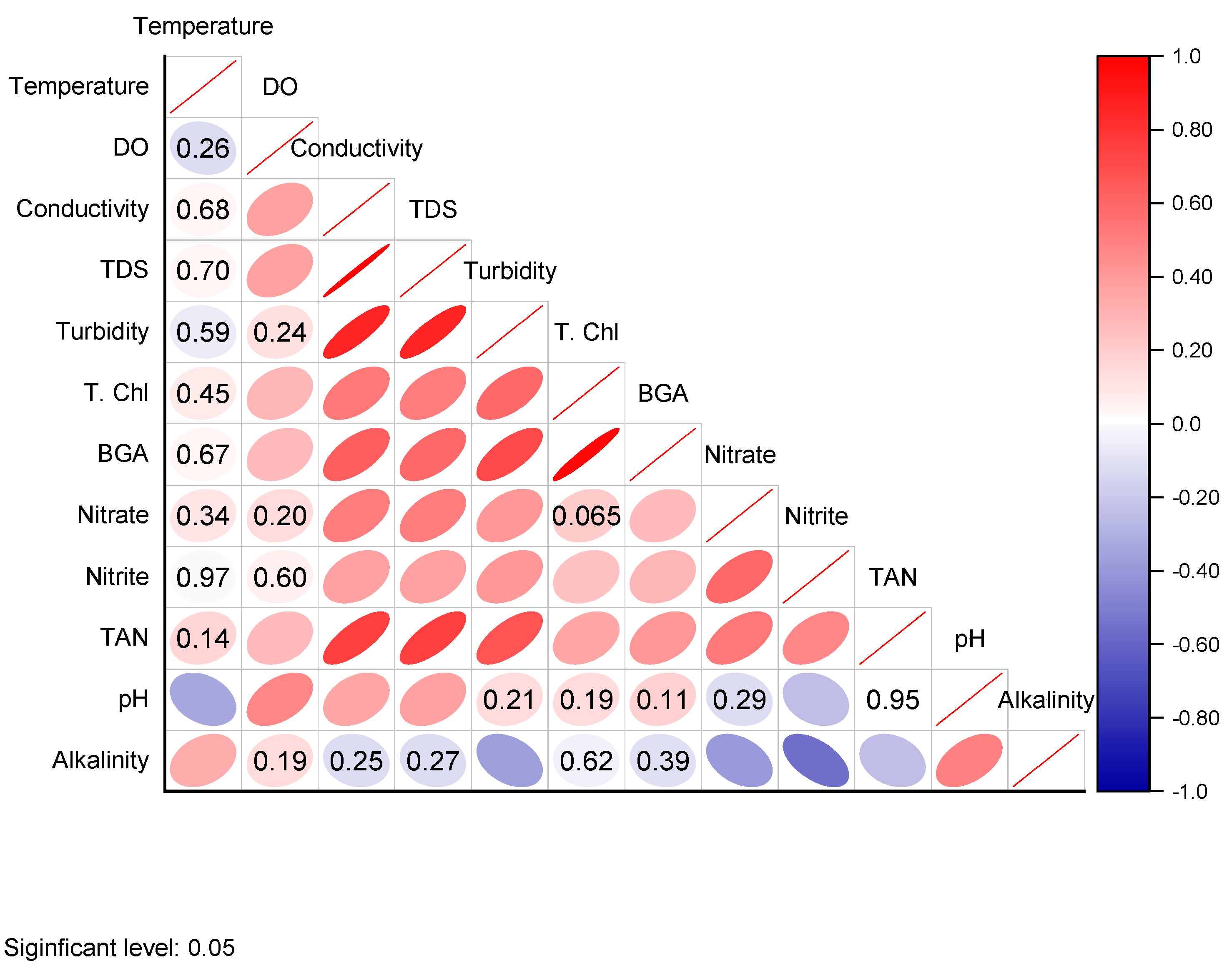
3.2. Factor Analysis
3.3. Effect on Growth and Survival of Ompok bimaculatus
3.4. Effect on Feed Utilisation
3.5. Effect on Digestive Enzyme Activities
3.6. Profitability Analysis
4. Discussion
4.1. Water Quality of the Culture System
4.2. Factor Analysis
4.3. Effect on Growth and Feed Utilization
4.4. Effect on Digestive Enzyme Activity
4.5. Profitability Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO (Food and Agriculture Organization of the United Nation). The State of World Fisheries and Aquaculture 2020; Sustainability in Action; FAO: Rome, Italy, 2020. [Google Scholar] [CrossRef]
- Piedrahita, R.H. Reducing the potential environmental impact of tank aquaculture effluents through intensification and recirculation. Aquaculture 2003, 226, 35–44. [Google Scholar] [CrossRef]
- Avnimelech, Y. Biofloc Technology: A Practical Hand Book; World Aquaculture Society: Baton Rouge, LA, USA, 2015. [Google Scholar]
- Ebeling, J.M.; Timmons, M.B.; Bisogni, J.J. Engineering analysis of the stoichiometry of photoautotrophic, autotrophic, and heterotrophic removal of ammonia–nitrogen in aquaculture systems. Aquaculture 2006, 257, 346–358. [Google Scholar] [CrossRef]
- Rafatnezhad, S.; Falahatkar, B.; Tolouei-Gilani, M.H. Effects of stocking density on haematological parameters, growth and fin erosion of great sturgeon (Huso huso) juveniles. Aquac. Res. 2008, 39, 1506–1513. [Google Scholar] [CrossRef]
- Qiang, J.; Bao, J.W.; He, J.; Tao, Y.F.; Habte-Tsion, H.M.; Xu, P. Growth, biochemical, fatty acid composition, and mRNA levels of hepatic enzymes in genetically improved farmed tilapia at different stocking densities. J. Appl. Ichthyol. 2017, 33, 757–766. [Google Scholar] [CrossRef]
- Liu, G.; Ye, Z.; Liu, D.; Zhao, J.; Sivaramasamy, E.; Deng, Y.; Zhu, S. Influence of stocking density on growth, digestive enzyme activities, immune responses, antioxidant of Oreochromis niloticus fingerlings in biofloc systems. Fish Shellfish Immunol. 2018, 81, 416–422. [Google Scholar] [CrossRef]
- Zaki, M.A.; Alabssawy, A.N.; Nour, A.E.A.M.; El-Basuini, M.F.; Dawood, M.A.; Alkahtani, S.; Abdel-Daim, M.M. The impact of stocking density and dietary carbon sources on the growth, oxidative status and stress markers of Nile tilapia (Oreochromis niloticus) reared under biofloc conditions. Aquac. Rep. 2020, 16, 100282. [Google Scholar] [CrossRef]
- El-Hawarry, W.N.; Shourbela, R.M.; Haraz, Y.G.; Khatab, S.A.; Dawood, M.A. The influence of carbon source on growth, feed efficiency, and growth-related genes in Nile tilapia (Oreochromis niloticus) reared under biofloc conditions and high stocking density. Aquaculture 2021, 542, 736919. [Google Scholar] [CrossRef]
- Manduca, L.G.; Silva, M.A.; Alvarenga, F.R.; Alves, G.F.O.; Ferreira, N.H.; Teixeira, E.A.; Fernandes, A.F.A.; Turra, E.M. Effects of different stocking densities on Nile tilapia performance and profitability of a biofloc system with a minimum water exchange. Aquaculture 2021, 530, 735814. [Google Scholar] [CrossRef]
- Debnath, S.; Ahmed, M.U.; Parvez, M.; Karmokar, A.K.; Ahsan, M. Effect of stocking density on growth performance and body composition of climbing perch (Anabas testudineus) in biofloc system. Aquac. Int. 2022, 30, 1089–1100. [Google Scholar] [CrossRef]
- Battisti, E.K.; Rabaioli, A.; Uczay, J.; Sutili, E.J.; Lazzari, R. Effect of stocking density on growth, haematological and biochemical parameters and antioxidant status of silver catfish cultured in biofloc system. Aquaculture 2020, 524, 735213. [Google Scholar] [CrossRef]
- Fauji, H.; Budiardi, T.; Ekasari, J. Growth performance and robustness of African Catfish Clarias gariepinus (Burchell) in biofloc-based nursery production with different stocking densities. Aquac. Res. 2018, 49, 1339–1346. [Google Scholar] [CrossRef]
- Basuki, F.; Yuniarti, T.; Harwanto, D.; Susilowati, T. Growth Performance of Catfish (Clarias Gariepinus Burchell, 1822) Cultured in High Density on the Biofloc System. In Proceedings of the IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2018; Volume 116, p. 012007. [Google Scholar]
- Arambam, K.; Singh, S.K.; Biswas, P.; Patel, A.B.; Jena, A.K.; Pandey, P.K. Influence of light intensity and photoperiod on embryonic development, survival and growth of threatened catfish Ompok bimaculatus early larvae. J. Fish Biol. 2020, 97, 740–752. [Google Scholar] [CrossRef] [PubMed]
- Debbarma, R.; Biswas, P.; Singh, S.K. An integrated biomarker approach to assess the welfare status of Ompok bimaculatus (Pabda) in biofloc system with altered C/N ratio and subjected to acute ammonia stress. Aquaculture 2021, 545, 737184. [Google Scholar] [CrossRef]
- Debbarma, R.; Meena, D.K.; Biswas, P.; Meitei, M.M.; Singh, S.K. Portioning of microbial waste into fish nutrition via frugal biofloc production: A sustainable paradigm for greening of environment. J. Clean. Prod. 2022, 334, 130246. [Google Scholar] [CrossRef]
- Avnimelech, Y. Carbon/nitrogen ratio as a control element in aquaculture systems. Aquaculture 1999, 176, 227–235. [Google Scholar] [CrossRef]
- Aljerf, L. Advanced highly polluted rainwater treatment process. J. Urban Environ. Eng. 2018, 12, 50–58. [Google Scholar] [CrossRef]
- APHA (American Public Health Association). Standard Methods for the Examination of Water and Wastewater, 21st ed.; Public Health Association: Washington, DC, USA, 2005. [Google Scholar]
- Avnimelech, Y. Biofloc Technology—A Practical Guide Book; World Aquaculture Society: Sorrento, LA, USA, 2009. [Google Scholar]
- Rick, W.; Stegbauer, H.P. α-Amylase measurement of reducing groups. In Methods of Enzymatic Analysis, 2nd ed.; Academic Press: Cambridge, MA, USA, 1974; Volume 2, pp. 885–890. [Google Scholar] [CrossRef]
- Drapeau, G. Protease from Staphylococcus aureus. In Methods in Enzymology; Academic Press: New York, NY, USA, 1974; p. 469. [Google Scholar]
- Cherry, I.S.; Crandall, L.A., Jr. The specificity of pancreatic lipase: Its appearance in the blood after pancreatic injury. Am. J. Physiol. 1932, 100, 266–273. [Google Scholar] [CrossRef]
- Wu, F.; Wen, H.; Tian, J.; Jiang, M.; Liu, W.; Yang, C.; Lu, X. Effect of stocking density on growth performance, serum biochemical parameters, and muscle texture properties of genetically improved farm tilapia, Oreochromis niloticus. Aquac. Int. 2018, 26, 1247–1259. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, Y.; Zhou, X.; Jiang, M. Effect of nitrate concentration on filamentous bulking under low level of dissolved oxygen in an airlift inner circular anoxic-aerobic incorporate reactor. J. Environ. Sci. 2013, 25, 1736–1744. [Google Scholar] [CrossRef]
- Sandoval-Vargas, L.Y.; Jiménez-Amaya, M.N.; Rodríguez-Pulido, J.; Guaje-Ramírez, D.N.; Ramírez-Merlano, J.A.; Medina-Robles, V.M. Applying biofloc technology in the culture of juvenile of Piaractus brachypomus (Cuvier, 1818): Effects on zootechnical performance and water quality. Aquac. Res. 2020, 51, 3865–3878. [Google Scholar] [CrossRef]
- De-Schryver, P.; Crab, R.; Defoirdt, T.; Boon, N.; Verstraete, W. The basics of bioflocs technology: The added value for aquaculture. Aquaculture 2008, 277, 125–137. [Google Scholar] [CrossRef]
- Khanjani, M.H.; Sajjadi, M.M.; Alizadeh, M.; Sourinejad, I. Nursery performance of Pacific white shrimp (Litopenaeus vannamei) cultivated in a biofloc system: The effect of adding different carbon sources. Aquac. Res. 2017, 48, 1491–1501. [Google Scholar] [CrossRef]
- Minabi, K.; Sourinejad, I.; Alizadeh, M.; Ghatrami, E.R.; Khanjani, M.H. Effects of different carbon to nitrogen ratios in the biofloc system on water quality, growth, and body composition of common carp (Cyprinus carpio) fingerlings. Aquac. Int. 2020, 28, 1883–1898. [Google Scholar] [CrossRef]
- Ekasari, J.; Maryam, S. Evaluation of biofloc technology application on water quality and production performance of red tilapia Oreochromis sp. cultured at different stocking densities. HAYATI J. Biosci. 2012, 19, 73–80. [Google Scholar] [CrossRef]
- Panigrahi, A.; Saranya, C.; Ambiganandam, K.; Sundaram, M.; Sivakumar, M.R. Evaluation of biofloc generation protocols to adopt high density nursery rearing of Penaeus vannamei for better growth performances, protective responses and immuno modulation in biofloc based technology. Aquaculture 2020, 522, 735095. [Google Scholar] [CrossRef]
- Luo, G.; Xu, J.; Meng, H. Nitrate accumulation in biofloc aquaculture systems. Aquaculture 2020, 520, 734675. [Google Scholar] [CrossRef]
- Azim, M.E.; Little, D.C. The biofloc technology (BFT) in indoor tanks: Water quality, biofloc composition, and growth and welfare of Nile tilapia (Oreochromis niloticus). Aquaculture 2008, 283, 29–35. [Google Scholar] [CrossRef]
- Kamilya, D.; Debbarma, M.; Pal, P.; Kheti, B.; Sarkar, S.; Singh, S.T. Biofloc technology application in indoor culture of Labeo rohita (Hamilton, 1822) fingerlings: The effects on inorganic nitrogen control, growth and immunity. Chemosphere 2017, 182, 8–14. [Google Scholar] [CrossRef]
- Haridas, H.; Verma, A.K.; Rathore, G.; Prakash, C.; Sawant, P.B.; Rani, A.M.B. Enhanced growth and immuno-physiological response of Genetically Improved Farmed Tilapia in indoor biofloc units at different stocking densities. Aquac. Res. 2017, 48, 4346–4355. [Google Scholar] [CrossRef]
- Adineh, H.; Naderi, M.; Hamidi, M.K.; Harsij, M. Biofloc technology improves growth, innate immune responses, oxidative status and resistance to acute stress in common carp under high stocking density. Fish Shellfish Immunol. 2019, 95, 440–448. [Google Scholar] [CrossRef]
- Aftabuddin, S.; Siddique, M.A.M.; Sein, A.; Dey, P.K.; Rashed-Un-Nabi, M.; Haque, M.A. First use of biofloc technology for Penaeus monodon culture in Bangladesh: Effects of stocking density on growth performance of shrimp, water quality and bacterial growth. Aquac. Rep. 2020, 18, 100518. [Google Scholar] [CrossRef]
- Pouey, J.F.; Piedras, S.N.; Rocha, C.B.; Tavares, R.A.; Santos, J.M.; Britto, A.P. Desempenho produtivo de juvenis de jundiá (Rhamdia quelen) submetidos a diferentes densidades de estocagem. Ars. Vet. 2011, 27, 241–245. [Google Scholar]
- Vijayan, M.M.; Ballantyne, J.S.; Leatherland, J.F. High stocking density alters the energy metabolism of brook charr, Salvelinus fontinalis. Aquaculture 1990, 88, 371–381. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.M.; Wang, L.B. Digestive enzyme and alkaline phosphatase activities during the early stages of Silurus soldatovi development. Zool. Res. 2010, 31, 627–632. [Google Scholar] [CrossRef]
- Najdegerami, E.H.; Bakhshi, F.; Lakani, F.B. Effects of biofloc on growth performance, digestive enzyme activities and liver histology of common carp (Cyprinus carpio) fingerlings in zero-water exchange system. Fish Physiol. Biochem. 2016, 42, 457–465. [Google Scholar] [CrossRef]
- Bakhshi, F.; Najdegerami, E.H.; Manaffar, R.; Tokmechi, A.; Farah, K.R.; ShalizarJalali, A. Growth performance, haematology, antioxidant status, immune response and histology of common carp (Cyprinus carpio L.) fed biofloc grown on different carbon sources. Aquac. Res. 2018, 49, 393–403. [Google Scholar] [CrossRef]
- Xu, W.J.; Pan, L.Q. Effects of bioflocs on growth performance, digestive enzyme activity and body composition of juvenile Litopenaeus vannamei in zero-water exchange tanks manipulating C/N ratio in feed. Aquaculture 2012, 356, 147–152. [Google Scholar] [CrossRef]
- Upadhyay, A.; Swain, H.S.; Das, B.K.; Ramteke, M.H.; Kumar, V.; Krishna, G.; Das, A.K. Stocking density matters in open water cage culture: Influence on growth, digestive enzymes, haemato-immuno and stress responses of Puntius sarana. Aquaculture 2021, 547, 737445. [Google Scholar] [CrossRef]
- Li, X.; Liu, Y.; Blancheton, J.P. Effect of stocking density on performances of juvenile turbot (Scophthalmus maximus) in recirculating aquaculture systems. Chin. J. Oceanol. Limnol. 2013, 31, 514–522. [Google Scholar] [CrossRef]
- Trenzado, C.E.; Carmona, R.; Merino, R.; García-Gallego, M.; Furné, M.; Domezain, A.; Sanz, A. Effect of dietary lipid content and stocking density on digestive enzymes profile and intestinal histology of rainbow trout (Oncorhynchus mykiss). Aquaculture 2018, 497, 10–16. [Google Scholar] [CrossRef]
- Avnimelech, Y. Feeding with microbial flocs by tilapia in minimal discharge bio-flocs technology ponds. Aquaculture 2007, 264, 140–147. [Google Scholar] [CrossRef]
- Luo, G.; Gao, Q.; Wang, C.; Liu, W.; Sun, D.; Li, L.; Tan, H. Growth, digestive activity, welfare, and partial cost-effectiveness of genetically improved farmed tilapia (Oreochromis niloticus) cultured in a recirculating aquaculture system and an indoor biofloc system. Aquaculture 2014, 422, 1–7. [Google Scholar] [CrossRef]
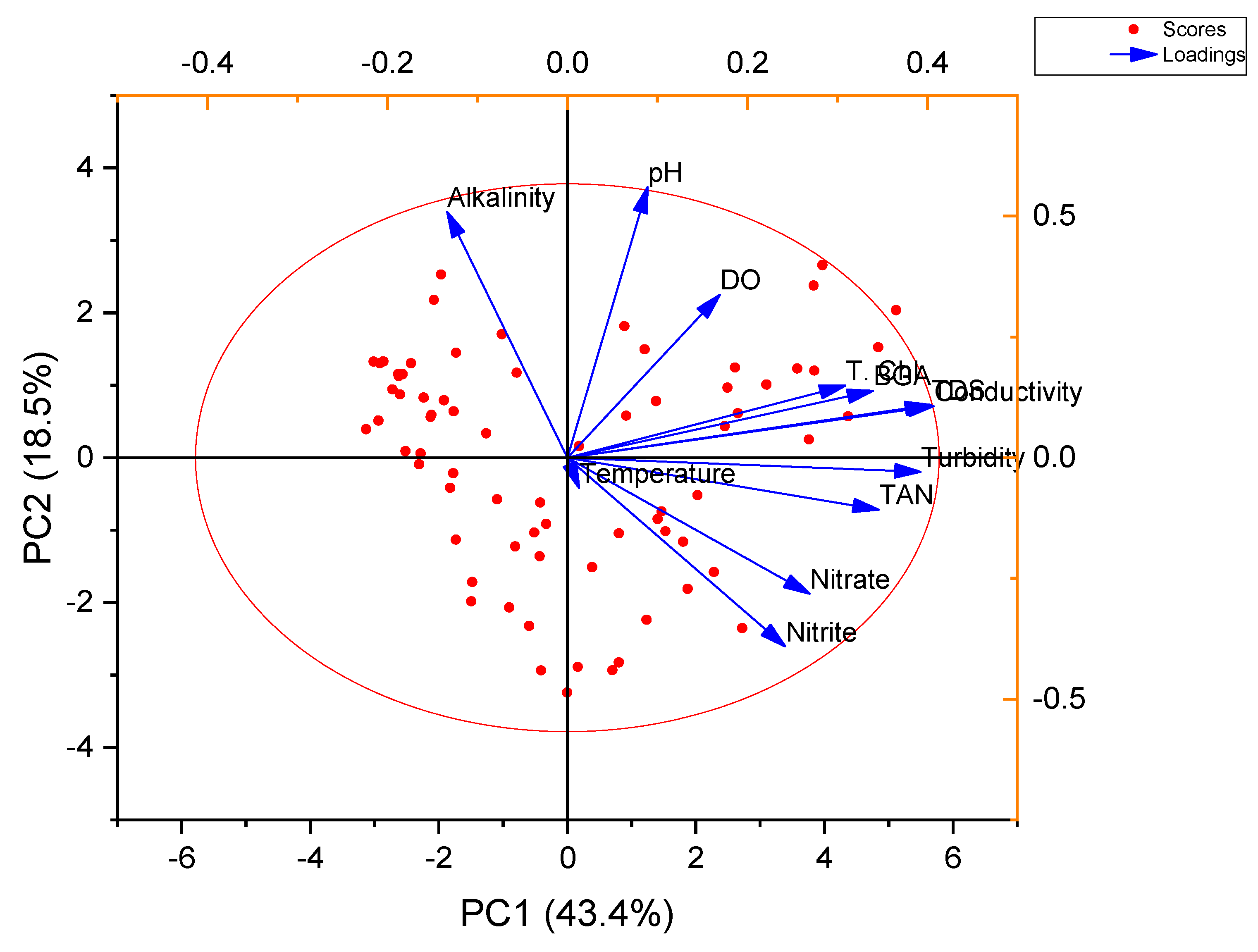
| Parameters | Factor 1 | Factor 2 | Factor 3 |
|---|---|---|---|
| TAN | 0.346 | −0.107 | 0.089 |
| Nitrite | 0.242 | −0.391 | −0.113 |
| Nitrate | 0.269 | −0.282 | −0.004 |
| Temperature | 0.013 | −0.063 | 0.811 |
| DO | 0.470 | 0.337 | −0.204 |
| pH | −0.189 | 0.559 | −0.276 |
| Conductivity | 0.401 | 0.106 | 8.801 |
| Alkalinity | −0.133 | 0.508 | 0.365 |
| Turbidity | 0.392 | −0.029 | −0.049 |
| T. Chl. | 0.309 | 0.149 | 0.205 |
| TDS | 0.404 | 0.109 | −0.009 |
| BGA | 0.339 | 0.138 | 0.160 |
| Eigenvalue | 5.202 | 2.221 | 1.345 |
| % of variance | 43.355 | 18.512 | 11.211 |
| % Cumulative | 43.355 | 61.867 | 73.078 |
| Possible interpretation | Stocking density influences DO primarily with a subsequent drop in pH due to increased CO2 release from animal respiration. A higher TDS is accompanied by heterotrophic conversion and drop in alkalinity. | Not interpreted as loading is low | Not interpreted as loading is low |
| Parameters | Experimental Groups | |||
|---|---|---|---|---|
| T1 | T2 | T3 | T4 | |
| Initial weight (g) | 1.21 ± 0.08 | 1.21 ± 0.08 | 1.21 ± 0.08 | 1.21 ± 0.08 |
| Final weight (g) | 7.29 ± 0.47 c | 4.16 ± 0.05 b | 3.74 ± 0.58 d | 2.66 ± 0.35 a |
| Net weight gain (g) | 6.06 ± 0.46 c | 2.94 ± 0.05 b | 2.51 ± 0.59 d | 1.44 ± 0.36 a |
| Total yield (g) | 121 ± 9.18 a | 118 ± 2.08 a | 150 ± 2.37 b | 115 ± 2.21 a |
| FCR | 1.11 ± 0.01 a | 1.62 ± 0.02 b | 1.64 ± 0.02 b | 1.91 ± 0.02 c |
| PER | 2.58 ± 0.02 c | 1.76 ± 0.02 b | 1.75 ± 0.03 b | 1.5 ± 0.02 a |
| Survival (%) | 95.3 ± 0.12 d | 76.0 ± 0.49 c | 69.4 ± 0.26 b | 49.6 ± 0.25 a |
| Enzymes | T1 | T2 | T3 | T4 | |
|---|---|---|---|---|---|
| Protease (U mg protein/min) | Liver | 0.08 ± 0.01 c | 0.04 ± 0.01 b | 0.01 ± 0.01 a | 0.01 ± 0.00 a |
| Gut | 0.28 ± 0.01 b | 0.09 ± 0.00 a | 0.07 ± 0.01 a | 0.08 ± 0.01 a | |
| Lipase (U mg protein/min) | Liver | 0.90 ± 0.03 c | 0.69 ± 0.02 b | 0.67 ± 0.01 b | 0.35 ± 0.03 a |
| Gut | 1.76 ± 0.14 b | 0.68 ± 0.23 a | 0.74 ± 0.14 a | 0.55 ± 0.03 a | |
| Amylase (U mg protein/min) | Liver | 0.12 ± 0.00 c | 0.06 ± 0.00 b | 0.05 ± 0.00 a | 0.05 ± 0.00 d |
| Gut | 0.41 ± 0.02 c | 0.18 ± 0.01 b | 0.17 ± 0.01 b | 0.07 ± 0.01 a |
| Parameters | Stocking Densities (g/L) | Significance | |||
|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | ||
| Seed stock cost | 0.314 ± 0.05 a | 0.629 ± 0.03 b | 0.943 ± 0.06 c | 1.256 ± 0.03 d | p < 0.05 |
| Feed cost | 0.086 ± 0.00 a | 0.108 ± 0.00 a | 0.161 ± 0.00 b | 0.166 ± 0.01 b | p < 0.05 |
| Molasses cost | 0.026 ± 0.00 a | 0.059 ± 0.00 b | 0.090 ± 0.00 c | 0.097 ± 0.00 c | p < 0.05 |
| Aeration cost | 0.859 ± 0.05 a | 1.583 ± 0.08 b | 2.872 ± 0.06 c | 3.472 ± 0.03 d | p < 0.05 |
| Probiotics cost | 0.050 ± 0.00 | 0.050 ± 0.00 | 0.050 ± 0.00 | 0.050 ± 0.00 | p > 0.05 |
| Raw salt cost | 0.0005 ± 0.00 | 0.0005 ± 0.00 | 0.001 ± 0.00 | 0.0013 ± 0.00 | p > 0.05 |
| Sodium bicarbonate cost | 0.002 ± 0.00 | 0.002 ± 0.00 | 0.002 ± 0.00 | 0.002 ± 0.00 | p > 0.05 |
| Total production cost | 1.340 ± 0.05 a | 2.438 ± 0.07 b | 4.111 ± 0.06 c | 5.043 ± 0.05 d | p < 0.05 |
| Gross income | 2.604 ± 0.203 a | 5.209 ± 0.00 b | 7.826 ± 0.00 c | 10.430 ± 0.00 d | p < 0.05 |
| Profit | 1.266 ± 0.05 a | 2.770 ± 0.07 b | 3.702 ± 0.06 c | 5.387 ± 0.04 d | p < 0.05 |
| Profit margin (%) | 1.214 ± 0.102 | 1.455 ± 0.086 | 1.150 ± 0.038 | 1.366 ± 0.025 | p > 0.05 |
| Parameters | Stocking Densities (g/L) | Significance | |||
|---|---|---|---|---|---|
| 0.5 | 1.0 | 1.5 | 2.0 | ||
| % harvested fish (>5 g) | 57.34 | 11.45 | 6.47 | 2.18 | |
| % harvested fish (3–5 g) | 36.99 | 44.26 | 48.96 | 39.12 | |
| % harvested fish (<3 g) | 5.67 | 44.29 | 44.57 | 58.70 | |
| Weighted average of fish price | 0.111 ± 0.00 c | 0.074 ± 0.00 b | 0.072 ± 0.00 d | 0.063 ± 0.00 a | p < 0.05 |
| Total production cost | 1.34 ± 0.05 a | 2.43 ± 0.07 b | 4.11 ± 0.06 c | 5.04 ± 0.05 d | p < 0.05 |
| Gross income | 2.221 ± 0.11 a | 2.949 ± 0.05 b | 4.289 ± 0.03 c | 5.00 ± 0.10 d | p < 0.05 |
| Profit | 0.884 ± 0.10 c | 0.504 ± 0.04 b | 0.176 ± 0.04 a | −0.043 ± 0.10 a | p < 0.05 |
| Profit margin (%) | 0.844 ± 0.09 c | 0.266 ± 0.03 b | 0.055 ± 0.01 a | −0.010 ± 0.02 a | p < 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Majhi, S.S.; Singh, S.K.; Biswas, P.; Debbarma, R.; Parhi, J.; Ngasotter, S.; Waikhom, G.; Meena, D.K.; Devi, A.G.; Mahanand, S.S.; et al. Effect of Stocking Density on Growth, Water Quality Changes and Cost Efficiency of Butter Catfish (Ompok bimaculatus) during Seed Rearing in a Biofloc System. Fishes 2023, 8, 61. https://doi.org/10.3390/fishes8020061
Majhi SS, Singh SK, Biswas P, Debbarma R, Parhi J, Ngasotter S, Waikhom G, Meena DK, Devi AG, Mahanand SS, et al. Effect of Stocking Density on Growth, Water Quality Changes and Cost Efficiency of Butter Catfish (Ompok bimaculatus) during Seed Rearing in a Biofloc System. Fishes. 2023; 8(2):61. https://doi.org/10.3390/fishes8020061
Chicago/Turabian StyleMajhi, Snigdha S., Soibam Khogen Singh, Pradyut Biswas, Reshmi Debbarma, Janmejay Parhi, Soibam Ngasotter, Gusheinzed Waikhom, Dharmendra Kumar Meena, Ayam Gangarani Devi, Sudhanshu S. Mahanand, and et al. 2023. "Effect of Stocking Density on Growth, Water Quality Changes and Cost Efficiency of Butter Catfish (Ompok bimaculatus) during Seed Rearing in a Biofloc System" Fishes 8, no. 2: 61. https://doi.org/10.3390/fishes8020061
APA StyleMajhi, S. S., Singh, S. K., Biswas, P., Debbarma, R., Parhi, J., Ngasotter, S., Waikhom, G., Meena, D. K., Devi, A. G., Mahanand, S. S., Xavier, K. A. M., & Patel, A. B. (2023). Effect of Stocking Density on Growth, Water Quality Changes and Cost Efficiency of Butter Catfish (Ompok bimaculatus) during Seed Rearing in a Biofloc System. Fishes, 8(2), 61. https://doi.org/10.3390/fishes8020061







